Identification of Oocyst-Driven Toxoplasma gondii Infections in Humans and Animals through Stage-Specific Serology—Current Status and Future Perspectives
Abstract
:1. Source Attribution of Toxoplasma gondii Infections Is Challenging but Relevant
1.1. A Quick Tour through the Life Cycle of Toxoplasma gondii
1.2. What Is the Relative Importance of Meat-Borne vs. Oocyst-Driven Transmission of T. gondii?
2. The Challenge of Differentiating between Meat-Borne and Oocyst-Driven T. gondii Infections
2.1. Overview of Serological Tests for the Diagnosis of T. gondii Infections in Humans and Animals
2.2. Investigating the Route of Infection—What Assays Are Available So Far?
2.3. What Antigens Are Already Described for Identification of Oocyst-Driven Infections?
2.3.1. Oocyst Wall Proteins
2.3.2. Sporozoite Proteins
3. Finding New Antigens for Identifying Oocyst-Driven T. gondii Infections by Experimental Approaches and In Silico Antigen Prediction
3.1. What Makes a Protein a Good Antigen?
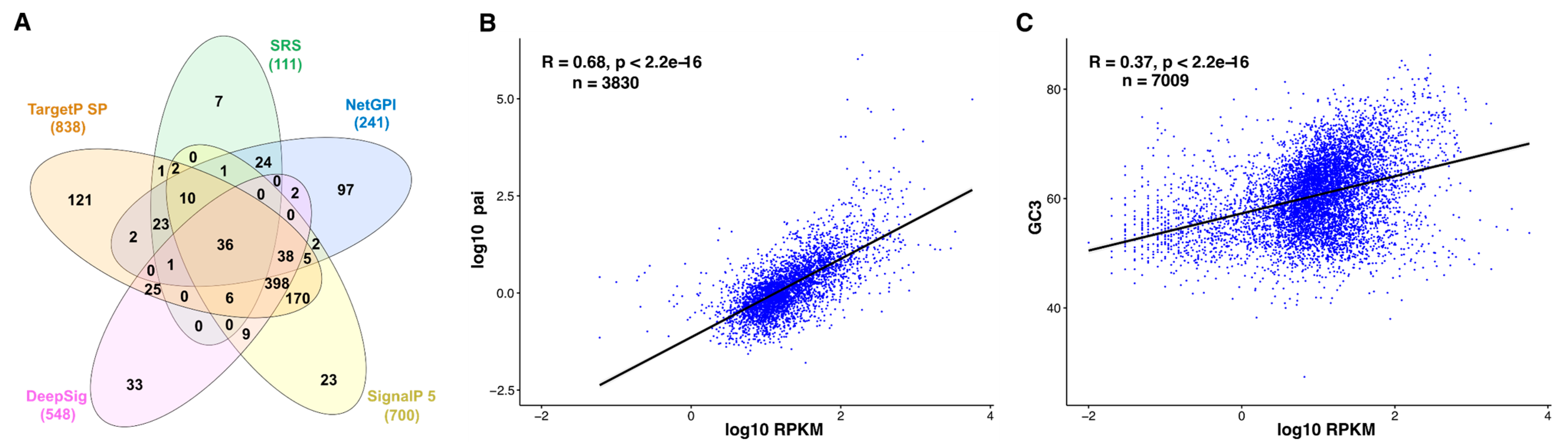
3.2. In Silico Prediction of the Surfaceome—Illustrating the Limits
3.3. Correlation between Tachyzoite mRNA and Protein Abundance—Indirect Quantitative Measures for Oocyst Proteomes?
3.4. Genome-Wide Prediction of Linear B-Cell Epitopes
3.5. Where Does This All Lead Us with Regard to the Prediction of Stage-Specific Antigens?
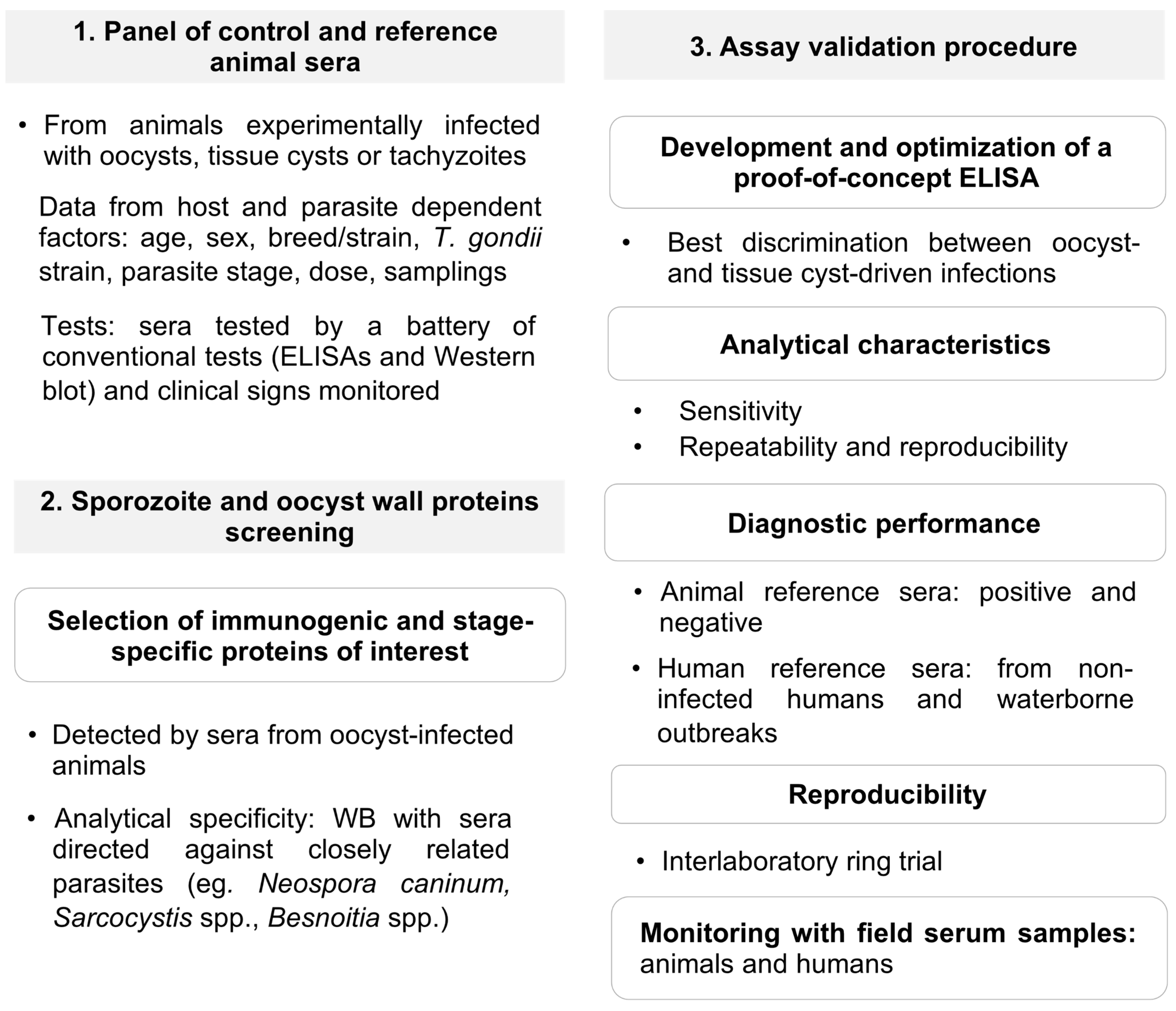
4. Verification of Protein Candidates to Identify Oocyst-Driven T. gondii Infections: The Rationale under a Proposed Workflow for Future Progress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robert-Gangneux, F.; Dardé, M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J.P.; Cerqueira-Cézar, C.K.; Murata, F.H.A.; Kwok, O.C.H.; Yang, Y.R.; Su, C. All about toxoplasmosis in cats: The last decade. Vet. Parasitol. 2020, 283, 109145. [Google Scholar] [CrossRef]
- Dubremetz, J.F.; Lebrun, M. Virulence factors of Toxoplasma gondii. Microbes Infect. 2012, 14, 1403–1410. [Google Scholar] [CrossRef]
- Cassini, A.; Colzani, E.; Pini, A.; Mangen, M.J.; Plass, D.; McDonald, S.A.; Maringhini, G.; van Lier, A.; Haagsma, J.A.; Havelaar, A.H.; et al. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): Results from the Burden of Communicable Diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Eur. Surv. 2018, 23, 17–00454. [Google Scholar] [CrossRef] [Green Version]
- Greigert, V.; Bittich-Fahmi, F.; Pfaff, A.W. Pathophysiology of ocular toxoplasmosis: Facts and open questions. PLoS Negl. Trop. Dis. 2020, 14, e0008905. [Google Scholar] [CrossRef]
- Johnson, H.J.; Koshy, A.A. Latent toxoplasmosis effects on rodents and humans: How much is real and how much is media hype? mBio 2020, 11, e02164-19. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J.P. Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 1998, 28, 1019–1024. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J.P.; Lindsay, D.S.; Speer, C.A. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 1998, 11, 267–299. [Google Scholar] [CrossRef] [Green Version]
- Frenkel, J.K. Toxoplasma in and around us. BioScience 1973, 23, 343–352. [Google Scholar] [CrossRef]
- Dubey, J.P. Infectivity and pathogenicity of Toxoplasma gondii oocysts for cats. J. Parasitol. 1996, 82, 957–961. [Google Scholar] [CrossRef]
- Miller, N.L.; Frenkel, J.K.; Dubey, J.P. Oral infections with Toxoplasma cysts and oocysts in felines, other mammals, and in birds. J. Parasitol. 1972, 58, 928–937. [Google Scholar] [CrossRef]
- Prestrud, K.W.; Åsbakk, K.; Oksanen, A.; Näreaho, A.; Jokelainen, P. Toxoplasma gondii in the Subarctic and Arctic. Acta Vet. Scand. 2010, 52, S7. [Google Scholar] [CrossRef] [Green Version]
- Rougier, S.; Montoya, J.G.; Peyron, F. Lifelong persistence of Toxoplasma cysts: A questionable dogma? Trends Parasitol. 2017, 33, 93–101. [Google Scholar] [CrossRef]
- Dubey, J.P. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 2009, 39, 877–882. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards; Koutsoumanis, K.; Allende, A.; Alvarez-Ordonez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. Public health risks associated with food-borne parasites. EFSA J. 2018, 16, e05495. [Google Scholar] [CrossRef] [PubMed]
- Delgado Betancourt, E.; Hamid, B.; Fabian, B.T.; Klotz, C.; Hartmann, S.; Seeber, F. From entry to early dissemination—Toxoplasma gondii’s initial encounter with its host. Front. Cell. Infect. Microbiol. 2019, 9, 46. [Google Scholar] [CrossRef]
- Tartarelli, I.; Tinari, A.; Possenti, A.; Cherchi, S.; Falchi, M.; Dubey, J.P.; Spano, F. During host cell traversal and cell-to-cell passage, Toxoplasma gondii sporozoites inhabit the parasitophorous vacuole and posteriorly release dense granule protein-associated membranous trails. Int. J. Parasitol. 2020, 50, 1099–1115. [Google Scholar] [CrossRef]
- Sasai, M.; Yamamoto, M. Innate, adaptive, and cell-autonomous immunity against Toxoplasma gondii infection. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hunter, C.A.; Sibley, L.D. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 2012, 10, 766–778. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, E.J.; Simon, A.; Bachand, N.; Stephen, C. Wildlife parasites in a One Health world. Trends Parasitol. 2015, 31, 174–180. [Google Scholar] [CrossRef] [PubMed]
- van der Giessen, J.; Deksne, G.; Gómez-Morales, M.A.; Troell, K.; Gomes, J.; Sotiraki, S.; Rozycki, M.; Kucsera, I.; Djurković-Djaković, O.; Robertson, L.J. Surveillance of foodborne parasitic diseases in Europe in a One Health approach. Parasit. Epidemiol. Control 2021, 13, e00205. [Google Scholar] [CrossRef]
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global status of Toxoplasma gondii infection: Systematic review and prevalence snapshots. Trop. Biomed. 2019, 36, 898–925. [Google Scholar]
- Hiszczyńska-Sawicka, E.; Gatkowska, J.M.; Grzybowski, M.M.; Długońska, H. Veterinary vaccines against toxoplasmosis. Parasitology 2014, 141, 1365–1378. [Google Scholar] [CrossRef]
- Stelzer, S.; Basso, W.; Benavides Silván, J.; Ortega-Mora, L.M.; Maksimov, P.; Gethmann, J.; Conraths, F.J.; Schares, G. Toxoplasma gondii infection and toxoplasmosis in farm animals: Risk factors and economic impact. Food Waterb. Parasitol. 2019, 15, e00037. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis in pigs—The last 20 years. Vet. Parasitol. 2009, 164, 89–103. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lunney, J.K.; Shen, S.K.; Kwok, O.C.; Ashford, D.A.; Thulliez, P. Infectivity of low numbers of Toxoplasma gondii oocysts to pigs. J. Parasitol. 1996, 82, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Oocyst shedding by cats fed isolated bradyzoites and comparison of infectivity of bradyzoites of the VEG strain Toxoplasma gondii to cats and mice. J. Parasitol. 2001, 87, 215–219. [Google Scholar] [CrossRef]
- Dubey, J.P. Comparative infectivity of oocysts and bradyzoites of Toxoplasma gondii for intermediate (mice) and definitive (cats) hosts. Vet. Parasitol. 2006, 140, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, T.; Sarvi, S.; Moosazadeh, M.; Daryani, A. Global prevalence of Toxoplasma gondii infection in the aborted fetuses and ruminants that had an abortion: A systematic review and meta-analysis. Vet. Parasitol. 2021, 290, 109370. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.; Berg, R.; Tagel, M.; Must, K.; Deksne, G.; Enemark, H.L.; Alban, L.; Johansen, M.V.; Nielsen, H.V.; Sandberg, M.; et al. Seroprevalence of Toxoplasma gondii in domestic pigs, sheep, cattle, wild boars, and moose in the Nordic-Baltic region: A systematic review and meta-analysis. Parasit. Epidemiol. Control 2019, 5, e00100. [Google Scholar] [CrossRef]
- Cook, A.J.; Gilbert, R.E.; Buffolano, W.; Zufferey, J.; Petersen, E.; Jenum, P.A.; Foulon, W.; Semprini, A.E.; Dunn, D.T. Sources of toxoplasma infection in pregnant women: European multicentre case-control study. European Research Network on Congenital Toxoplasmosis. Brit. Med. J. 2000, 321, 142–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hald, T.; Aspinall, W.; Devleesschauwer, B.; Cooke, R.; Corrigan, T.; Havelaar, A.H.; Gibb, H.J.; Torgerson, P.R.; Kirk, M.D.; Angulo, F.J.; et al. World Health Organization estimates of the relative contributions of food to the burden of disease due to selected foodborne hazards: A structured expert elicitation. PLoS ONE 2016, 11, e0145839. [Google Scholar] [CrossRef] [Green Version]
- Pinto-Ferreira, F.; Caldart, E.T.; Pasquali, A.K.S.; Mitsuka-Breganó, R.; Freire, R.L.; Navarro, I.T. Patterns of transmission and sources of infection in outbreaks of human toxoplasmosis. Emerg. Infect. Dis. 2019, 25, 2177–2182. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, C.; Heinkel, S.-B.; Zacharias, N.; Mertens, F.-M.; Christoffels, E.; Gayer, U.; Koch, C.; Kistemann, T. Infectious rain? Evaluation of human pathogen concentrations in stormwater in separate sewer systems. Water Sci. Technol. 2019, 80, 1022–1030. [Google Scholar] [CrossRef]
- Roghmann, M.C.; Faulkner, C.T.; Lefkowitz, A.; Patton, S.; Zimmerman, J.; Morris, J.G., Jr. Decreased seroprevalence for Toxoplasma gondii in Seventh Day Adventists in Maryland. Am. J. Trop. Med. Hyg. 1999, 60, 790–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, E.M.; Banerjee, S.N. The seroepidemiology of toxoplasmosis in the lower Fraser Valley of British Columbia. Can. J. Infect. Dis. 1994, 5, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Hall, S.M.; Pandit, A.; Golwilkar, A.; Williams, T.S. How do Jains get toxoplasma infection? Lancet 1999, 354, 486–487. [Google Scholar] [CrossRef]
- de Wit, L.A.; Croll, D.A.; Tershy, B.; Correa, D.; Luna-Pasten, H.; Quadri, P.; Kilpatrick, A.M. Potential public health benefits from cat eradications on islands. PLoS Negl. Trop. Dis. 2019, 13, e0007040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Shapiro, K.; VanWormer, E. Dynamics and epidemiology of Toxoplasma gondii oocyst shedding in domestic and wild felids. Transb. Emerg. Dis. 2021. [Google Scholar] [CrossRef]
- Dubey, J.P. Outbreaks of clinical toxoplasmosis in humans: Five decades of personal experience, perspectives and lessons learned. Parasites Vect. 2021, 14, 263. [Google Scholar] [CrossRef]
- Almeria, S.; Dubey, J.P. Foodborne transmission of Toxoplasma gondii infection in the last decade. An overview. Res. Vet. Sci. 2021, 135, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterb. Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef]
- Opsteegh, M.; Kortbeek, T.M.; Havelaar, A.H.; van der Giessen, J.W. Intervention strategies to reduce human Toxoplasma gondii disease burden. Clin. Infect. Dis. 2015, 60, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.L.; Dubey, J.P. Waterborne toxoplasmosis--recent developments. Exp. Parasitol. 2010, 124, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Minuzzi, C.E.; Fernandes, F.D.; Portella, L.P.; Bräunig, P.; Sturza, D.A.F.; Giacomini, L.; Salvagni, E.; Ribeiro, J.D.S.; Silva, C.R.; Difante, C.M.; et al. Contaminated water confirmed as source of infection by bioassay in an outbreak of toxoplasmosis in South Brazil. Transb. Emerg. Dis. 2021, 68, 767–772. [Google Scholar] [CrossRef]
- Wyrosdick, H.M.; Schaefer, J.J. Toxoplasma gondii: History and diagnostic test development. Anim. Health Res. Rev. 2015, 16, 150–162. [Google Scholar] [CrossRef]
- Teimouri, A.; Mohtasebi, S.; Kazemirad, E.; Keshavarz, H. Role of Toxoplasma gondii IgG avidity testing in discriminating between acute and chronic toxoplasmosis in pregnancy. J. Clin. Microbiol. 2020, 58, e00505-20. [Google Scholar] [CrossRef] [PubMed]
- Dard, C.; Fricker-Hidalgo, H.; Brenier-Pinchart, M.-P.; Pelloux, H. Relevance of and new developments in serology for toxoplasmosis. Trends Parasitol. 2016, 32, 492–506. [Google Scholar] [CrossRef]
- Ybañez, R.H.D.; Ybañez, A.P.; Nishikawa, Y. Review on the current trends of toxoplasmosis serodiagnosis in humans. Front. Cell. Infect. Microbiol. 2020, 10, 204. [Google Scholar] [CrossRef]
- Vieira, F.P.; Alves Mda, G.; Martins, L.M.; Rangel, A.L.; Dubey, J.P.; Hill, D.; Bahia-Oliveira, L.M. Waterborne toxoplasmosis investigated and analysed under hydrogeological assessment: New data and perspectives for further research. Mem. Inst. Oswaldo Cruz 2015, 110, 929–935. [Google Scholar] [CrossRef]
- Holec-Gasior, L. Toxoplasma gondii recombinant antigens as tools for serodiagnosis of human toxoplasmosis: Current status of studies. Clin. Vacc. Immunol. 2013, 20, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Felgner, J.; Juarez, S.; Hung, C.; Liang, L.I.; Jain, A.; Döşkaya, M.; Felgner, P.L.; Caner, A.; Gürüz, Y.; Davies, D.H. Identification of Toxoplasma gondii antigens associated with different types of infection by serum antibody profiling. Parasitology 2015, 142, 827–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomares, C.; Montoya, J.G. Laboratory diagnosis of congenital toxoplasmosis. J. Clin. Microbiol. 2016, 54, 2448–2454. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wang, Z.D.; Huang, S.Y.; Zhu, X.Q. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites Vect. 2015, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Lind, P.; Haugegaard, J.; Wingstrand, A.; Henriksen, S.A. The time course of the specific antibody response by various ELISAs in pigs experimentally infected with Toxoplasma gondii. Vet. Parasitol. 1997, 71, 1–15. [Google Scholar] [CrossRef]
- Payne, R.A.; Joynson, D.H.; Wilsmore, A.J. Enzyme-linked immunosorbent assays for the measurement of specific antibodies in experimentally induced ovine toxoplasmosis. Epidemiol. Infect. 1988, 100, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suaréz-Aranda, F.; Galisteo, A.J.; Hiramoto, R.M.; Cardoso, R.P.; Meireles, L.R.; Miguel, O.; Andrade, H.F., Jr. The prevalence and avidity of Toxoplasma gondii IgG antibodies in pigs from Brazil and Peru. Vet. Parasitol. 2000, 91, 23–32. [Google Scholar] [CrossRef]
- Basso, W.; Grimm, F.; Ruetten, M.; Djokic, V.; Blaga, R.; Sidler, X.; Deplazes, P. Experimental Toxoplasma gondii infections in pigs: Humoral immune response, estimation of specific IgG avidity and the challenges of reproducing vertical transmission in sows. Vet. Parasitol. 2017, 236, 76–85. [Google Scholar] [CrossRef]
- Sager, H.; Gloor, M.; Tenter, A.; Maley, S.; Hässig, M.; Gottstein, B. Immunodiagnosis of primary Toxoplasma gondii infection in sheep by the use of a P30 IgG avidity ELISA. Parasitol. Res. 2003, 91, 171–174. [Google Scholar] [CrossRef]
- Lundén, A. Immune responses in sheep after immunization with Toxoplasma gondii antigens incorporated into iscoms. Vet. Parasitol. 1995, 56, 23–35. [Google Scholar] [CrossRef]
- Caballero-Ortega, H.; Quiroz-Romero, H.; Olazarán-Jenkins, S.; Correa, D. Frequency of Toxoplasma gondii infection in sheep from a tropical zone of Mexico and temporal analysis of the humoral response changes. Parasitology 2008, 135, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Coss, C.; Dubey, J.P.; Wroblewski, K.; Sautter, M.; Hosten, T.; Muñoz-Zanzi, C.; Mui, E.; Withers, S.; Boyer, K.; et al. Identification of a sporozoite-specific antigen from Toxoplasma gondii. J. Parasitol. 2011, 97, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Wang, Z.-D.; El-Ashram, S.; Liu, Q. Toxoplasma gondii oocyst-driven infection in pigs, chickens and humans in northeastern China. BMC Vet. Res. 2019, 15, 366. [Google Scholar] [CrossRef] [Green Version]
- Santana, S.S.; Gebrim, L.C.; Carvalho, F.R.; Barros, H.S.; Barros, P.C.; Pajuaba, A.C.; Messina, V.; Possenti, A.; Cherchi, S.; Reiche, E.M.; et al. CCp5A Protein from Toxoplasma gondii as a serological marker of oocyst-driven infections in humans and domestic animals. Front. Microbiol. 2015, 6, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Döşkaya, M.; Caner, A.; Can, H.; İz, S.G.; Gedik, Y.; Döşkaya, A.D.; Kalantari-Dehaghi, M.; Gürüz, Y. Diagnostic value of a rec-ELISA using Toxoplasma gondii recombinant SporoSAG, BAG1, and GRA1 proteins in murine models infected orally with tissue cysts and oocysts. PLoS ONE 2014, 9, e108329. [Google Scholar] [CrossRef]
- Fabian, B.T.; Lepenies, B.; Schares, G.; Dubey, J.P.; Spano, F.; Seeber, F. Expanding the known repertoire of c-type lectin receptors binding to Toxoplasma gondii oocysts using a modified high-resolution immunofluorescence assay. mSphere 2021, 6, e01341-20. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.J.; Birch-Andersen, A.; Siim, J.C.; Hutchison, W.M. An ultrastructural study on the excystation of the sporozoites of Toxoplasma gondii. Acta Pathol. Microbiol. Scand. B 1979, 87, 277–283. [Google Scholar] [CrossRef]
- Ferguson, D.J.; Hutchison, W.M.; Siim, J.C. The ultrastructural development of the macrogamete and formation of the oocyst wall of Toxoplasma gondii. Acta Pathol. Microbiol. Scand. B 1975, 83, 491–505. [Google Scholar] [CrossRef]
- Bushkin, G.G.; Motari, E.; Magnelli, P.; Gubbels, M.J.; Dubey, J.P.; Miska, K.B.; Bullitt, E.; Costello, C.E.; Robbins, P.W.; Samuelson, J. β-1,3-glucan, which can be targeted by drugs, forms a trabecular scaffold in the oocyst walls of Toxoplasma and Eimeria. mBio 2012, 3, 00258-12. [Google Scholar] [CrossRef] [Green Version]
- Bushkin, G.G.; Motari, E.; Carpentieri, A.; Dubey, J.P.; Costello, C.E.; Robbins, P.W.; Samuelson, J. Evidence for a structural role for acid-fast lipids in oocyst walls of Cryptosporidium, Toxoplasma, and Eimeria. mBio 2013, 4, e00387-13. [Google Scholar] [CrossRef] [Green Version]
- Possenti, A.; Cherchi, S.; Bertuccini, L.; Pozio, E.; Dubey, J.P.; Spano, F. Molecular characterisation of a novel family of cysteine-rich proteins of Toxoplasma gondii and ultrastructural evidence of oocyst wall localisation. Int. J. Parasitol. 2010, 40, 1639–1649. [Google Scholar] [CrossRef]
- Salman, D.; Okuda, L.H.; Ueno, A.; Dautu, G.; Zhang, F.; Igarashi, M. Evaluation of novel oocyst wall protein candidates of Toxoplasma gondii. Parasitol. Int. 2017, 66, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Spano, F.; Puri, C.; Ranucci, L.; Putignani, L.; Crisanti, A. Cloning of the entire COWP gene of Cryptosporidium parvum and ultrastructural localization of the protein during sexual parasite development. Parasitology 1997, 114, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Burrells, A.; Opsteegh, M.; Pollock, K.G.; Alexander, C.L.; Chatterton, J.; Evans, R.; Walker, R.; McKenzie, C.A.; Hill, D.; Innes, E.A.; et al. The prevalence and genotypic analysis of Toxoplasma gondii from individuals in Scotland, 2006–2012. Parasit. Vect. 2016, 9, 324. [Google Scholar] [CrossRef] [Green Version]
- Mangiavacchi, B.M.; Vieira, F.P.; Bahia-Oliveira, L.M.; Hill, D. Salivary IgA against sporozoite-specific embryogenesis-related protein (TgERP) in the study of horizontally transmitted toxoplasmosis via T. gondii oocysts in endemic settings. Epidemiol. Infect. 2016, 144, 2568–2577. [Google Scholar] [CrossRef] [Green Version]
- Crawford, J.; Lamb, E.; Wasmuth, J.; Grujic, O.; Grigg, M.E.; Boulanger, M.J. Structural and functional characterization of SporoSAG: A SAG2-related surface antigen from Toxoplasma gondii. J. Biol. Chem. 2010, 285, 12063–12070. [Google Scholar] [CrossRef] [Green Version]
- Fritz, H.M.; Bowyer, P.W.; Bogyo, M.; Conrad, P.A.; Boothroyd, J.C. Proteomic analysis of fractionated Toxoplasma oocysts reveals clues to their environmental resistance. PLoS ONE 2012, 7, e29955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, H.M.; Buchholz, K.R.; Chen, X.; Durbin-Johnson, B.; Rocke, D.M.; Conrad, P.A.; Boothroyd, J.C. Transcriptomic analysis of Toxoplasma development reveals many novel functions and structures specific to sporozoites and oocysts. PLoS ONE 2012, 7, e29998. [Google Scholar] [CrossRef]
- Jung, C.; Lee, C.Y.; Grigg, M.E. The SRS superfamily of Toxoplasma surface proteins. Int. J. Parasitol. 2004, 34, 285–296. [Google Scholar] [CrossRef]
- Possenti, A.; Fratini, F.; Fantozzi, L.; Pozio, E.; Dubey, J.P.; Ponzi, M.; Pizzi, E.; Spano, F. Global proteomic analysis of the oocyst/sporozoite of Toxoplasma gondii reveals commitment to a host-independent lifestyle. BMC Genom. 2013, 14, 183. [Google Scholar] [CrossRef] [Green Version]
- Radke, J.R.; Gubbels, M.J.; Jerome, M.E.; Radke, J.B.; Striepen, B.; White, M.W. Identification of a sporozoite-specific member of the Toxoplasma SAG superfamily via genetic complementation. Mol. Microbiol. 2004, 52, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Dessens, J.T.; Sinden, R.E.; Claudianos, C. LCCL proteins of apicomplexan parasites. Trends Parasitol. 2004, 20, 102–108. [Google Scholar] [CrossRef]
- Kasper, L.H.; Bradley, M.S.; Pfefferkorn, E.R. Identification of stage-specific sporozoite antigens of Toxoplasma gondii by monoclonal antibodies. J. Immunol. 1984, 132, 443–449. [Google Scholar] [PubMed]
- Kasper, L.H.; Ware, P.L. Recognition and characterization of stage-specific oocyst/sporozoite antigens of Toxoplasma gondii by human antisera. J. Clin. Investig. 1985, 75, 1570–1577. [Google Scholar] [CrossRef] [Green Version]
- Hruzik, A.; Asif, A.R.; Gross, U. Identification of Toxoplasma gondii SUB1 antigen as a marker for acute infection by use of an innovative evaluation method. J. Clin. Microbiol. 2011, 49, 2419–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, G.Y.; Zhang, J.Z.; Yin, G.R.; Zhang, J.H.; Meng, X.L.; Zhao, F. Toxoplasma gondii: Proteomic analysis of antigenicity of soluble tachyzoite antigen. Exp. Parasitol. 2009, 122, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yeng, C.; Osman, E.; Mohamed, Z.; Noordin, R. Detection of immunogenic parasite and host-specific proteins in the sera of active and chronic individuals infected with Toxoplasma gondii. Electrophoresis 2010, 31, 3843–3849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Döşkaya, M.; Juarez, S.; Caner, A.; Jasinskas, A.; Tan, X.; Hajagos, B.E.; Bradley, P.J.; Korkmaz, M.; Gürüz, Y.; et al. Identification of potential serodiagnostic and subunit vaccine antigens by antibody profiling of toxoplasmosis cases in Turkey. Mol. Cell. Proteom. 2011, 10, 6916. [Google Scholar] [CrossRef] [Green Version]
- Döşkaya, M.; Liang, L.; Jain, A.; Can, H.; İz, S.G.; Felgner, P.L.; Döşkaya, A.D.; Davies, D.H.; Gürüz, A.Y. Discovery of new Toxoplasma gondii antigenic proteins using a high throughput protein microarray approach screening sera of murine model infected orally with oocysts and tissue cysts. Parasites Vect. 2018, 11, 393. [Google Scholar] [CrossRef]
- Kassegne, K.; Zhou, X.-N.; Abe, E.M.; Chen, J.-H. Immunomic approaches for antigen discovery of human parasites. Expert Rev. Proteom. 2016, 13, 1091–1101. [Google Scholar] [CrossRef]
- Jaenisch, T.; Heiss, K.; Fischer, N.; Geiger, C.; Bischoff, F.R.; Moldenhauer, G.; Rychlewski, L.; Sié, A.; Coulibaly, B.; Seeberger, P.H.; et al. High-density peptide arrays help to identify linear immunogenic B-cell epitopes in individuals naturally exposed to malaria infection. Mol. Cell. Proteom. 2019, 18, 642–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arranz-Solís, D.; Carvalheiro, C.G.; Zhang, E.R.; Grigg, M.E.; Saeij, J.P.J. Toxoplasma GRA peptide-specific serologic fingerprints discriminate among major strains causing toxoplasmosis. Front. Cell. Infect. Microbiol. 2021, 11, 621738. [Google Scholar] [CrossRef]
- Kong, J.T.; Grigg, M.E.; Uyetake, L.; Parmley, S.; Boothroyd, J.C. Serotyping of Toxoplasma gondii infections in humans using synthetic peptides. J. Infect. Dis. 2003, 187, 1484–1495. [Google Scholar] [CrossRef] [Green Version]
- Maksimov, P.; Zerweck, J.; Maksimov, A.; Hotop, A.; Gross, U.; Pleyer, U.; Spekker, K.; Däubener, W.; Werdermann, S.; Niederstrasser, O.; et al. Peptide microarray analysis of in silico-predicted epitopes for serological diagnosis of Toxoplasma gondii infection in humans. Clin. Vacc. Immunol. 2012, 19, 865–874. [Google Scholar] [CrossRef]
- Arranz-Solís, D.; Cordeiro, C.; Young, L.H.; Dardé, M.L.; Commodaro, A.G.; Grigg, M.E.; Saeij, J.P.J. Serotyping of Toxoplasma gondii infection using peptide membrane arrays. Front. Cell. Infect. Microbiol. 2019, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Felgner, P.L. A systems biology approach for diagnostic and vaccine antigen discovery in tropical infectious diseases. Curr. Opin. Infect. Dis. 2015, 28, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Camponovo, F.; Campo, J.J.; Le, T.Q.; Oberai, A.; Hung, C.; Pablo, J.V.; Teng, A.A.; Liang, X.; Sim, B.K.L.; Jongo, S. Proteome-wide analysis of a malaria vaccine study reveals personalized humoral immune profiles in Tanzanian adults. eLife 2020, 9, e53080. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Campo, J.J.; Le, T.Q.; Liang, X.; Bentley, S.D.; Hanage, W.P.; Lipsitch, M. Diverse evolutionary patterns of pneumococcal antigens identified by pangenome-wide immunological screening. Proc. Natl. Acad. Sci. USA 2017, 114, E357–E366. [Google Scholar] [CrossRef] [Green Version]
- Da Cunha, J.P.; Galante, P.A.; de Souza, J.E.; de Souza, R.F.; Carvalho, P.M.; Ohara, D.T.; Moura, R.P.; Oba-Shinja, S.M.; Marie, S.K.; Silva, W.A., Jr.; et al. Bioinformatics construction of the human cell surfaceome. Proc. Natl. Acad. Sci. USA 2009, 106, 16752–16757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Qin, H.; Ye, M. An overview on enrichment methods for cell surface proteome profiling. J. Sep. Sci. 2020, 43, 292–312. [Google Scholar] [CrossRef]
- Che, F.-Y.; Madrid-Aliste, C.; Burd, B.; Zhang, H.; Nieves, E.; Kim, K.; Fiser, A.; Angeletti, R.H.; Weiss, L.M. Comprehensive proteomic analysis of membrane proteins in Toxoplasma gondii. Mol. Cell. Proteom. 2011, 10, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harb, O.S.; Roos, D.S. ToxoDB: Functional genomics resource for Toxoplasma and related organisms. Methods Mol. Biol. 2020, 2071, 27–47. [Google Scholar] [CrossRef]
- Tu, V.; Mayoral, J.; Sugi, T.; Tomita, T.; Han, B.; Ma, Y.F.; Weiss, L.M. Enrichment and proteomic characterization of the cyst wall from in vitro Toxoplasma gondii cysts. mBio 2019, 10, e00469-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, V.; Tomita, T.; Sugi, T.; Mayoral, J.; Han, B.; Yakubu, R.R.; Williams, T.; Horta, A.; Ma, Y.; Weiss, L.M. The Toxoplasma gondii cyst wall interactome. mBio 2020, 11, e02699-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barylyuk, K.; Koreny, L.; Ke, H.; Butterworth, S.; Crook, O.M.; Lassadi, I.; Gupta, V.; Tromer, E.; Mourier, T.; Stevens, T.J.; et al. A comprehensive subcellular atlas of the Toxoplasma proteome via hyperLOPIT provides spatial context for protein functions. Cell Host Microbe 2020, 28, 752–766. [Google Scholar] [CrossRef]
- Mulvey, C.M.; Breckels, L.M.; Geladaki, A.; Britovšek, N.K.; Nightingale, D.J.H.; Christoforou, A.; Elzek, M.; Deery, M.J.; Gatto, L.; Lilley, K.S. Using hyperLOPIT to perform high-resolution mapping of the spatial proteome. Nat. Protoc. 2017, 12, 1110–1135. [Google Scholar] [CrossRef]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Profiti, G.; Casadio, R. BUSCA: An integrative web server to predict subcellular localization of proteins. Nucl. Acids Res. 2018, 46, W459–W466. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Sønderby, C.K.; Sønderby, S.K.; Nielsen, H.; Winther, O. DeepLoc: Prediction of protein subcellular localization using deep learning. Bioinformatics 2017, 33, 3387–3395. [Google Scholar] [CrossRef]
- Weiss, L.M.; Fiser, A.; Angeletti, R.H.; Kim, K. Toxoplasma gondii proteomics. Expert Rev. Proteom. 2009, 6, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Venkat, A.; Bainbridge, R.E.; Reese, M.L.; Le Roch, K.G.; Ay, F.; Boyle, J.P. Third-generation sequencing revises the molecular karyotype for Toxoplasma gondii and identifies emerging copy number variants in sexual recombinants. Genome Res. 2021, 31, 834–851. [Google Scholar] [CrossRef]
- Lee, V.V.; Judd, L.M.; Jex, A.R.; Holt, K.E.; Tonkin, C.J.; Ralph, S.A. Direct Nanopore sequencing of mRNA reveals landscape of transcript isoforms in apicomplexan parasites. mSystems 2021, 6, e01081-20. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, M.; Spano, F.; Magini, A.; Emiliani, C.; Carruthers, V.B.; Di Cristina, M. Alternative splicing mechanisms orchestrating post-transcriptional gene expression: Intron retention and the intron-rich genome of apicomplexan parasites. Curr. Genet. 2015, 62, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Garfoot, A.L.; Wilson, G.M.; Coon, J.J.; Knoll, L.J. Proteomic and transcriptomic analyses of early and late-chronic Toxoplasma gondii infection shows novel and stage specific transcripts. BMC Genom. 2019, 20, 859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Tsirigos, K.D.; Peters, C.; Shu, N.; Käll, L.; Elofsson, A. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res. 2015, 43, W401–W407. [Google Scholar] [CrossRef]
- Tsirigos, K.D.; Govindarajan, S.; Bassot, C.; Västermark, Å.; Lamb, J.; Shu, N.; Elofsson, A. Topology of membrane proteins—predictions, limitations and variations. Curr. Opin. Struct. Biol. 2018, 50, 9–17. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Salvatore, M.; Emanuelsson, O.; Winther, O.; von Heijne, G.; Elofsson, A.; Nielsen, H. Detecting sequence signals in targeting peptides using deep learning. Life Sci. Allianc. 2019, 2, 429. [Google Scholar] [CrossRef] [Green Version]
- Gíslason, M.H.; Nielsen, H.; Almagro Armenteros, J.J.; Johansen, A.R. Prediction of GPI-anchored proteins with pointer neural networks. Curr. Res. Biotechnol. 2021, 3, 6–13. [Google Scholar] [CrossRef]
- Pierleoni, A.; Martelli, P.L.; Casadio, R. PredGPI: A GPI-anchor predictor. BMC Bioinform. 2008, 9, 392. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Zheng, Y.; Li, H.; Luo, X.; He, Z.; Cao, S.; Shi, Y.; Zhao, Q.; Xue, Y.; Zuo, Z.; et al. GPS-Lipid: A robust tool for the prediction of multiple lipid modification sites. Sci. Rep. 2016, 6, 28249. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, L.P. IPC—Isoelectric Point Calculator. Biol. Dir. 2016, 11, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaharieva, N.; Dimitrov, I.; Flower, D.; Doytchinova, I. Immunogenicity prediction by VaxiJen: A ten year overview. J. Proteom. Bioinform. 2017, 10, 298–310. [Google Scholar] [CrossRef]
- Manavalan, B.; Govindaraj, R.G.; Shin, T.H.; Kim, M.O.; Lee, G. iBCE-EL: A new ensemble learning framework for improved linear B-cell epitope prediction. Front. Immunol. 2018, 9, 1695. [Google Scholar] [CrossRef] [Green Version]
- Cornejo-Granados, F.; Hurtado-Ramirez, J.M.; Hernandez-Pando, R.; Ochoa-Leyva, A. Secret-AAR: A web server to assess the antigenic density of proteins and homology search against bacterial and parasite secretome proteins. Genomics 2019, 111, 1514–1516. [Google Scholar] [CrossRef] [PubMed]
- Morotti, A.L.M.; Martins-Teixeira, M.B.; Carvalho, I. Protozoan parasites glycosylphosphatidylinositol anchors: Structures, functions and trends for drug discovery. Curr. Med. Chem. 2017, 24, 1–21. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Hassan, M.A.; Vasquez, J.J.; Guo-Liang, C.; Meissner, M.; Siegel, T.N. Comparative ribosome profiling uncovers a dominant role for translational control in Toxoplasma gondii. BMC Genom. 2017, 18, 961. [Google Scholar] [CrossRef] [Green Version]
- Liu, E.W.; Skinner, J.; Tran, T.M.; Kumar, K.; Narum, D.L.; Jain, A.; Ongoiba, A.; Traoré, B.; Felgner, P.L.; Crompton, P.D. Protein-specific features associated with variability in human antibody responses to Plasmodium falciparum malaria antigens. Am. J. Trop. Med. Hyg. 2018, 98, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Trötschel, C.; Poetsch, A. Current approaches and challenges in targeted absolute quantification of membrane proteins. Proteomics 2015, 15, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Buccitelli, C.; Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qin, B.; Nikolay, R.; Spahn, C.M.T.; Zhang, G. Translatomics: The global view of translation. Int. J. Mol. Sci. 2019, 20, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamaliddin, C.; Guillochon, E.; Salnot, V.; Rombaut, D.; Huguet, S.; Guillonneau, F.; Houze, S.; Cot, M.; Deloron, P.; Argy, N.; et al. Comprehensive analysis of transcript and protein relative abundance during blood stages of Plasmodium falciparum infection. J. Proteom. Res. 2021, 20, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, L.; Wang, Y.; Chen, L.; Li, H.; Xie, Z. RPFdb v2.0: An updated database for genome-wide information of translated mRNA generated from ribosome profiling. Nucl. Acids Res. 2019, 47, D230–D234. [Google Scholar] [CrossRef] [PubMed]
- Jeacock, L.; Faria, J.; Horn, D. Codon usage bias controls mRNA and protein abundance in trypanosomatids. eLife 2018, 7, 1247. [Google Scholar] [CrossRef]
- Forrest, M.E.; Pinkard, O.; Martin, S.; Sweet, T.J.; Hanson, G.; Coller, J. Codon and amino acid content are associated with mRNA stability in mammalian cells. PLoS ONE 2020, 15, e0228730. [Google Scholar] [CrossRef]
- Hia, F.; Yang, S.F.; Shichino, Y.; Yoshinaga, M.; Murakawa, Y.; Vandenbon, A.; Fukao, A.; Fujiwara, T.; Landthaler, M.; Natsume, T.; et al. Codon bias confers stability to human mRNAs. EMBO Rep. 2019, 20, 589. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, Q.; Zhao, F. Synonymous but not silent: The codon usage code for gene expression and protein folding. Annu. Rev. Biochem. 2021, 90, 375–401. [Google Scholar] [CrossRef]
- Ferdous, S.; Kelm, S.; Baker, T.S.; Shi, J.; Martin, A.C.R. B-cell epitopes: Discontinuity and conformational analysis. Mol. Immunol. 2019, 114, 643–650. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, M.C.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-2.0: Improving sequence-based B-cell epitope prediction using conformational epitopes. Nucl. Acids Res. 2017, 45, W24–W29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galanis, K.A.; Nastou, K.C.; Papandreou, N.C.; Petichakis, G.N.; Pigis, D.G.; Iconomidou, V.A. Linear B-cell epitope prediction for in silico vaccine design: A performance review of methods available via command-line interface. Int. J. Mol. Sci. 2021, 22, 3210. [Google Scholar] [CrossRef]
- Sidik, S.M.; Huet, D.; Ganesan, S.M.; Huynh, M.H.; Wang, T.; Nasamu, A.S.; Thiru, P.; Saeij, J.P.J.; Carruthers, V.B.; Niles, J.C.; et al. A genome-wide CRISPR screen in Toxoplasma identifies essential apicomplexan genes. Cell 2016, 166, 1423–1435.e12. [Google Scholar] [CrossRef] [Green Version]
- Sangare, L.O.; Olafsson, E.B.; Wang, Y.; Yang, N.; Julien, L.; Camejo, A.; Pesavento, P.; Sidik, S.M.; Lourido, S.; Barragan, A.; et al. In vivo CRISPR screen identifies TgWIP as a Toxoplasma modulator of dendritic cell migration. Cell Host Microbe 2019, 26, 478–492.e8. [Google Scholar] [CrossRef]
- Seidi, A.; Muellner-Wong, L.S.; Rajendran, E.; Tjhin, E.T.; Dagley, L.F.; Aw, V.Y.; Faou, P.; Webb, A.I.; Tonkin, C.J.; van Dooren, G.G. Elucidating the mitochondrial proteome of Toxoplasma gondii reveals the presence of a divergent cytochrome c oxidase. eLife 2018, 7, e38131. [Google Scholar] [CrossRef]
- Basso, W.; Hartnack, S.; Pardini, L.; Maksimov, P.; Koudela, B.; Venturini, M.C.; Schares, G.; Sidler, X.; Lewis, F.I.; Deplazes, P. Assessment of diagnostic accuracy of a commercial ELISA for the detection of Toxoplasma gondii infection in pigs compared with IFAT, TgSAG1-ELISA and Western blot, using a Bayesian latent class approach. Int. J. Parasitol. 2013, 43, 565–570. [Google Scholar] [CrossRef]
- Garcia, J.L.; Gennari, S.M.; Navarro, I.T.; Machado, R.Z.; Headley, S.A.; Vidotto, O.; da Silva Guimarães Junior, J.; Bugni, F.M.; Igarashi, M. Evaluation of IFA, MAT, ELISAs and immunoblotting for the detection of anti-Toxoplasma gondii antibodies in paired serum and aqueous humour samples from experimentally infected pigs. Res. Vet. Sci. 2008, 84, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Fillaux, J.; Guigon, A.; Lavergne, R.A.; Villard, O.; Villena, I.; Marty, P.; Pomares, C. Serological diagnosis of Toxoplasma gondii: Analysis of false-positive IgG results and implications. Parasite 2020, 27, 7. [Google Scholar] [CrossRef] [Green Version]
- Huertas-López, A.; Martínez-Subiela, S.; Cerón, J.J.; Vázquez-Calvo, Á.; Pazmiño-Bonilla, E.D.; López-Ureña, N.M.; Martínez-Carrasco, C.; Álvarez-García, G. Development and validation of a time-resolved fluorescence immunoassay for the detection of anti-Toxoplasma gondii antibodies in goats. Vet. Parasitol. 2021, 293, 109432. [Google Scholar] [CrossRef]
- Newberry, K.M.; Colling, A. Quality standards and guidelines for test validation for infectious diseases in veterinary laboratories. Rev. Sci. Tech. 2021, 40, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Banoo, S.; Bell, D.; Bossuyt, P.; Herring, A.; Mabey, D.; Poole, F.; Smith, P.G.; Sriram, N.; Wongsrichanalai, C.; Linke, R.; et al. Evaluation of diagnostic tests for infectious diseases: General principles. Nat. Rev. Microbiol. 2010, 8, S17–S29. [Google Scholar] [CrossRef] [PubMed]
| Proteins (MW) | Assays Developed * | Ref. Test | N/E | Host-Dependent Factors | Parasite-Dependent Factors | Results | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal Species | Nº | Age | Sex | Samplings | T. gondii Strain | Stage: TZ, TC, Oo | Dose | ||||||
| OWP1 (40KDa) | ELISA/WB | Soluble TZ ELISA | N | Pig | 44 | na | M | 1 sampling | - | - * | - | OWP1: no recognition | [64] |
| E | Pig | 3 | 6.5–7.5 w | F and M | 28 dpi | RH | TZ | 106 | OWP1: no recognition | ||||
| E | Pig | 3 | 6.5–7.5 w | F and M | 28 dpi | VEG | Oo | 1.5 × 104 | OWP1: no recognition | ||||
| N | Chicken | 113 | na | na | 1 sampling | - | - ** | - | OWP1: recognition OWP1 ELISA: 74% + TZ ELISA: 80% + | ||||
| E | Chicken | na | na | na | 1 sampling | RH | TZ | 100 µg (two 15d-interval boosters) | OWP1: no recognition | ||||
| E | Chicken | na | na | na | 1 sampling | VEG | TC | 100 | OWP1: no recognition | ||||
| OWP8 (65KDa) | ELISA/WB | recGRA7 ELISA | N | Pig *** | 90 | na | na | 1 sampling | - | - | - | OWP8 ELISA: 12.2% + GRA7 ELISA: 16.7% + | [63] |
| N | Chicken | 96 | na | na | 1 sampling | - | - | - | OWP8 ELISA: 13.5% + GRA7 ELISA: 10.4% + | ||||
| Proteins (MW) | Assays Developed * | Ref. Test | Cohorts Studied | Results | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nº | Age | Sex | Origin | Seropositive (Tests) | Other Relevant Data | Samplings | |||||
| Oocyst Wall Proteins | |||||||||||
| OWP8 (65 kDa) | ELISA/WB | recGRA7 ELISA | 169 | na | na | Hospital | na | - | One sampling | OWP8 ELISA: 3.6% + GRA7 ELISA: 14.7% + | [63] |
| Sporozoite Proteins | |||||||||||
| ERP (11 kDa) | ELISA/WB | Conv. tests | 6 | na | na | Laboratory employees | yes (IgM/IgG WBs) | Outbreak | From 1 m post exposure till 8 mpi | ERP: recognition by 100% by WB at 1 mpi and detectable Abs for 5–6 mpi ** | [62] |
| 4 | na | na | Chronically infected people (IgM− and IgG+) | yes (dye test and IFAT) | - | One sampling | ERP WB: no recognition | ||||
| 11 | na | na | Visitors to a horse stable | yes (IgM/IgG+, dye test/IFAT) | Outbreak | Between 78 and 149 days after onset of symptoms | ERP WB: 82%+ | ||||
| 182 | 18–43 yr | na | Settings where the infection is prevalent | yes (IgG ELISA/avidity ELISA) | - | One sampling | ERP WB: 60+ (29 and 31 acute and chronic infections) ERP ELISA: 44+ (23 and 21 acute and chronic infections) | ||||
| 10 | adults and two 2 yr siblings | na | 8 sera from Amish family (1 serum from a congenitally infected child) | yes (IgA, IgM and IgG conv. tests) with the exception of 1 sibling | - | One sampling | ERP WB: 6 adults + | ||||
| 76 | na | F | Congenital infections | yes (IgG/IgM+) | 3 mothers acquired acute toxoplasmosis during an outbreak | 2.5 m after childbirth | ERP WB: 78% + 2.5 m after birth | ||||
| 59 | na | F | Chronically infected people | yes (58 IgG+; 1 IgG+ and IgM+) | - | One sampling | WB ERP: no recognition | ||||
| ELISA | Sabin Feldman Dye test and ELISA | 10 | na | na | Blood donors | yes (seroconversion in 2nd sampling) | - | Two consecutive samplings | ERP ELISA: 1+ no recognition in 2nd sampling | [74] | |
| ELISA | Conv. IgG/IgM tests | 476 *** 380 | 0–28 yr | 177M/299F 161M/219F | Endemic area of toxoplasmosis Public schools (students, parents, school staff) | yes (249 IgM+ and/or IgG+) | Serum and saliva pairwise comparisons **** | One sampling | Divergent results among conventional ELISA and TgERP ELISA for saliva and sera in all age groups. Prevalence values similar (between 66.6–68.7%) for both ELISAs in 15–21 yr age group | [75] | |
| ELISA | Conv. IgG/IgM tests | 128 | na | na | Areas with groundwater vulnerability (unconfined aquifers) | 111 (IgG+) All individuals: IgM+ | - | One sampling | ERP ELISA: 63+ >OD values in younger people | [50] | |
| CCp5A (50 kDa) | ELISA/WB | Conv. tests | 78 | na | na | Outbreak | yes | Acute clinical signs, ocular disease; attributed to contaminated water | One sampling | CCp5A ELISA: higher IgG/IgM levels than in pregnant women TZ ELISA: higher IgM levels than in pregnant women | [64] |
| 78 | na | F | Pregnant women | yes (IgG+ and IgM+) | - | One sampling | CCp5A ELISA: lower IgG/IgM levels than in the outbreak; 80% IgM+ (evidence of recent exposure to T. gondii) TZ ELISA: IgG levels higher than in the outbreak | ||||
| CCp5A (17 kDa fragment) | ELISA/WB | recGRA7 ELISA | 169 | na | na | Hospital | na | No previous clinical/serological data | One sampling | CCp5A ELISA: 3% + GRA7 ELISA: 14.7% + | [63] |
| SporoSAG (25.6 kDa) | ELISA | recSAG1 ELISA/recSRS2 ELISA | 13 | na | na | Waterborne transmission | yes (SAG1+) | - | One sampling | SporoSAG: no recognition by anti IgA, IgM and IgGs | [76] |
| 1 | na | na | Infected with type II strain oocysts (control serum) | yes | - | One sampling | SporoSAG: no recognition by anti IgA, IgM and IgGs | ||||
| 6 | na | F | Pregnant women | yes | Presumably infected by meat route | One sampling | SporoSAG: no recognition by anti IgA, IgM and IgGs | ||||
| Proteins (MW) | Assays Developed * | Ref. Test | Host-Dependent Factors | Parasite-Dependent Factors | Results | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N/E | Species (Strain) | Nº | Age | Sex | Sampling Period | T. gondii Strain | Stage: TZ, BZ, TC, Oo | Dose | |||||
| ERP (11 kDa) | ELISA/WB | MAT/ELISA | E | Pig | 10 | 5 m | na | 9 mpi | VEG | Oo | 1000 | ERP WB: recognition for 6–8 mpi | [62] |
| MAT/ELISA | E | Pig | 10 | 5 m | na | 9 mpi | VEG | TC | 5000 | ERP WB: no recognition | |||
| - | E | Mice (Swiss Webster) | na | na | na | 60 dpi | ME49 | Oo | 50 | ERP WB: recognition | |||
| - | E | Mice (Swiss Webster) | na | na | na | 60 dpi | ME49 | TC | na | ERP WB: no recognition | |||
| ELISA | IgG/IgM tests | N | Chicken | 198 | na | na | 1 sampling | - | - ** | - | ERP: recognition by 49% | [50] | |
| CCp5A (50 kDa) | ELISA/WB | Soluble TZ ELISA | N | Pig | 44 | na | M | 1 sampling | - | - | - | CCp5A ELISA: 100% + TZ ELISA: 100% + | [64] |
| E | Pig | 3 | 6.5–7.5 w | F and M | 28 dpi | RH | TZ | 106 | CCp5A WB: no recognition TZ ELISA: seroconversion | ||||
| E | Pig | 3 | 6.5–7.5 w | F and M | 28 dpi | VEG | Oo | 1.5 × 104 | CCp5A ELISA: recognition; Abs peaked at 7dpi and decrease at 28dpi TZ ELISA: seroconversion | ||||
| N | Chicken | 113 | na | na | 1 sampling | - | - | - | CCp5A ELISA: 70% + TZ ELISA: 80% + | ||||
| E | Chicken | na | na | na | 1 sampling | RH | TZ | 100 ug (two 15d boosters) | CCp5A WB: no recognition | ||||
| E | Chicken | na | na | na | 1 sampling | VEG | TC | 100 | CCp5A WB: no recognition | ||||
| E | Mice (Balb/c) | 5 | 8–12 w | na | 60 dpi | VEG | Oo | 50 | CCp5A WB: recognition anti CCp5A IgMs peaked at 15 dpi TZ ELISA: seroconversion | ||||
| E | Mice (Balb/c) | 5 | 8–12 w | na | 60 dpi | VEG | TC | 50 | WB/ELISA CCp5A: no recognition TZ ELISA: seroconversion | ||||
| CCp5A (17 kDa fragment) | ELISA/WB | recGRA7 ELISA | N | Pig *** | 90 | na | na | 1 sampling | - | - | - | CCp5A ELISA: 12.2% + GRA7 ELISA: 16.7% + | [63] |
| N | Chicken | 96 | na | na | 1 sampling | - | - | - | CCp5A ELISA: 9.4% + GRA7 ELISA: 10.4% + | ||||
| SporoSAG (25.6 kDa) | ELISA | recSAG1 ELISA/recSRS2 ELISA | E | Mice | 1 | na | na | 1 sampling | ME49 | Oo | 100 | SporoSAG ELISA: no recognition by anti IgA, IgM and IgG antibodies. | [76] |
| E | Mice | 3 | na | na | 1 sampling | ME49 | Oo **** | 100 | SporoSAG ELISA: no recognition by anti IgA, IgM and IgG antibodies. | ||||
| E | Mice | 4 | na | na | 1 sampling | ME49 | BZ | 20 | SporoSAG: no recognition by anti IgA, IgM and IgG antibodies. | ||||
| SporoSAG (23.78 kDa) | ELISA | recGRA1 ELISA | E | Mice (Swiss strain) | 6 | na | na | 120 dpi | PRU | Oo | 8–10 | SporoSAG ELISA: IgM peaked at 1, 10 and 15 dpi IgG peaked at 40 and 120 dpi | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez García, G.; Davidson, R.; Jokelainen, P.; Klevar, S.; Spano, F.; Seeber, F. Identification of Oocyst-Driven Toxoplasma gondii Infections in Humans and Animals through Stage-Specific Serology—Current Status and Future Perspectives. Microorganisms 2021, 9, 2346. https://doi.org/10.3390/microorganisms9112346
Álvarez García G, Davidson R, Jokelainen P, Klevar S, Spano F, Seeber F. Identification of Oocyst-Driven Toxoplasma gondii Infections in Humans and Animals through Stage-Specific Serology—Current Status and Future Perspectives. Microorganisms. 2021; 9(11):2346. https://doi.org/10.3390/microorganisms9112346
Chicago/Turabian StyleÁlvarez García, Gema, Rebecca Davidson, Pikka Jokelainen, Siv Klevar, Furio Spano, and Frank Seeber. 2021. "Identification of Oocyst-Driven Toxoplasma gondii Infections in Humans and Animals through Stage-Specific Serology—Current Status and Future Perspectives" Microorganisms 9, no. 11: 2346. https://doi.org/10.3390/microorganisms9112346
APA StyleÁlvarez García, G., Davidson, R., Jokelainen, P., Klevar, S., Spano, F., & Seeber, F. (2021). Identification of Oocyst-Driven Toxoplasma gondii Infections in Humans and Animals through Stage-Specific Serology—Current Status and Future Perspectives. Microorganisms, 9(11), 2346. https://doi.org/10.3390/microorganisms9112346







