Abstract
Inflammasomes are cytoplasmic multiprotein complexes formed by the host’s immune system as a response to microbial infection and cellular damage. Many studies have revealed various regulators of NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome activation, while it has been recently shown that NLRP3 is implicated in COVID-19 pathogenesis. At the same time, probiotics counteract the inflammatory process and modulate cytokine release, thus influencing both innate and adaptive immune systems. Herein, we review the immunomodulatory potential of probiotics on the assembly of NLRP3 inflammasome, as well as the pathophysiological mechanisms supporting the use of probiotic bacteria for SARS-CoV-2 infection management, presenting evidence from preclinical studies of the last decade: in vivo, ex vivo, and mixed trials. Data show that probiotics intake is related to NLRP3 inflammasome attenuation and lower levels of inflammation markers, highlighting the beneficial effects of probiotics on inflammatory conditions. Currently, none of the ongoing clinical trials evaluating the effectiveness of probiotics intake in humans with COVID-19 has been completed. However, evidence from preclinical studies indicates that probiotics may block virus invasion and replication through their metabolites, bacteriocins, and their ability to block Angiotensin-Converting Enzyme 2 (ACE2), and by stimulating the immune response through NLRP3 inflammasome regulation. In this review, the beneficial effects of probiotics in the inflammatory process through NLRP3 inflammasome attenuation are presented. Furthermore, probiotics may target SARS-CoV-2 both by blocking virus invasion and replication and by stimulating the immune response through NLRP3 inflammasome regulation. Heterogeneity of the results—due to, among others, different bacterial strains and their metabolites, forms, dosage, and experimental designs—indicates the need for more extensive research.
1. Introduction
Viral infection triggers host innate immune responses indicated by cytokine production and inflammasome activation [1]. Inflammasomes are cytoplasmic multiprotein complexes which consist of a sensor protein, the adaptor molecule apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and the effector protein caspase-1. Active caspase-1 proceeds to cleave the precursors pro-interleukin (IL)-1β and pro-IL-18 into mature forms IL-1β and IL-18, respectively, inducing pyroptosis. Inflammasomes are vital for the maintenance of intestinal homeostasis and gut-associated physiological inflammatory responses in humans. The NLRP3 (NLR family pyrin domain containing 3) inflammasome is assembled in both gut immune and epithelial cells [2] and it is the most widely studied inflammasome.
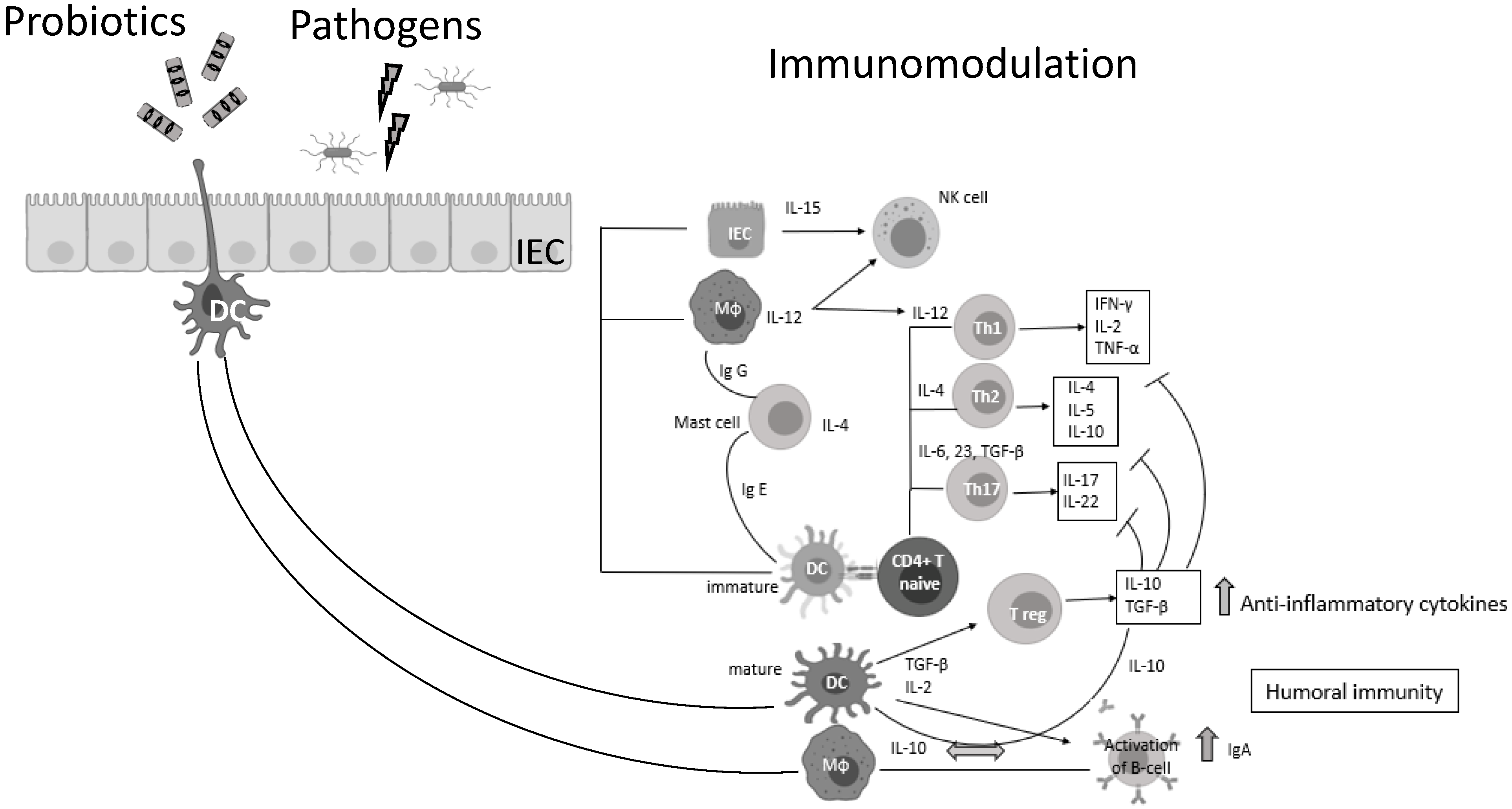
Probiotic bacteria (PB) are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [3,4]. Lactobacilli and Bifidobacteria are the most common probiotics, but the yeast Saccharomyces boulardii and Bacillus species are also widely known [4]. PB—generally regarded as safe (GRAS)—have beneficial effects in various clinical conditions, such as the prevention of antibiotic-associated diarrhea, constipation, necrotizing enterocolitis, sepsis, and allergies in infants, while recently have shown promising results in oral health and periodontal therapy [5,6,7,8,9,10,11]. Lactic acid bacteria (LAB) can prevent gastrointestinal dysbiosis and reduce the risk of developing secondary infections, while other strains and their metabolites exhibit antiviral activities [12,13], such as enhancement of the barrier function by stimulating mucin secretion, antimicrobial activity by competing with microbial pathogens for nutrients and adhesion to epithelial cells producing antimicrobial substances such as bacteriocins and stimulation of mucosal epithelial cells to secrete defensins. Moreover, PB have immunomodulatory activity by interacting with dendritic cells (DCs), monocytes, and lymphocytes (Figure 1) [14].
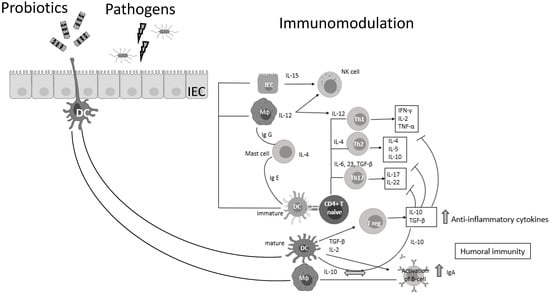
Figure 1.
Probiotic bacteria can modulate cytokine release, thus influencing both innate and adaptive immune response. Part of this figure was created with BioRender (https://biorender.com, accessed on 2 October 2021).
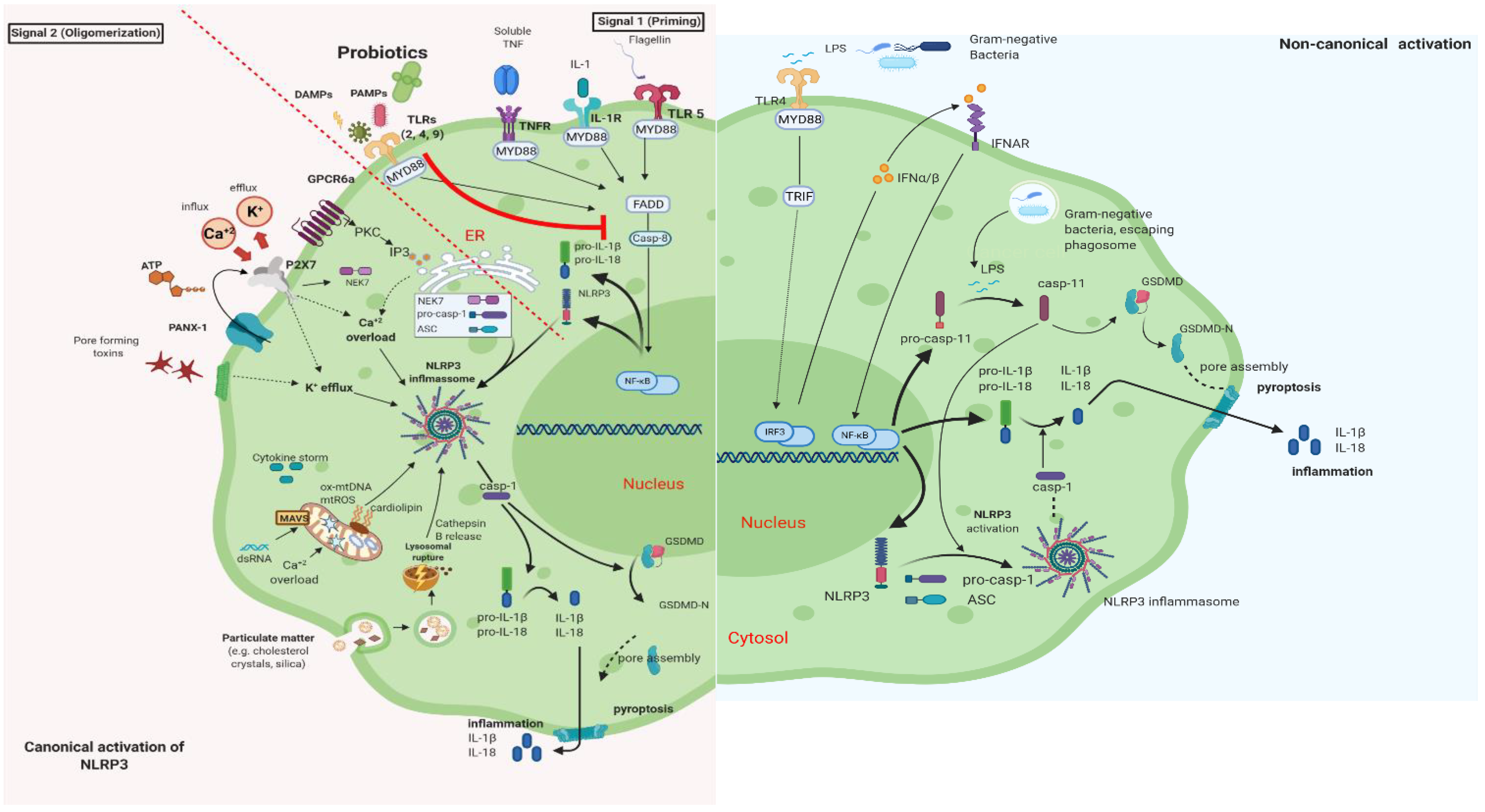
It is well documented that PB can regulate inflammation in two ways: (1) indirectly, by producing short-chain fatty acids (SCFAs) and (2) directly, by binding to innate immune system receptors Toll-like (membrane glycoproteins TLR 2, 4, 9) and by triggering important signaling pathways. One of these pathways includes the transcription factor NF-κB, which is associated with the activation of NOD-like receptors (nucleotide-binding oligomerization domain-like receptors), that affect the formation of inflammasomes, thus the inflammatory response [15]. Overactivation of NLRP3 inflammasome has been linked to the pathogenesis of inflammatory bowel disease, cryopyrin-associated periodic syndromes, type 2 diabetes mellitus, atherosclerosis, and neurodegenerative diseases [16]. PB interact with the host by germline-encoded host sensors, namely, pattern recognition receptors (PRRs) [17], that detect microbial structures—pathogen-associated molecular patterns (PAMPs) and components of the host’s cells released during cell damage or death named damage-associated molecular patterns (DAMPs) [18,19]. PRRs are expressed by most innate immune effector cells (dendritic cells, macrophages, monocytes, neutrophils, and epithelial cells). The mechanism of triggering the immune response depends on the kind of antigenic molecule [20]. Many recent studies focus on the complex mechanisms of NLRP3 activation stemming from pathogenic bacteria and their products, such as extracellular ATP and pore-forming toxins (Figure 2).
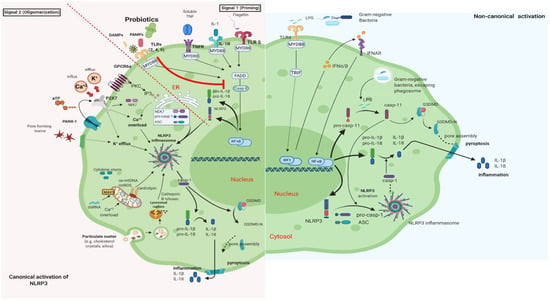
Figure 2.
Canonical and Non-Canonical activation of NLRP3 inflammasome. The macrophage in an animal model (transgenic mice-expressing the human angiotensin I-converting enzyme 2 (ACE2) receptor driven by the cytokeratin-18 (K18) gene promoter (K18-hACE2)) [21,22]. (i) The canonical pathway of NLRP3 requires a two-step process: priming and activation. Priming (signal 1) is the upregulation of the NLRP3 inflammasome components, NLRP3, pro-ILβ, and pro-caspase. This transcriptional upregulation is stimulated by PAMPs (bacterial, fungal, viral) or DAMPs which are recognized by PRRs such as TLRs. Additionally, cytokines such as TNF or IL-1 stimulate this upregulation by engaging TNFR and IL1-R, respectively. The activation (signal 2) is provided by various stress signals such as ion or plasma perturbations, ATP, lysosomal rupture, particulate matter, mitochondrial antiviral-signaling protein, mitochondrial reactive oxygen species, ox-mtDNA, etc. Oligomerization of NLRP3 inflammasome activates caspase-1, which in turn cleaves pro-ILβ, pro-IL18, and GSDMD and induces pyroptosis and inflammation [23]. (ii) Gram-negative bacteria (e.g., lipopolysaccharide (LPS) or outer membrane vesicle from bacteria) can activate the non-canonical inflammasome pathway that involves NLRP3-dependent caspase-4/5 in humans (known as caspase-11 in murine models) [24]. Caspase-11, when it is secreted at normal rates, protects against bacterial infections, but its excessive activation can cause tissue damage and pyroptosis which is activated in both pathways through cytosolic gasdermin (GSDMD), a member of the gasdermin family [25]. GSDMD cleavage generates a N-terminal domain that is capable of forming plasma membrane pores and a C-terminal domain that acts as an inhibitor of cytolysis [26]. The cleaved N-terminal domain of GSDMD oligomerizes and forms pores on the host cell membrane, leading to pyroptosis and further activation of NLRP3 by triggering K+ efflux [27,28]. This figure was created with BioRender (https://biorender.com, accessed on 2 October 2021).
In this review, we aim to investigate the impact PB have on stimulating the assembly of NLRP3 inflammasome, as well as the pathophysiological mechanisms supporting the use of PB in COVID-19.
We assessed preclinical studies focusing on the activation of NLRP3 by PB during the decade 2010–2020. A literature research using the terms “probiotics”, “inflammasome” and “NLRP3” and their combination in PubMed and Cochrane databases revealed twenty-two publications in English language. We used evidence from the original articles only and we included studies identified by manual search of the reference lists of the aforementioned articles. Similarly, we assessed the available literature in PubMed and Cochrane databases and in ClinicalTrials.gov (accessed on 3 December 2020) using the terms “NLRP3, COVID-19, probiotics” “SARS-CoV-2”, and their combination on the use of probiotics as immunomodulators of COVID-19 infection management through NLRP3 inflammasome manipulation.
2. Probiotic Bacteria and NLRP3 Activation
We did not detect any publication investigating the role of PB on NLRP3 inflammasome activation in humans in vivo. A total of fifteen studies (in vivo, ex vivo) providing data regarding the NLRP3 inflammasome stimulation by PBs in a variety of cellular and animal models were included in this review. More specifically, four studies on mammals (three porcine, one canine), seven on laboratory animals (four rats, three mice), one on rodents (Syrian hamsters), and three on cell cultures, in which inflammatory biomarkers were measured. Treatment included probiotic supplements (regardless of viability, strains, forms, dosages, and duration of treatment). The effect of probiotics on alterations in inflammation markers (IL-1β, TNF-a, IL-6, IL-18) and any immunomodulatory potential related to intervention were considered as endpoints.
Heterogeneity was the main feature of the included studies. Different types of trials, interventions, subjects, and cell cultures and a variety of PB strains and metabolites, dosages, and duration precluded adequate studies classification (Table 1, Table S1).

Table 1.
Trials (in vivo, ex vivo) with probiotic administration and NLRP3 activation in animals and cell cultures.
Pigs fed on either a basal diet or a Clostridium butyricum-supplemented diet were given orally enterotoxigenic Escherichia coli (ETEC) K88 or saline. The results of this Chinese study showed that C. butyricum decreased IL-1β and IL-18 levels in serum and gut tissue, whereas IL-10 levels were increased. C. butyricum promoted the accumulation of intestinal NLRP3 mRNA and inhibited ETEC K88-induced caspase-1 and NLRP3 increase [35]. In an Italian study that investigated behavioral and obesity effects in hamsters fed with high fat diet (HFD), IL-1β, NLRP3, caspase-1, and NF-kB levels were measured in the presence or absence of a multispecies probiotic formulation. Hamsters were subjected to unpredictable chronic mild stress. Consequently, PB decreased hypothalamic expression and hematic circulating levels of all above inflammatory markers, while HFD increased them [30]. In a study published in 2020, three purified bacteriocins produced by L. helveticus: PJ4, L. brevis: DT24, and L. animalis: TSU4 were administered in mice as a treatment strategy for HFD induced obesity. Subjects were divided into five groups and all groups were fed with HFD—except for controls. Three HFD groups received three bacteriocins, respectively. Results showed that the inflammatory mediators (IL-1β, IL-6, TNF-α) were significantly increased in the HFD group without bacteriocins administration. The DT24 group did not show any change in cytokines levels, while PJ4 and TSU4 groups showed a significant reduction; PJ4 was more effective than TSU4 in decreasing inflammatory biomarkers. Notably, increased NLRP3 expression was observed in all groups, especially in HFD compared to controls [41]. University of Florida researchers evaluated the effects of Lactobacillus johnsonii N6.2 in combination with rosmarinic acid, a natural antioxidant with anti-inflammatory properties, on inflammasome assembly in ileal tissue of diabetes-prone rats. Their findings confirm that L. johnsonii suppresses NLRP3 and caspase-1 maturation lowering overall intestinal inflammation [31]. When the effect of oral preadministration of L. johnsonii L531 in piglets with Salmonella infantis-induced enteritis was evaluated, S. infantis activated NLRP3 and NF-κB signaling in the jejunum and ileal tissue, L. johnsonii L531 administration before the challenge reduced the severity of intestinal inflammation and prevented the excessive expression of NLRP3 and caspase-1 through the elimination of damaged mitochondria and accelerated autophagic degradation [33]. Similarly, in rats with cecal ligation and puncture-induced sepsis, the administration of a different strain of Lactobacillus (L. rhamnosus GG, LGG) decreased IL-1β, NLRP3, IL-6, and TNF-a levels in liver tissues indicating the anti-inflammatory role of LGG. Moreover, B. fragilis ZY-312 probiotic therapy reduced liver injury following experimental sepsis in neonatal rats with necrotizing enterocolitis (NEC) induced by Cronobacter sakazakii [4]. Fan et al. evaluated the effects of C. sakazakii on intestinal barrier function and the protective role of ZY-312. They showed that the expression of NLRP3, caspase-1 (p10, p20), IL-1, and gasdermin D were significantly increased in the C. sakazakii group. Meanwhile, ZY-312 ameliorated the deleterious effects of C. sakazakii on intestinal integrity and attenuated clinical symptoms (weight loss, loss of appetite, abdominal flatulence) and intestinal inflammation [40]. The anti-inflammatory effects of Roseburia intestinalis-derived flagellin (a subunit protein of the flagellum, a whip-like appendage that allows bacterial motility) have been investigated in a dextran sulfate sodium (DSS) induced colitis model in mice [43]. The mRNA levels of NLRP3 and IL 1β were upregulated in the DSS group compared with the control group, but treatment with probiotic Roseburia intestinalis flagellin remarkably alleviated the intestinal inflammation inhibiting the increase of proinflammatory cytokines (IL 1β, IL 18, IL 6, and TNF α) levels in serum and decreasing the NLRP3 activation in colonic tissues.
Chung et al. [36]. assessed the immunomodulatory effects of heat-killed Enterococcus faecalis, a commensal Gram+ lactic acid bacterium, and its potential protective role on intestinal inflammation in murine models of DSS-induced colitis and colitis-associated colorectal cancer. E. faecalis ameliorated the severity of inflammation and attenuated NLRP3 activation in THP-1-derived macrophages while inducing the expression of pro-and mature IL-1β but did not affect the amount of active caspase-1 [36]. In another detailed analysis, microbe-derived antioxidant (MA) fermented by Bacillus subtilis, Lactobacillus, and beer yeast was used in mother rats and offspring. MA supplementation attenuated HFD-induced NLRP3 activation in the liver and decreased IL-1β and IL-18 gene expression in HFD-induced hepatic lipid disorders during pregnancy and lactation and improved hepatic function [37].
2.1. Ex Vivo Trials
In 2011, investigators cloned and sequenced porcine NLRP3 cDNA isolated from ileal Peyer’s patches. Then, they examined the expression of NLRP3 in diverse tissues (spleen, esophagus, duodenum, jejunum, ileum, Peyer’s patches, colon, and mesenteric lymph nodes (MLNs) from newborn and adult porcine); they also examined the ability of two Lactobacilli strains (L. delbrueckii subsp. bulgaricus NIAI B6 and L. gasseri JCM1131T) to evoke the expression of NLRP3 in the gut-associated lymphoid tissues (GALT) of the subjects showing that the two strains of lactic acid bacteria can enhance NLRP3 expression in adult and newborn GALT [38]. Later, one different team investigated the potency of L. rhamnosus GR-1 to prevent E. coli adhesion and described the effects of L. rhamnosus GR-1 on ameliorating E. coli-induced mastitis and cell damage in primary bovine mammary epithelial cells (BMECs), as well as the NLRP3 inflammasome activation. They showed that NLRP3 expression and caspase-1 were increased during E. coli infection, while L. rhamnosus GR-1 pretreatment ameliorated E. coli-induced mastitis. Preincubation of BMECs with probiotic strains had no direct killing effect on E. coli but reduced the adhesion levels to about 50% of that observed in BMECs infected with E. coli. BMECs treated with L. rhamnosus GR-1 did not exhibit increased NLRP3 activation compared with untreated controls, whereas IL-6, IL-8, and TNF-a production was downregulated [32]. Treatment with probiotic Enterococcus faecium NCIMB 10415 (E. faecium) was examined in porcine monocyte-derived dendritic cells (MoDC) infected by ETEC to elicit NLRP3 inflammasome activation [34]. Inflammasome activation normally requires a two-step process (priming and activation) to induce its transcription; in this case, the research team studied the E. faecium potency on primed cells with LPS (mono- and co-incubated; n = 5 independent experiments) and unprimed cells (mono- and co-incubated; n = 4 independent experiments). In a co-incubation experiment, MoDC were pretreated with E. faecium for 1 h and then were challenged with ETEC for 1 h as well, as in the ETEC mono-incubation. The expression of NLRP3 components was measured in MoDC after 1.5, 6, and 20 h of stimulation. The complex design of the experiments showed that priming of MoDC with LPS for 3 h induced an increased mRNA expression of IL-1β, IL-18, caspase-1, and NLRP3. In the unprimed cells, the mRNA expression of IL-1β, IL-18, and NLRP3 was significantly increased in cultures incubated with ETEC, but at the 6th and 20th h. The pathogenic ETEC strain stimulates a time-dependent inflammasome response, possibly because LPS is present in the outer membrane of ETEC, unlike the E. faecium that did not stimulate NLRP3.
2.2. Mixed Trials
In 2015, Schmitz et al. [29]. assessed the intestinal expression of caspase-1, IL-1β, IL-18, and NLRP3 in canines with chronic enteropathy (CE) compared to controls when treated with E. faecium in vivo and ex vivo. In in vivo experiments, all groups were fed a standardized diet. Samples were collected from duodenal and colonic biopsies. Results showed that inflammasome-related genes in the duodenum were not significantly different between dogs with probiotic or placebo treatment, whilst they were expressed at a much higher level in the colon than in the duodenum (both in healthy and CE dogs). Interestingly, IL-1β protein expression in CE group was decreased with dietary treatment (not with PB). In ex vivo culture of duodenal biopsies (macrophage DH82 cells), the samples were stimulated with different TLR ligands and E. faecium. Incubation with E. faecium increased caspase-1 levels compared to stimulation with pure TLR ligands (PBS, Flagellin, Pam3CSK4, LPS), but did not affect the NLRP3 expression independently of disease status. Challenge with pure TLR ligands showed minor effects on mRNA levels of the NLRP3 components apart from IL-18 [29].
In another mixed trial, German scientists investigated the expression of NLRP3 components in sows and piglets’ intestines (jejunal, ileal, and colonic tissues) and analyzed the influence of age and long-term supplementation with the E. faecium NCIMB 10,415 [42] on NLRP3 expression. NLRP3 expression in tissues was higher in 29-day-old piglets compared to 70-day-old growing pigs, indicating that age is a factor that affects NLRP3 inflammasome activation. Furthermore, they examined cell cultures (intestinal epithelial cells) which first were challenged with ETEC and then inoculated with E. faecium NCIMB 10415. Expression of NLRP3 was slightly higher in epithelia mono-incubated with E. faecium or ETEC compared with epithelia incubated with E. faecium and ETEC whereas IL-1β and IL-18 did not differ significantly between the treatment groups but tended to be higher in epithelia incubated with ETEC.
To sum up, most of the above-mentioned trials showed NLRP3 attenuation and decreased levels of inflammation markers -caspase-1, IL-1β, IL-6, IL-18, and TNF-a-after PB administration/incubation (Table 1). The age of the subjects, the exact time in which the samples were analyzed, and the condition of treatment or pretreatment of different pathogens and challenges are of concern in interpreting the results. Beneficial effects of PB seem to be affected by many factors, including different bacterial strains and their metabolites, forms (viable or nonviable3. Involvement of SARS-CoV-2 in NLRP3 Activation.
Although COVID-19 pathogenesis remains elusive, emerging evidence indicates the role of NLRP3 inflammasome involvement in its pathogenesis [44,45].
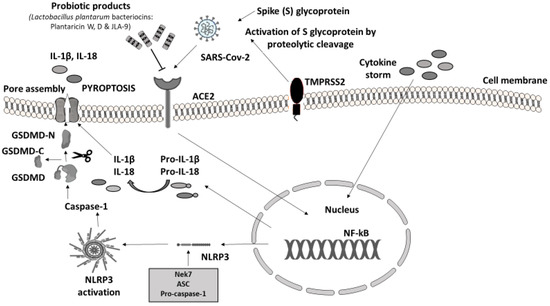
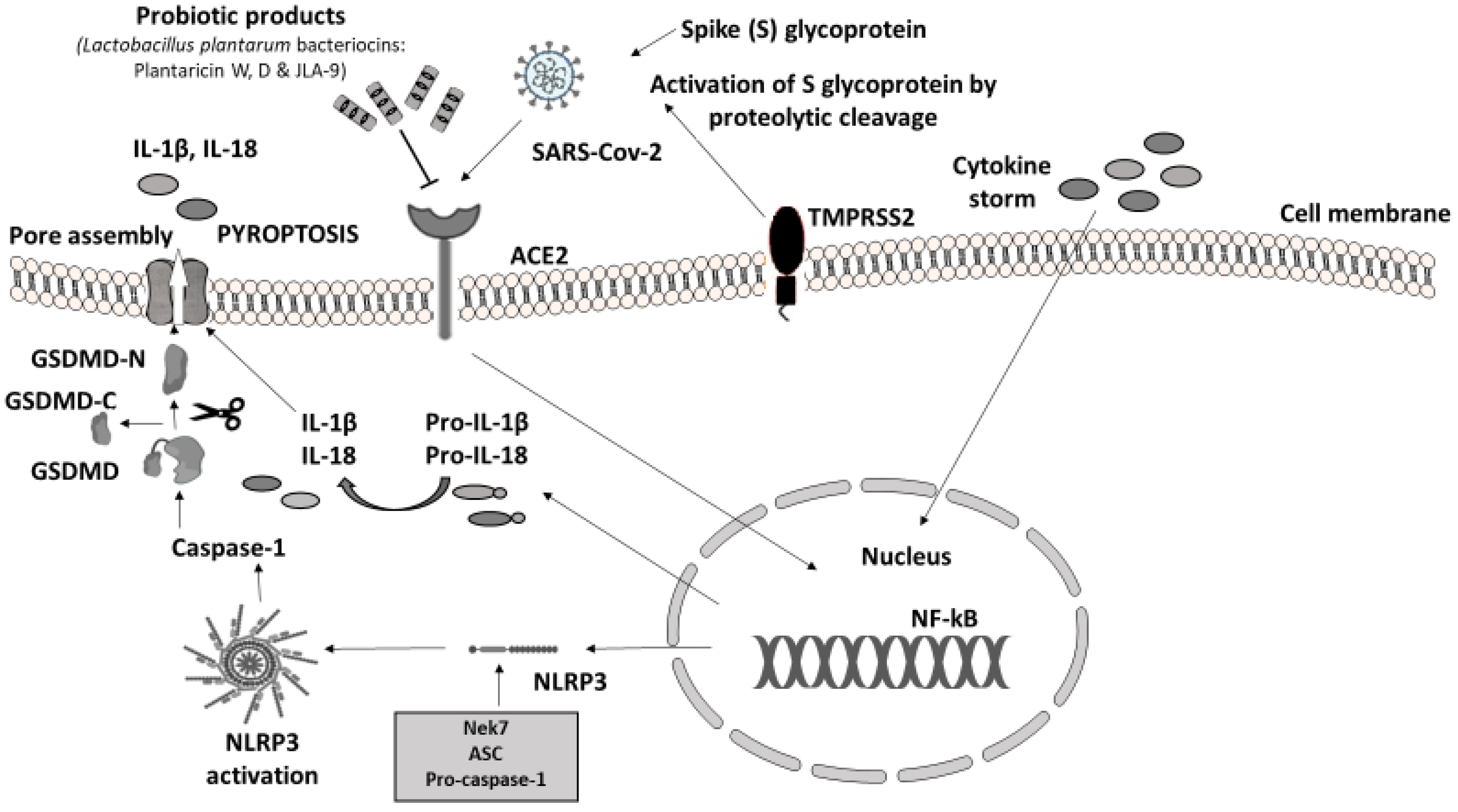
In humans, the main clinical manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are severe acute respiratory failure and macrophage activation syndrome (MAS) [46]. The immune response to SARS-CoV-2 is driven by inflammatory alveolar and monocyte-derived macrophages, which are activated by PAMPs and DAMPs, released by infected pneumocytes [47]. The massive release of cytokines, produced by the triggered innate immune system, leads to NLRP3 hyperactivation through viroporins. Viroporins are a group of viral proteins (protein E, open reading frame 3a (ORF3a), and ORF8a) with ion channel activity that takes part in virus replication and disease pathogenesis [48]. SARS-CoV-2 was confirmed to evoke gut inflammation in intestinal epithelial cells (IECs) and epithelial alveolar cells by invading in the same way. The virus invades the cells of the host through angiotensin-converting enzyme 2 (ACE2) receptors, expressed in both the respiratory and the GI tract. The viral protein S (Spike S glycoprotein) is activated by the transmembrane protease serine 2 (TMPRSS2) leading to the proinflammatory cytokine cascade. The downregulation of ACE2 caused by SAR-CoV-2 leads to alter gut microbiota, increased intestinal permeability, and inflammation directly linked to gastrointestinal symptoms and diarrhea in patients with COVID-19 [49,50,51].
It is already known that there is bidirectional crosstalk between gut and lung, named the gut–lung axis, which is involved in immune homeostasis. The underlying mechanism linking dysbiosis of gut microbiota with several respiratory diseases and dysbiosis of the lung microbiota is not fully understood. A potential mechanism could be the leaky gut and the migration of bacterial products or particles to the lung, stimulating the immune response. Additionally, blood or lymphatic mediated circulation of immune cells or inflammatory cytokines from the GI tract to the lung can induce inflammatory response [21]. The antiviral activity of probiotics against common respiratory viruses (including influenza, rhinovirus, and respiratory syncytial virus) is already confirmed by clinical and experimental studies [22,52]. LAB may offer protection from airway infection indirectly, through interaction with GALT, eliciting an enhancement of respiratory immunity. Furthermore, the protective role of probiotics is associated with the activation of proinflammatory natural killer (NK) cells and macrophages within the airway mucosa [53].
4. Conclusions
To conclude, PB may target SARS-CoV-2 in two different ways: by blocking virus invasion and replication through their metabolites, bacteriocins, and their ability to block ACE2 and by stimulating the immune response through NLRP3 regulation. While it is difficult to extrapolate the results from experimental studies with different strains and different types of samples in humans, in most of the above trials, PB were able to shield the host’s immunity system. PB administration as a preventive method and therapeutic modality through the NLRP3 inflammasome manipulation should be tested as a safe and affordable solution.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9112376/s1; Table S1. In vivo and ex vivo studies in animals and cell cultures-evaluating NLRP3 activation with probiotic administration.
Author Contributions
A.N.K. conceived the idea. K.D.T. supervised the work. M.D.N. developed the search strategy. K.D.S. contributed to the analysis and interpretation of the results. A.N.K. wrote the manuscript. A.N.K. and I.A.P. designed the figures. All authors contributed to the development of the selection criteria, strategy assessment, and data extraction criteria and discussed the results. All authors read, provided feedback, and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ASC | Adaptor molecule apoptosis-associated speck-like protein |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| BMECs | Bovine mammary epithelial cells |
| CARD | Caspase Activation and Recruitment Domain |
| COVID-19 | Coronavirus Disease 2019 |
| CE | Chronic Enteropathy |
| DAMPs | Damage-Associated Molecular Patterns |
| DC | Dendritic Cells |
| GRAS | Generally Regarded as Safe |
| GALT | Gut-Associated Lymphoid Tissue |
| GSDMD | Gasdermin D |
| HDPs | Host Defense Peptides |
| HFD | High Fat Diet |
| HK | Heat Killed |
| IECs | Intestinal Epithelial Cells |
| Ig | Immunoglobulin |
| IL | Interleukin |
| LAB | Lactic Acid Bacteria |
| LPS | Lipopolysaccharide |
| MA | Microbe-Derived Antioxidant |
| MAS | Macrophage Activation Syndrome |
| MΦ | Macrophage |
| NF-κB | Nuclear Factor-kappa B |
| NK | Natural killer cells |
| NLRP3 | NLR family pyrin domain containing 3 |
| NLRs | Nucleotide-Binding Oligomerization Domain-Like Receptors |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PB | Probiotic Bacteria |
| PPs | Peyer’s patches |
| PRRs | Pattern Recognition Receptors |
| SCFA | Short-Chain Fatty Acids |
| TLR | Toll-like Receptors |
References
- Llewellyn, A.; Foey, A. Probiotic Modulation of Innate Cell Pathogen Sensing and Signaling Events. Nutrients 2017, 9, 1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zmora, N.; Levy, M.; Pevsner-Fishcer, M.; Elinav, E. Inflammasomes and intestinal inflammation. Mucosal Immunol. 2017, 10, 865–883. [Google Scholar] [CrossRef]
- Morais, A.H.A.; Maciel, B.L.L.; da Silva-Maia, J.K.; Passos, T.S. Can Probiotics and Diet Promote Beneficial Immune Modulation and Purine Control in Coronavirus Infection? Nutrients 2020, 12, 1737. [Google Scholar] [CrossRef]
- Ding, L.; Gong, Y.; Yang, Z.; Zou, B.; Liu, X.; Zhang, B.; Li, J. Lactobacillus rhamnosus GG Ameliorates Liver Injury and Hypoxic Hepatitis in Rat Model of CLP-Induced Sepsis. Dig. Dis. Sci. 2019, 64, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Blaabjerg, S.; Artzi, D.M.; Aabenhus, R. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients—A Systematic Review and Meta-Analysis. Antibiotics 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.; Hu, J. Positive Effect of Probiotics on Constipation in Children: A Systematic Review and Meta-Analysis of Six Randomized Controlled Trials. Front. Cell. Infect. Microbiol. 2017, 7, 153. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.M.; Underwood, M.A. Probiotics and necrotizing enterocolitis. Semin. Pediatric Surg. 2018, 27, 39–46. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Hu, H.-J.; Liu, C.-Y.; Shakya, S.; Li, Z.-Y. Probiotics for Preventing Late-Onset Sepsis in Preterm Neonates: A PRISMA-Compliant Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 2016, 95, e2581. [Google Scholar] [CrossRef]
- Wang, H.; Anvari, S.; Anagnostou, K. The Role of Probiotics in Preventing Allergic Disease. Children 2019, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Nanavati, G.; Prasanth, T.; Kosala, M.; Bhandari, S.K.; Banotra, P. Effect of Probiotics and Prebiotics on Oral Health. Dent. J. Adv. Stud. 2021, 9, 1–6. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms 2021, 9, 69. [Google Scholar] [CrossRef]
- Ermolenko, E.; Gromova, L.; Borschev, Y.; Voeikova, A.; Karaseva, A.; Ermolenko, K.; Gruzdkov, A.; Suvorov, A. Influence of Different Probiotic Lactic Acid Bacteria on Microbiota and Metabolism of Rats with Dysbiosis. Biosci. Microbiota Food Health 2013, 32, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Kassaa, I.; Hober, D.; Hamze, M.; Chihib, N.E.; Drider, D. Antiviral potential of lactic acid bacteria and their bacteriocins. Probiotics Antimicrob. Proteins 2014, 6, 177–185. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24880436 (accessed on 6 May 2021). [CrossRef] [PubMed]
- Tiwari, S.K.; Dicks, L.M.T.; Popov, I.; Karaseva, A.; Ermakov, A.; Suvorov, A.; Tagg, J.R.; Weeks, R.; Chikindas, M.L. Probiotics at War Against Viruses: What Is Missing From the Picture? Front. Microbiol. 2020, 11, 1–21. [Google Scholar] [CrossRef]
- Lescheid, D.W. Probiotics as regulators of inflammation: A review. Funct. Foods Health Dis. 2014, 4, 299. [Google Scholar] [CrossRef]
- Zahid, A.; Li, B.; Kombe, A.J.K.; Jin, T.; Tao, J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front. Immunol. 2019, 10, 2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorente, C.G.; Muñoz, S.; Gil, A. Role of Toll-like receptors in the development of immunotolerance mediated by probiotics. Proc. Nutr. Soc. 2010, 69, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Mosser, D.M. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv. Physiol. Educ. 2013, 37, 284–291. [Google Scholar] [CrossRef]
- Wang, L.; Hauenstein, A.V. The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol. Asp. Med. 2020, 76, 100889. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Anwar, F.; Altayb, H.N.; Al-Abbasi, F.A.; Al-Malki, A.L.; Kamal, M.A.; Kumar, V. Antiviral effects of probiotic metabolites on COVID-19. J. Biomol. Struct. Dyn. 2020, 39, 4175–4184. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Aolymat, I.; Al-Holy, M.; Ayyash, M.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Osaili, T.; Apostolopoulos, V.; Liu, S.-Q.; Shah, N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. npj Sci. Food 2020, 4, 17. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [Green Version]
- Wagatsuma, K.; Nakase, H. Contradictory Effects of NLRP3 Inflammasome Regulatory Mechanisms in Colitis. Int. J. Mol. Sci. 2020, 21, 8145. [Google Scholar] [CrossRef]
- Russo, A.J.; Behl, B.; Banerjee, I.; Rathinam, V.A. Emerging Insights into Noncanonical Inflammasome Recognition of Microbes. J. Mol. Biol. 2018, 430, 207–216. [Google Scholar] [CrossRef]
- Carty, M.; Kearney, J.; Shanahan, K.A.; Hams, E.; Sugisawa, R.; Connolly, D.; Doran, C.G.; Muñoz-Wolf, N.; Gürtler, C.; Fitzgerald, K.; et al. Cell Survival and Cytokine Release after Inflammasome Activation Is Regulated by the Toll-IL-1R Protein SARM. Immunity 2019, 50, 1412–1424.e6. [Google Scholar] [CrossRef] [PubMed]
- Evavold, C.; Ruan, J.; Tan, Y.; Xia, S.; Wu, H.; Kagan, J.C. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 2018, 48, 35–44.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, I.; Behl, B.; Mendonca, M.; Shrivastava, G.; Russo, A.J.; Menoret, A.; Ghosh, A.; Vella, A.T.; Vanaja, S.K.; Sarkar, S.; et al. Gasdermin D Restrains Type I Interferon Response to Cytosolic DNA by Disrupting Ionic Homeostasis. Immunity 2018, 49, 413–426.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, S.; Werling, D.; Allenspach, K. Effects of Ex-Vivo and In-Vivo Treatment with Probiotics on the Inflammasome in Dogs with Chronic Enteropathy. PLoS ONE 2015, 10, e0120779. [Google Scholar] [CrossRef]
- Avolio, E.; Fazzari, G.; Zizza, M.; De Lorenzo, A.; Di Renzo, L.; Alò, R.; Facciolo, R.M.; Canonaco, M. Probiotics modify body weight together with anxiety states via pro-inflammatory factors in HFD-treated Syrian golden hamster. Behav. Brain Res. 2019, 356, 390–399. [Google Scholar] [CrossRef]
- Teixeira, L.; Kling, D.; Lorca, G.; Gonzalez, C. Lactobacillus johnsonii N6.2 diminishes caspase-1 maturation in the gastrointestinal system of diabetes prone rats. Benef. Microbes 2018, 9, 527–539. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, M.-C.; Yang, J.; Wang, J.-F.; Zhu, Y.-H. Lactobacillus rhamnosus GR-1 Ameliorates Escherichia coli-Induced Inflammation and Cell Damage via Attenuation of ASC-Independent NLRP3 Inflammasome Activation. Appl. Environ. Microbiol. 2016, 82, 1173–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, B.; Yu, J.; He, T.; Liu, X.; Su, J.; Wang, M.; Wang, J.; Zhu, Y. Lactobacillus johnsonii L531 ameliorates enteritis via elimination of damaged mitochondria and suppression of SQSTM1-dependent mitophagy in a Salmonella infantis model of piglet diarrhea. FASEB J. 2019, 34, 2821–2839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loss, H.; Aschenbach, J.R.; Ebner, F.; Tedin, K.; Lodemann, U. Effects of a pathogenic ETEC strain and a probiotic Enterococcus faecium strain on the inflammasome response in porcine dendritic cells. Veter. Immunol. Immunopathol. 2018, 203, 78–87. [Google Scholar] [CrossRef]
- Li, H.-H.; Li, Y.-P.; Zhu, Q.; Qiao, J.-Y.; Wang, W.-J. Dietary supplementation with Clostridium butyricum helps to improve the intestinal barrier function of weaned piglets challenged with enterotoxigenic Escherichia coli K88. J. Appl. Microbiol. 2018, 125, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-C.; Ouyang, C.-N.; Yuan, S.-N.; Lin, H.-C.; Huang, K.-Y.; Wu, P.-S.; Liu, C.-Y.; Tsai, K.-J.; Loi, L.-K.; Chen, Y.-J.; et al. Pretreatment with a Heat-Killed Probiotic Modulates the NLRP3 Inflammasome and Attenuates Colitis-Associated Colorectal Cancer in Mice. Nutrients 2019, 11, 516. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Xu, X.; Zhao, S.; Sho, T.; Luo, W.; Zhang, J.; Xu, W.; Hon, K.; Xu, J. Inclusion of microbe-derived antioxidant during pregnancy and lactation attenuates high-fat diet-induced hepatic oxidative stress, lipid disorders, and NLRP3 inflammasome in mother rats and offspring. Food Nutr. Res. 2019, 63, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tohno, M.; Shimosato, T.; Aso, H.; Kitazawa, H. Immunobiotic Lactobacillus strains augment NLRP3 expression in newborn and adult porcine gut-associated lymphoid tissues. Veter. Immunol. Immunopathol. 2011, 144, 410–416. [Google Scholar] [CrossRef]
- Bai, L.; Kumar, S.; Verma, S.; Seshadri, S. Bacteriocin PJ4 from probiotic lactobacillus reduced adipokine and inflammasome in high fat diet induced obesity. 3 Biotech 2020, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Chen, Z.; Lin, R.; Liu, Y.; Wu, X.; Puthiyakunnon, S.; Wang, Y.; Zhu, B.; Zhang, Q.; Bai, Y.; et al. Bacteroides fragilis Strain ZY-312 Defense against Cronobacter sakazakii-Induced Necrotizing Enterocolitis In Vitro and in a Neonatal Rat Model. mSystems 2019, 4, e00305-19. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Pan, S.; Luo, W.; Shen, Z.; Meng, X.; Xiao, M.; Tan, B.; Nie, K.; Tong, T.; Wang, X. Roseburia intestinalis-derived flagellin ameliorates colitis by targeting miR-223-3p-mediated activation of NLRP3 inflammasome and pyroptosis. Mol. Med. Rep. 2020, 22, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Aschenbach, J.R.; Tedin, K.; Pieper, R.; Loss, H.; Lodemann, U. Characterization of Inflammasome Components in Pig Intestine and Analysis of the Influence of Probiotic Enterococcus Faecium during an Escherichia Coli Challenge. Immunol. Investig. 2017, 46, 742–757. [Google Scholar] [CrossRef] [PubMed]
- A Hajam, I.; Dar, P.; Shahnawaz, I.; Jaume, J.C.; Lee, J.H. Bacterial flagellin—A potent immunomodulatory agent. Exp. Mol. Med. 2017, 49, e373. [Google Scholar] [CrossRef]
- Freeman, T.; Swartz, T.H. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front. Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chitre, S.A.; Akinyemi, I.A.; Loeb, J.C.; Lednicky, J.A.; McIntosh, M.T.; McIntosh, S.B. SARS-CoV-2 viroporin triggers the NLRP3 inflammatory pathway. bioRxiv 2020, 357731. [Google Scholar] [CrossRef]
- Kerget, B.; Kerget, F.; Aksakal, A.; Aşkın, S.; Sağlam, L.; Akgün, M. Evaluation of alpha defensin, IL-1 receptor antagonist, and IL-18 levels in COVID-19 patients with macrophage activation syndrome and acute respiratory distress syndrome. J. Med. Virol. 2020, 93, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Ben Said, L.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef] [PubMed]
- Shah, A. Novel Coronavirus-Induced NLRP3 Inflammasome Activation: A Potential Drug Target in the Treatment of COVID-19. Front. Immunol. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Effenberger, M.; Grabherr, F.; Mayr, L.; Schwaerzler, J.; Nairz, M.; Seifert, M.; Hilbe, R.; Seiwald, S.; Scholl-Buergi, S.; Fritsche, G.; et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut 2020, 69, 1543–1544. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Li, Z.; Cui, X.; Xiao, J.; Zhan, J.; et al. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020, 69, 1010–1018. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Shaikh, A.; Singh, R.; Misra, A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 241–246. [Google Scholar] [CrossRef]
- Villena, J.; Kitazawa, H. Editorial: Immunobiotics—Interactions of Beneficial Microbes with the Immune System. Front. Immunol. 2017, 8, 1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortaz, E.; Adcock, I.; Folkerts, G.; Barnes, P.J.; Vos, A.P.; Garssen, J. Probiotics in the Management of Lung Diseases. Mediat. Inflamm. 2013, 2013, 751068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef] [PubMed]
- Serkedjieva, J.; Danova, S.; Ivanova, I. Antiinfluenza Virus Activity of a Bacteriocin Produced by Lactobacillus delbrueckii. Appl. Biochem. Biotechnol. 2000, 88, 285–298. [Google Scholar] [CrossRef]
- Wachsman, M.B.; Castilla, V.; De Ruiz Holgado, A.P.; De Torres, R.A.; Sesma, F.; Coto, C.E. Enterocin CRL35 inhibits late stages of HSV-1 and HSV-2 replication in vitro. Antivir. Res. 2003, 58, 17–24. [Google Scholar] [CrossRef]
- Salman, J.A.-S.; Mahmood, N.N.; Abdulsattar, B.O.; Abid, H.A. The Effectiveness of Probiotics against Viral Infections: A Rapid Review with Focus on SARS-CoV-2 Infection. Open Access Maced. J. Med. Sci. 2020, 8, 496–508. [Google Scholar] [CrossRef]
- Forman, H.J. Hydrogen Peroxide: The Good, The Bad, and The Ugly. In Oxidants in Biology; Springer: Dordrecht, The Netherlands, 2008; pp. 1–17. [Google Scholar]
- De Brito, L.P.; da Silva, J.N., Jr.; de Barros, P.D.S.; da Silva, E.C.; de Calaça, P.R.; Soares, M.T.C.V.; Porto, A.L.F. Can postbiotics show antiviral effects against SARS-CoV-2? Res. Soc. Dev. 2021, 10, e17259. [Google Scholar] [CrossRef]
- American Veterinary Medical Association. SARS-CoV-2 in Animals 2020. Available online: https://www.avma.org/resources-tools/animal-health-and-welfare/covid-19/sars-cov-2-animals-including-pets (accessed on 6 May 2021).
- Fuglsang, A.; Rattray, F.P.; Nilsson, D.; Nyborg, N.C. Lactic acid bacteria: Inhibition of angiotensin converting enzyme in vitro and in vivo. Antonie Van Leeuwenhoek 2003, 83, 27–34. [Google Scholar] [CrossRef]
- Wu, D.; Yang, X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020, 53, 368–370. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Gohil, K.; Samson, R.; Dastager, S.; Dharne, M. Probiotics in the prophylaxis of COVID-19: Something is better than nothing. 3 Biotech 2021, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Xue, J.; Yang, J.; Chen, X.; Shao, Y.; Kwok, L.-Y.; Bilige, M.; Mang, L.; Zhang, H. Angiotensin-converting enzyme inhibitory activity of Lactobacillus helveticus strains from traditional fermented dairy foods and antihypertensive effect of fermented milk of strain H9. J. Dairy Sci. 2014, 97, 6680–6692. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Gonzalez, C.; Gibson, T.; Jauregi, P. Novel probiotic-fermented milk with angiotensin I-converting enzyme inhibitory peptides produced by Bifidobacterium bifidum MF 20/5. Int. J. Food Microbiol. 2013, 167, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, H.; Koning, C.; Mulder, L.; Rombouts, F.; Beynen, A. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).