Molecular Rationale of Insect-Microbes Symbiosis—From Insect Behaviour to Mechanism
Abstract
:1. Introduction
2. A Glimpse of Insect–Microbe Niche Foundations
2.1. Morphology and Physiochemical Conditions of Niches
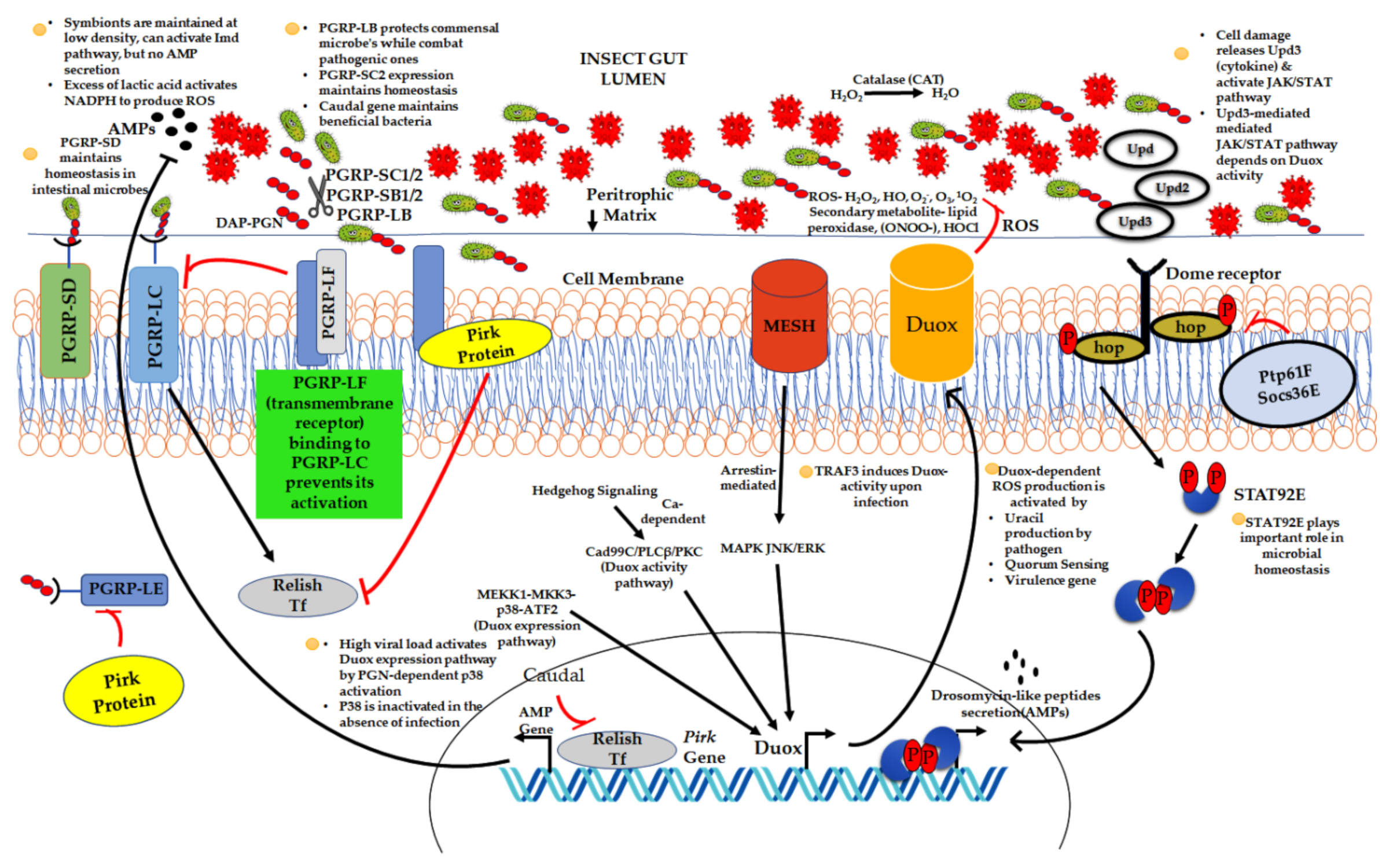
2.2. Impact of Host Immune System on Resident Symbionts
3. Microbial Symbiont: A Stealthy Modulator of Insect–Plant Interactions
4. Microbiome Sabotaging the Vector Competence of Insect Hosts
4.1. Arthropod Vector and its Symbiotic Microbiota
4.2. Insect as a Carrier of Plant and Mammalian Pathogens
4.3. Tripartite Interaction of Symbionts–Arthropod-Borne Pathogens–Insect Vectors
Wolbachia: A Panoply of Tactics for Vector-Borne Disease Control
5. The Extended Microbial Contribution in Insect–Microbiome Interaction: A Quantum Leap
5.1. Vitamin B Provisioning in Insect Nutrition
5.2. Microbial Secondary Metabolite-Driven Insect Community Interactions
5.3. Microbiome-Shaping Insect Behaviour
5.4. Gut Microbiota Linking Insects’ Nervous System, Physiology, and Behaviour
6. Insect Symbiosis: Implication and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Microbiology: Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Metzker, M.L. Sequencing technologies the next generation. Nat. Rev. Genet. 2010, 11, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Shpigler, H.Y.; Saul, M.C.; Corona, F.; Block, L.; Ahmed, A.C.; Zhao, S.D.; Robinson, G.E. Erratum: Deep evolutionary conservation of autism-related genes. Proc. Natl. Acad. Sci. USA 2019, 116, 17600. [Google Scholar] [CrossRef] [Green Version]
- Feltzin, V.; Wan, K.; Celniker, S.; Bonini, N. Role and impact of the gut microbiota in a Drosophila model for parkinsonism. bioRxiv 2019, 718825. [Google Scholar] [CrossRef]
- De Cock, M.; Virgilio, M.; Vandamme, P.; Augustinos, A.; Bourtzis, K.; Willems, A.; De Meyer, M. Impact of sample preservation and manipulation on insect gut microbiome profiling. A Test Case With Fruit Flies (Diptera, Tephritidae). Front. Microbiol. 2019, 10, 2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberti, J.; Engel, P. The gut microbiota—Brain axis of insects. Curr. Opin. Insect Sci. 2020, 39, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; O’Connell, R.M.; Mazmanian, S.K. Coordination of tolerogenic immune responses by the commensal microbiota. J. Autoimmun. 2010, 34, J220–J225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Frago, E.; Dicke, M.; Godfray, H.C.J. Insect symbionts as hidden players in insect-plant interactions. Trends Ecol. Evol. 2012, 27, 705–711. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects-diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Oliver, K.M.; Martinez, A.J. How resident microbes modulate ecologically-important traits of insects. Curr. Opin. Insect Sci. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lewis, Z.; Lizé, A. Insect behaviour and the microbiome. Curr. Opin. Insect Sci. 2015, 9, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Jing, T.Z.; Qi, F.H.; Wang, Z.Y. Most dominant roles of insect gut bacteria: Digestion, detoxification, or essential nutrient provision? Microbiome 2020, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, B.; Aksoy, S. Microbiome influences on insect host vector competence. Trends Parasitol. 2011, 27, 514–522. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, T.; Kikuchi, Y.; Shimada, M.; Fukatsu, T. Obligate symbiont involved in pest status of host insect. Proc. R. Soc. B Biol. Sci. 2007, 274, 1979–1984. [Google Scholar] [CrossRef] [Green Version]
- Nikoh, N.; Hosokawa, T.; Oshima, K.; Hattori, M.; Fukatsu, T. Reductive evolution of bacterial genome in insect gut environment. Genome Biol. Evol. 2011, 3, 702–714. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, T.; Koga, R.; Matsumoto, S.; Fukatsu, T. Interspecific symbiont transfection confers a novel ecological trait to the recipient insect. Biol. Lett. 2011, 7, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Barr, K.L.; Hearne, L.B.; Briesacher, S.; Clark, T.L.; Davis, G.E. Microbial symbionts in insects influence down-regulation of defense genes in maize. PLoS ONE 2010, 5, e11339. [Google Scholar] [CrossRef]
- Vorburger, C.; Gehrer, L.; Rodriguez, P. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol. Lett. 2010, 6, 109–111. [Google Scholar] [CrossRef] [Green Version]
- Nikoh, N.; Hosokawa, T.; Moriyama, M.; Oshima, K.; Hattori, M.; Fukatsu, T. Evolutionary origin of insect-Wolbachia nutritional mutualism. Proc. Natl. Acad. Sci. USA 2014, 111, 10257–10262. [Google Scholar] [CrossRef] [Green Version]
- Hansen, A.K.; Trumble, J.T.; Stouthamer, R.; Paine, T.D. A New Huanglongbing Species, “ Candidatus Liberibacter psyllaurous,” found to infect tomato and potato, is vectored by the Psyllid Bactericera cockerelli (Sulc). Appl. Environ. Microbiol. 2008, 74, 5862–5865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casteel, C.L.; Hansen, A.K.; Walling, L.L.; Paine, T.D. Manipulation of plant defense responses by the tomato psyllid (Bactericerca cockerelli) and its associated endosymbiont Candidatus Liberibacter Psyllaurous. PLoS ONE 2012, 7, e35191. [Google Scholar] [CrossRef]
- Kaiser, W.; Huguet, E.; Casas, J.; Commin, C.; Giron, D. Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. Proc. R. Soc. B Biol. Sci. 2010, 277, 2311–2319. [Google Scholar] [CrossRef] [Green Version]
- Morin, S.; Ghanim, M.; Zeidan, M.; Czosnek, H.; Verbeek, M.; van den Heuvel, J.F.J.M. A GroEL homologue from endosymbiotic bacteria of the whitefly Bemisia tabaci Is implicated in the circulative transmission of tomato yellow leaf curl virus. Virology 1999, 256, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottlieb, Y.; Zchori-Fein, E.; Mozes-Daube, N.; Kontsedalov, S.; Skaljac, M.; Brumin, M.; Sobol, I.; Czosnek, H.; Vavre, F.; Fleury, F.; et al. The transmission efficiency of tomato yellow leaf curl virus by the whitefly Bemisia tabaci Is Correlated with the Presence of a Specific Symbiotic Bacterium Species. J. Virol. 2010, 84, 9310–9317. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.; Schrader, L.; Gil, R.; Manzano-Marín, A.; Flórez, L.; Wheeler, D.; Werren, J.H.; Latorre, A.; Heinze, J.; Kaltenpoth, M.; et al. A novel intracellular mutualistic bacterium in the invasive ant Cardiocondyla obscurior. ISME J. 2016, 10, 376–388. [Google Scholar] [CrossRef] [Green Version]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarborough, C.L. Aphid Protected from pathogen by endosymbiont. Science 2005, 310, 1781. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, J.P.; McDonald, B.R.; Moran, N.A. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc. Natl. Acad. Sci. USA 2009, 106, 15394–15399. [Google Scholar] [CrossRef] [Green Version]
- Shigenobu, S.; Watanabe, H.; Hattori, M.; Sakaki, Y.; Ishikawa, H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 2000, 407, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Weiss, B.L.; Wang, J.; Aksoy, S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 2011, 9, e1000619. [Google Scholar] [CrossRef] [Green Version]
- Dale, C.; Welburn, S. The endosymbionts of tsetse flies: Manipulating host–parasite interactions. Int. J. Parasitol. 2001, 31, 628–631. [Google Scholar] [CrossRef]
- Pons, I.; Renoz, F.; Noël, C.; Hance, T. Circulation of the cultivable symbiont Serratia symbiotica in Aphids Is Mediated by Plants. Front. Microbiol. 2019, 10, 764. [Google Scholar] [CrossRef] [Green Version]
- Bando, H.; Okado, K.; Guelbeogo, W.M.; Badolo, A.; Aonuma, H.; Nelson, B.; Fukumoto, S.; Xuan, X.; Sagnon, N.; Kanuka, H. Intra-specific diversity of Serratia marcescens in Anopheles mosquito midgut defines Plasmodium transmission capacity. Sci. Rep. 2013, 3, 1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colgan, L.J.; Erbilgin, N. Tree-mediated interactions between the jack pine budworm and a mountain pine beetle fungal associate. Ecol. Entomol. 2011, 36, 425–434. [Google Scholar] [CrossRef]
- Adams, A.S.; Six, D.L. Detection of host habitat by parasitoids using cues associated with mycangial fungi of the mountain pine beetle, Dendroctonus ponderosae. Can. Entomol. 2008, 140, 124–127. [Google Scholar] [CrossRef] [Green Version]
- DiGuistini, S.; Wang, Y.; Liao, N.Y.; Taylor, G.; Tanguay, P.; Feau, N.; Henrissat, B.; Chan, S.K.; Hesse-Orce, U.; Alamouti, S.M.; et al. Genome and transcriptome analyses of the mountain pine beetle-fungal symbiont Grosmannia clavigera, a lodgepole pine pathogen. Proc. Natl. Acad. Sci. USA 2011, 108, 2504–2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulcr, J.; Mann, R.; Stelinski, L.L. The scent of a partner: Ambrosia beetles are attracted to volatiles from their fungal symbionts. J. Chem. Ecol. 2011, 37, 1374–1377. [Google Scholar] [CrossRef]
- Kopac, S.M.; Klassen, J.L. Can they make it on their own? Hosts, microbes, and the holobiont niche. Front. Microbiol. 2016, 7, 1647. [Google Scholar] [CrossRef]
- Borges, R.M. Co-niche construction between hosts and symbionts: Ideas and evidence. J. Genet. 2017, 96, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, S.; Klassen, J. Editorial overview: Hidden players: Microbes reshape the insect niche. Curr. Opin. Insect Sci. 2020, 39, vi–ix. [Google Scholar] [CrossRef] [PubMed]
- Currie, C.R.; Poulsen, M.; Mendenhall, J.; Boomsma, J.J.; Billen, J. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 2006, 311, 81–83. [Google Scholar] [CrossRef] [Green Version]
- Hulcr, J.; Rountree, N.R.; Diamond, S.E.; Stelinski, L.L.; Fierer, N.; Dunn, R.R. Mycangia of Ambrosia Beetles Host Communities of Bacteria. Microb. Ecol. 2012, 64, 784–793. [Google Scholar] [CrossRef] [Green Version]
- Yek, S.H.; Mueller, U.G. The metapleural gland of ants. Biol. Rev. 2011, 86, 774–791. [Google Scholar] [CrossRef]
- Mason, C.J. Complex Relationships at the intersection of insect gut microbiomes and plant defenses. J. Chem. Ecol. 2020, 46, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Calusinska, M.; Marynowska, M.; Bertucci, M.; Untereiner, B.; Klimek, D.; Goux, X.; Sillam-Dussès, D.; Gawron, P.; Halder, R.; Wilmes, P.; et al. Integrative omics analysis of the termite gut system adaptation to Miscanthus diet identifies lignocellulose degradation enzymes. Commun. Biol. 2020, 3, 275. [Google Scholar] [CrossRef]
- Větrovský, T.; Soukup, P.; Stiblik, P.; Votýpková, K.; Chakraborty, A.; Larrañaga, I.O.; Sillam-Dussès, D.; Lo, N.; Bourguignon, T.; Baldrian, P.; et al. Termites host specific fungal communities that differ from those in their ambient environments. Fungal Ecol. 2020, 48, 100991. [Google Scholar] [CrossRef]
- Soukup, P.; Větrovský, T.; Stiblik, P.; Votýpková, K.; Chakraborty, A.; Sillam-Dussès, D.; Kolařík, M.; Odriozola, I.; Lo, N.; Baldrian, P.; et al. Termites are associated with external species-specific bacterial communities. Appl. Environ. Microbiol. 2021, 87, e02042-20. [Google Scholar] [CrossRef]
- Edwards, M.J.; Jacobs-Lorena, M. Permeability and disruption of the peritrophic matrix and caecal membrane from Aedes aegypti and Anopheles gambiae mosquito larvae. J. Insect Physiol. 2000, 46, 1313–1320. [Google Scholar] [CrossRef]
- Pauchet, Y.; Muck, A.; Svatoš, A.; Heckel, D.G.; Preiss, S. Mapping the larval midgut lumen proteome of Helicoverpa armigera, a generalist herbivorous insect. J. Proteome Res. 2008, 7, 1629–1639. [Google Scholar] [CrossRef]
- Smith, T.E.; Moran, N.A. Coordination of host and symbiont gene expression reveals a metabolic tug-of-war between aphids and Buchnera. Proc. Natl. Acad. Sci. USA 2020, 117, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Teh, B.S.; Sun, C.; Hu, S.; Lu, X.; Boland, W.; Shao, Y. Biodiversity and activity of the gut microbiota across the life history of the insect herbivore Spodoptera littoralis. Sci. Rep. 2016, 6, 29505. [Google Scholar] [CrossRef] [PubMed]
- Ceja-Navarro, J.A.; Nguyen, N.H.; Karaoz, U.; Gross, S.R.; Herman, D.J.; Andersen, G.L.; Bruns, T.D.; Pett-Ridge, J.; Blackwell, M.; Brodie, E.L. Compartmentalized microbial composition, oxygen gradients and nitrogen fixation in the gut of Odontotaenius disjunctus. ISME J. 2014, 8, 6–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.H.; Scully, E.D.; Peiffer, M.; Geib, S.M.; Rosa, C.; Hoover, K.; Felton, G.W. Host plant species determines symbiotic bacterial community mediating suppression of plant defenses. Sci. Rep. 2017, 7, 39690. [Google Scholar] [CrossRef]
- Ivens, A.B.F.; Gadau, A.; Kiers, E.T.; Kronauer, D.J.C. Can social partnerships influence the microbiome? Insights from ant farmers and their trophobiont mutualists. Mol. Ecol. 2018, 27, 1898–1914. [Google Scholar] [CrossRef] [Green Version]
- Lucas, J.M.; Madden, A.A.; Penick, C.A.; Epps, M.J.; Marting, P.R.; Stevens, J.L.; Fergus, D.J.; Dunn, R.R.; Meineke, E.K. Azteca ants maintain unique microbiomes across functionally distinct nest chambers. Proc. R. Soc. B Biol. Sci. 2019, 286, 20191026. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.; Yao, Z.; Raza, M.F.; Cai, Z.; Zhang, H. Regulatory mechanisms of microbial homeostasis in insect gut. Insect Sci. 2021, 28, 286–301. [Google Scholar] [CrossRef]
- Chakraborty, A.; Roy, A. Microbial influence on plant–insect interaction. In Plant-Pest Interactions: From Molecular Mechanisms to Chemical Ecology; Springer: Singapore, 2021; pp. 337–363. [Google Scholar]
- Hammer, T.J.; Bowers, M.D. Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia 2015, 179, 1–14. [Google Scholar] [CrossRef]
- Jones, A.G.; Mason, C.J.; Felton, G.W.; Hoover, K. Host plant and population source drive diversity of microbial gut communities in two polyphagous insects. Sci. Rep. 2019, 9, 2792. [Google Scholar] [CrossRef] [Green Version]
- Mason, C.J.; Rubert-Nason, K.F.; Lindroth, R.L.; Raffa, K.F. Aspen defense chemicals influence midgut bacterial community composition of gypsy moth. J. Chem. Ecol. 2015, 41, 75–84. [Google Scholar] [CrossRef]
- Hammer, T.J.; Janzen, D.H.; Hallwachs, W.; Jaffe, S.P.; Fierer, N. Caterpillars lack a resident gut microbiome. Proc. Natl. Acad. Sci. USA 2017, 114, 9641–9646. [Google Scholar] [CrossRef] [Green Version]
- Mason, C.J.; Hoover, K.; Felton, G.W. Effects of maize (Zea mays) genotypes and microbial sources in shaping fall armyworm (Spodoptera frugiperda) gut bacterial communities. Sci. Rep. 2021, 11, 4429. [Google Scholar] [CrossRef]
- Casteel, C.L.; Hansen, A.K. Evaluating insect-microbiomes at the plant-insect interface. J. Chem. Ecol. 2014, 40, 836–847. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015, 6, 7618. [Google Scholar] [CrossRef] [Green Version]
- Mason, C.J.; Ray, S.; Shikano, I.; Peiffer, M.; Jones, A.G.; Luthe, D.S.; Hoover, K.; Felton, G.W. Plant defenses interact with insect enteric bacteria by initiating a leaky gut syndrome. Proc. Natl. Acad. Sci. USA 2019, 116, 15991–15996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Bosch, T.J.M.; Welte, C.U. Detoxifying symbionts in agriculturally important pest insects. Microb. Biotechnol. 2017, 10, 531–540. [Google Scholar] [CrossRef]
- Berasategui, A.; Salem, H.; Paetz, C.; Santoro, M.; Gershenzon, J.; Kaltenpoth, M.; Schmidt, A. Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Mol. Ecol. 2017, 26, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Tago, K.; Hayatsu, M.; Kikuchi, Y. Detoxifying symbiosis: Microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat. Prod. Rep. 2018, 35, 434–454. [Google Scholar] [CrossRef] [PubMed]
- De Fine Licht, H.H.; Schitøt, M.; Rogowska-Wrzesinska, A.; Nygaard, S.; Roepstorff, P.; Boomsma, J.J. Laccase detoxification mediates the nutritional alliance between leaf-cutting ants and fungus-garden symbionts. Proc. Natl. Acad. Sci. USA 2013, 110, 583–587. [Google Scholar] [CrossRef] [Green Version]
- Welte, C.U.; de Graaf, R.M.; van den Bosch, T.J.M.; Op den Camp, H.J.M.; van Dam, N.M.; Jetten, M.S.M. Plasmids from the gut microbiome of cabbage root fly larvae encode SaxA that catalyses the conversion of the plant toxin 2-phenylethyl isothiocyanate. Environ. Microbiol. 2016, 18, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Gurr, G.M.; Vasseur, L.; Zheng, D.; Zhong, H.; Qin, B.; Lin, J.; Wang, Y.; Song, F.; Li, Y.; et al. Metagenomic sequencing of diamondback moth gut microbiome unveils key holobiont adaptations for herbivory. Front. Microbiol. 2017, 8, 663. [Google Scholar] [CrossRef]
- Chakraborty, A.; Ashraf, M.Z.; Modlinger, R.; Synek, J.; Schlyter, F.; Roy, A. Unravelling the gut bacteriome of Ips (Coleoptera: Curculionidae: Scolytinae): Identifying core bacterial assemblage and their ecological relevance. Sci. Rep. 2020, 10, 18572. [Google Scholar] [CrossRef]
- Chakraborty, A.; Modlinger, R.; Ashraf, M.Z.; Synek, J.; Schlyter, F.; Roy, A. Core Mycobiome and Their Ecological Relevance in the Gut of Five Ips Bark Beetles (Coleoptera: Curculionidae: Scolytinae). Front. Microbiol. 2020, 11, 568853. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Schmidt, A.; Wadke, N.; Wright, L.P.; Schneider, B.; Bohlmann, J.; Brand, W.A.; Fenning, T.M.; Gershenzon, J.; Paetz, C. A common fungal associate of the spruce bark beetle metabolizes the stilbene defenses of Norway spruce. Plant Physiol. 2013, 162, 1324–1336. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Lu, Y.; Yang, F.; Zeng, L.; Liang, G.; Xu, Y. Transmission modes of a pesticide-degrading symbiont of the oriental fruit fly Bactrocera dorsalis (Hendel). Appl. Microbiol. Biotechnol. 2017, 101, 8543–8556. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Guo, Z.; Riegler, M.; Xi, Z.; Liang, G.; Xu, Y. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel). Microbiome 2017, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramya, S.L.; Venkatesan, T.; Murthy, K.S.; Jalali, S.K.; Varghese, A. Degradation of acephate by Enterobacter asburiae, Bacillus cereus and Pantoea agglomerans isolated from diamondback moth Plutella xylostella (L), a pest of cruciferous crops. J. Environ. Biol. 2016, 37, 611–618. [Google Scholar]
- De Almeida, L.G.; De Moraes, L.A.B.; Trigo, J.R.; Omoto, C.; Cônsoli, F.L. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: A potential source for biotechnological exploitation. PLoS ONE 2017, 12, e0174754. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Tomitaka, Y.; Shimoda, T.; Seo, S.; Sakurai, T.; Kugimiya, S.; Tsuda, S.; Kobayashi, M. Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant Cell Physiol. 2012, 53, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.H.; Rosa, C.; Scully, E.D.; Peiffer, M.; Tooker, J.F.; Hoover, K.; Luthe, D.S.; Felton, G.W. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc. Natl. Acad. Sci. USA 2013, 110, 15728–15733. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chung, S.H.; Peiffer, M.; Rosa, C.; Hoover, K.; Zeng, R.; Felton, G.W. Herbivore oral secreted bacteria trigger distinct defense responses in preferred and non-preferred host plants. J. Chem. Ecol. 2016, 42, 463–474. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Sumioka, H.; Takiguchi, M.; Uemura, T.; Kihara, Y.; Shinya, T.; Galis, I.; Arimura, G. Phytohormone-dependent plant defense signaling orchestrated by oral bacteria of the herbivore Spodoptera litura. New Phytol. 2021, 231, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, D.; Dettner, K.; Boland, W. Gut bacteria may be involved in interactions between plants, herbivores and their predators: Microbial biosynthesis of N-acylglutamine surfactants as elicitors of plant volatiles. Biol. Chem. 2000, 381, 755–762. [Google Scholar] [CrossRef]
- Hill, C.A.; Kafatos, F.C.; Stansfield, S.K.; Collins, F.H. Arthropod-borne diseases: Vector control in the genomics era. Nat. Rev. Microbiol. 2005, 3, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Cirimotich, C.M.; Dong, Y.; Garver, L.S.; Sim, S.; Dimopoulos, G. Mosquito immune defenses against Plasmodium infection. Dev. Comp. Immunol. 2010, 34, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Xi, Z.; Ramirez, J.L.; Dimopoulos, G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008, 4, e1000098. [Google Scholar] [CrossRef]
- Minard, G.; Mavingui, P.; Moro, C.V. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasites Vectors 2013, 6, 146. [Google Scholar] [CrossRef] [Green Version]
- Mereghetti, V.; Chouaia, B.; Montagna, M. New insights into the microbiota of moth pests. Int. J. Mol. Sci. 2017, 18, 2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guégan, M.; Zouache, K.; Démichel, C.; Minard, G.; Tran Van, V.; Potier, P.; Mavingui, P.; Valiente Moro, C. The mosquito holobiont: Fresh insight into mosquito-microbiota interactions. Microbiome 2018, 6, 49. [Google Scholar] [CrossRef]
- Strand, M.R. Composition and functional roles of the gut microbiota in mosquitoes. Curr. Opin. Insect Sci. 2018, 28, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Degnan, P.H.; Lazarus, A.B.; Wernegreen, J.J. Genome sequence of Blochmannia pennsylvanicus indicates parallel evolutionary trends among bacterial mutualists of insects. Genome Res. 2005, 15, 1023–1033. [Google Scholar] [CrossRef] [Green Version]
- Akman, L.; Yamashita, A.; Watanabe, H.; Oshima, K.; Shiba, T.; Hattori, M.; Aksoy, S. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat. Genet. 2002, 32, 402–407. [Google Scholar] [CrossRef]
- Degnan, P.H.; Yu, Y.; Sisneros, N.; Wing, R.A.; Moran, N.A. Hamiltonella defensa, genome evolution of protective bacterial endosymbiont from pathogenic ancestors. Proc. Natl. Acad. Sci. USA 2009, 106, 9063–9068. [Google Scholar] [CrossRef] [Green Version]
- Akman, L.; Rio, R.V.M.; Beard, C.B.; Aksoy, S. Genome size determination and coding capacity of Sodalis glossinidius, an enteric symbiont of tsetse flies, as revealed by hybridization to Escherichia coli gene arrays. J. Bacteriol. 2001, 183, 4517–4525. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.A.; Clancy, D.; Duncan, J. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity 1996, 76, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, K.T.; Hoffmann, A.A. Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet. Res. 2002, 80, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.J.; Palmer, M.R.; Rand, D.M. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity 2004, 93, 379–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownlie, J.C.; Cass, B.N.; Riegler, M.; Witsenburg, J.J.; Iturbe-Ormaetxe, I.; McGraw, E.A.; O’Neill, S.L. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 2009, 5, e1000368. [Google Scholar] [CrossRef] [Green Version]
- Kremer, N.; Voronin, D.; Charif, D.; Mavingui, P.; Mollereau, B.; Vavre, F. Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog. 2009, 5, e1000630. [Google Scholar] [CrossRef]
- Ikeya, T.; Broughton, S.; Alic, N.; Grandison, R.; Partridge, L. The endosymbiont Wolbachia increases insulin/IGF-like signalling in Drosophila. Proc. R. Soc. B Biol. Sci. 2009, 276, 3799–3807. [Google Scholar] [CrossRef] [Green Version]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef] [Green Version]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The Endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaser, R.L.; Meola, M.A. The native Wolbachia Endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to west nile virus infection. PLoS ONE 2010, 5, e11977. [Google Scholar] [CrossRef] [Green Version]
- Nazni, W.A.; Hoffmann, A.A.; NoorAfizah, A.; Cheong, Y.L.; Mancini, M.V.; Golding, N.; Kamarul, G.M.R.; Arif, M.A.K.; Thohir, H.; NurSyamimi, H.; et al. Establishment of Wolbachia strain wAlbB in malaysian populations of aedes aegypti for dengue control. Curr. Biol. 2019, 29, 4241–4248.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, S.B.; Riback, T.I.S.; Sylvestre, G.; Costa, G.; Peixoto, J.; Dias, F.B.S.; Tanamas, S.K.; Simmons, C.P.; Dufault, S.M.; Ryan, P.A.; et al. Effectiveness of wolbachia-infected mosquito deployments in reducing the incidence of dengue and other aedes-borne diseases in niterói, brazil: A quasi-experimental study. PLoS Negl. Trop. Dis. 2021, 15, e0009556. [Google Scholar] [CrossRef] [PubMed]
- Ant, T.H.; Herd, C.S.; Geoghegan, V.; Hoffmann, A.A.; Sinkins, S.P. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018, 14, e1006815. [Google Scholar] [CrossRef]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I.; et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N. Engl. J. Med. 2021, 384, 2177–2186. [Google Scholar] [CrossRef]
- Fraser, J.E.; De Bruyne, J.T.; Iturbe-Ormaetxe, I.; Stepnell, J.; Burns, R.L.; Flores, H.A.; O’Neill, S.L. Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog. 2017, 13, e1006751. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, Y.; Pang, X.; Xiao, X.; Zhang, R.; Cheng, G. Regulation of antimicrobial peptides in Aedes aegypti Aag2 Cells. Front. Cell. Infect. Microbiol. 2017, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Royet, J.; Dziarski, R. Peptidoglycan recognition proteins: Pleiotropic sensors and effectors of antimicrobial defences. Nat. Rev. Microbiol. 2007, 5, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Xiao, X.; Liu, Y.; Zhang, R.; Liu, J.; Liu, Q.; Wang, P.; Cheng, G. Mosquito C-type lectins maintain gut microbiome homeostasis. Nat. Microbiol. 2016, 1, 16023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Yang, L.; Pang, X.; Zhang, R.; Zhu, Y.; Wang, P.; Gao, G.; Cheng, G. A Mesh-Duox pathway regulates homeostasis in the insect gut. Nat. Microbiol. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Oliveira, J.H.M.; Gonçalves, R.L.S.; Lara, F.A.; Dias, F.A.; Gandara, A.C.P.; Menna-Barreto, R.F.S.; Edwards, M.C.; Laurindo, F.R.M.; Silva-Neto, M.A.C.; Sorgine, M.H.F.; et al. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011, 7, e1001320. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Albiter, H.; Sant’Anna, M.R.V.; Genta, F.A.; Dillon, R.J. Reactive oxygen species-mediated immunity against Leishmania mexicana and Serratia marcescens in the phlebotomine sand fly Lutzomyia longipalpis. J. Biol. Chem. 2012, 287, 23995–24003. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Zhou, G.; Wu, J.; Bian, G.; Lu, P.; Raikhel, A.S.; Xi, Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2012, 109, E23–E31. [Google Scholar] [CrossRef] [Green Version]
- Hurst, G.D.D.; Anbutsu, H.; Kutsukake, M.; Fukatsu, T. Hidden from the host: Spiroplasma bacteria infecting Drosophila do not cause an immune response, but are suppressed by ectopic immune activation. Insect Mol. Biol. 2003, 12, 93–97. [Google Scholar] [CrossRef]
- Hutchence, K.J.; Fischer, B.; Paterson, S.; Hurst, G.D.D. How do insects react to novel inherited symbionts? A microarray analysis of Drosophila melanogaster response to the presence of natural and introduced Spiroplasma. Mol. Ecol. 2011, 20, 950–958. [Google Scholar] [CrossRef]
- Zug, R.; Hammerstein, P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. 2015, 90, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Molina-Cruz, A.; Gupta, L.; Rodrigues, J.; Barillas-Mury, C. A Peroxidase/Dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 2010, 327, 1644–1648. [Google Scholar] [CrossRef] [Green Version]
- Louis, C.; Nigro, L. Ultrastructural evidence of Wolbachia rickettsiales in Drosophila simulans and their relationships with unidirectional cross-incompatibility. J. Invertebr. Pathol. 1989, 54, 39–44. [Google Scholar] [CrossRef]
- Zug, R.; Hammerstein, P. Wolbachia and the insect immune system: What reactive oxygen species can tell us about the mechanisms of Wolbachia-host interactions. Front. Microbiol. 2015, 6, 1201. [Google Scholar] [CrossRef] [Green Version]
- Cleton, N.; Koopmans, M.; Reimerink, J.; Godeke, G.J.; Reusken, C. Come fly with me: Review of clinically important arboviruses for global travelers. J. Clin. Virol. 2012, 55, 191–203. [Google Scholar] [CrossRef]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.-P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.R.; Catteruccia, F. Vector biology meets disease control: Using basic research to fight vector-borne diseases. Nat. Microbiol. 2019, 4, 20–34. [Google Scholar] [CrossRef]
- Gonzales-Ceron, L.; Santillan, F.; Rodriguez, M.H.; Mendez, D.; Hernandez-Avila, J.E. Bacteria in midguts of field-collected Anopheles albimanus Block Plasmodium vivax Sporogonic Development. J. Med. Entomol. 2003, 40, 371–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Aguilar, R.; Xi, Z.; Warr, E.; Mongin, E.; Dimopoulos, G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006, 2, 0513–0525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pais, R.; Lohs, C.; Wu, Y.; Wang, J.; Aksoy, S. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl. Environ. Microbiol. 2008, 74, 5965–5974. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, R.G.; Fang, J.; Kang, S.; Angleró-Rodríguez, Y.I.; Dong, Y.; Dimopoulos, G. Aminopeptidase secreted by Chromobacterium sp. Panama inhibits dengue virus infection by degrading the E protein. PLoS Negl. Trop. Dis. 2018, 12, e0006443. [Google Scholar] [CrossRef]
- Apte-Deshpande, A.; Paingankar, M.; Gokhale, M.D.; Deobagkar, D.N. Serratia odorifera a midgut inhabitant of aedes aegypti mosquito enhances its susceptibility to dengue-2 virus. PLoS ONE 2012, 7, e40401. [Google Scholar] [CrossRef]
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 2011, 332, 855–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narasimhan, S.; Rajeevan, N.; Liu, L.; Zhao, Y.O.; Heisig, J.; Pan, J.; Eppler-Epstein, R.; Deponte, K.; Fish, D.; Fikrig, E. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe 2014, 15, 58–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruzinova, K.; Sadlova, J.; Seblova, V.; Homola, M.; Votypka, J.; Volf, P. Comparison of bloodmeal digestion and the peritrophic matrix in four sand fly species differing in susceptibility to leishmania donovani. PLoS ONE 2015, 10, e0128203. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, F.H.; Gendrin, M.; Wyer, C.A.S.; Christophides, G.K. Microbiota-induced peritrophic matrix regulates midgut homeostasis and prevents systemic infection of malaria vector mosquitoes. PLoS Pathog. 2017, 13, e1006391. [Google Scholar] [CrossRef]
- Aksoy, S. Tsetse peritrophic matrix influences for trypanosome transmission. J. Insect Physiol. 2019, 118, 103919. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Yang, G.; Aksoy, S. Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission. Proc. Natl. Acad. Sci. USA 2009, 106, 12133–12138. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, J.; Brayner, F.A.; Alves, L.C.; Dixit, R.; Barillas-Mury, C. Hemocyte Differentiation Mediates Innate Immune Memory in Anopheles gambiae Mosquitoes. Science 2010, 329, 1353–1355. [Google Scholar] [CrossRef] [Green Version]
- Fraser, J.E.; O’Donnell, T.B.; Duyvestyn, J.M.; O’Neill, S.L.; Simmons, C.P.; Flores, H.A. Novel phenotype of Wolbachia strain wPip in Aedes aegypti challenges assumptions on mechanisms of Wolbachia-mediated dengue virus inhibition. PLoS Pathog. 2020, 16, e1008410. [Google Scholar] [CrossRef]
- Lindsey, A.; Bhattacharya, T.; Newton, I.; Hardy, R. Conflict in the intracellular lives of endosymbionts and viruses: A mechanistic look at Wolbachia-Mediated Pathogen-blocking. Viruses 2018, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Frentiu, F.D.; Moreira, L.A.; O’Neill, S.L.; Asgari, S. Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 108, 9250–9255. [Google Scholar] [CrossRef] [Green Version]
- Rancès, E.; Ye, Y.H.; Woolfit, M.; McGraw, E.A.; O’Neill, S.L. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012, 8, e1002548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Bian, G.; Pan, X.; Xi, Z. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl. Trop. Dis. 2012, 6, e1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Hussain, M.; O’Neill, S.L.; Asgari, S. Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc. Natl. Acad. Sci. USA 2013, 110, 10276–10281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef]
- Geoghegan, V.; Stainton, K.; Rainey, S.M.; Ant, T.H.; Dowle, A.A.; Larson, T.; Hester, S.; Charles, P.D.; Thomas, B.; Sinkins, S.P. Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nat. Commun. 2017, 8, 526. [Google Scholar] [CrossRef]
- Caragata, E.P.; Rancès, E.; Hedges, L.M.; Gofton, A.W.; Johnson, K.N.; O’Neill, S.L.; McGraw, E.A. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 2013, 9, e1003459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, C.; Islam, M.N.; Ye, Y.H.; Chotiwan, N.; Graham, B.; Belisle, J.T.; Kouremenos, K.A.; Dayalan, S.; Tull, D.L.; Klatt, S.; et al. Dengue virus dominates lipid metabolism modulations in Wolbachia-coinfected Aedes aegypti. Commun. Biol. 2020, 3, 518. [Google Scholar] [CrossRef] [PubMed]
- Chotiwan, N.; Andre, B.G.; Sanchez-Vargas, I.; Islam, M.N.; Grabowski, J.M.; Hopf-Jannasch, A.; Gough, E.; Nakayasu, E.; Blair, C.D.; Belisle, J.T.; et al. Dynamic remodeling of lipids coincides with dengue virus replication in the midgut of Aedes aegypti mosquitoes. PLOS Pathog. 2018, 14, e1006853. [Google Scholar] [CrossRef] [Green Version]
- Manokaran, G.; Flores, H.A.; Dickson, C.T.; Narayana, V.K.; Kanojia, K.; Dayalan, S.; Tull, D.; McConville, M.J.; Mackenzie, J.M.; Simmons, C.P. Modulation of acyl-carnitines, the broad mechanism behind Wolbachia-mediated inhibition of medically important flaviviruses in Aedes aegypti. Proc. Natl. Acad. Sci. USA 2020, 117, 24475–24483. [Google Scholar] [CrossRef] [PubMed]
- Haqshenas, G.; Terradas, G.; Paradkar, P.N.; Duchemin, J.B.; McGraw, E.A.; Doerig, C. A Role for the insulin receptor in the suppression of dengue virus and zika virus in Wolbachia-infected mosquito cells. Cell Rep. 2019, 26, 529–535.e3. [Google Scholar] [CrossRef] [Green Version]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines. Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molloy, J.C.; Sommer, U.; Viant, M.R.; Sinkins, S.P. Wolbachia modulates lipid metabolism in Aedes albopictus mosquito cells. Appl. Environ. Microbiol. 2016, 82, 3109–3120. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, S.L.; Ryan, P.A.; Turley, A.P.; Wilson, G.; Hurst, T.P.; Retzki, K.; Brown-Kenyon, J.; Hodgson, L.; Kenny, N.; Cook, H.; et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 2019, 3, 1547. [Google Scholar] [CrossRef]
- Tantowijoyo, W.; Andari, B.; Arguni, E.; Budiwati, N.; Nurhayati, I.; Fitriana, I.; Ernesia, I.; Daniwijaya, E.W.; Supriyati, E.; Yusdiana, D.H.; et al. Stable establishment of WMEL Wolbachia in Aedes aegypti populations in Yogyakarta, Indonesia. PLoS Negl. Trop. Dis. 2020, 14, e0008157. [Google Scholar] [CrossRef]
- Gesto, J.S.M.; Ribeiro, G.S.; Rocha, M.N.; Dias, F.B.S.; Peixoto, J.; Carvalho, F.D.; Pereira, T.N.; Moreira, L.A. Reduced competence to arboviruses following the sustainable invasion of Wolbachia into native Aedes aegypti from Southeastern Brazil. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Carr, J.P.; Donnelly, R.; Tungadi, T.; Murphy, A.M.; Jiang, S.; Bravo-Cazar, A.; Yoon, J.Y.; Cunniffe, N.J.; Glover, B.J.; Gilligan, C.A. Viral Manipulation of plant stress responses and host interactions with insects. Adv. Virus Res. 2018, 102, 177–197. [Google Scholar] [CrossRef]
- Kaur, N.; Hasegawa, D.K.; Ling, K.S.; Wintermantel, W.M. Application of genomics for understanding plant virus-insect vector interactions and insect vector control. Phytopathology 2016, 106, 1213–1222. [Google Scholar] [CrossRef] [Green Version]
- Frank, J.H.; Frank, J.H.; Thomas, M.C.; Yousten, A.A.; Howard, F.W.; Giblin-davis, R.M.; Heppner, J.B.; Zuparko, R.L.; Sánchez, N.E.; Luna, M.G.; et al. Plant Viruses and Insects. In Encyclopedia of Entomology; Springer: Dordrecht, The Netherlands, 2008; pp. 2938–2945. [Google Scholar]
- Gong, J.-T.; Li, Y.; Li, T.-P.; Liang, Y.; Hu, L.; Zhang, D.; Zhou, C.-Y.; Yang, C.; Zhang, X.; Zha, S.-S.; et al. Stable introduction of plant-virus-inhibiting Wolbachia into Planthoppers for Rice Protection. Curr. Biol. 2020, 30, 4837–4845. [Google Scholar] [CrossRef]
- Ramalho, M.O.; Duplais, C.; Orivel, J.; Dejean, A.; Gibson, J.C.; Suarez, A.V.; Moreau, C.S. Development but not diet alters microbial communities in the Neotropical arboreal trap jaw ant Daceton armigerum: An exploratory study. Sci. Rep. 2020, 10, 7350. [Google Scholar] [CrossRef]
- Segers, F.H.I.D.; Kaltenpoth, M.; Foitzik, S. Abdominal microbial communities in ants depend on colony membership rather than caste and are linked to colony productivity. Ecol. Evol. 2019, 9, 13450–13467. [Google Scholar] [CrossRef] [Green Version]
- Salem, H.; Bauer, E.; Strauss, A.S.; Vogel, H.; Marz, M.; Kaltenpoth, M. Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, A.E. The B vitamin nutrition of insects: The contributions of diet, microbiome and horizontally acquired genes. Curr. Opin. Insect Sci. 2017, 23, 65–69. [Google Scholar] [CrossRef]
- Kaltenpoth, M.; Göttler, W.; Herzner, G.; Strohm, E. Symbiotic bacteria protect wasp larvae from fungal infestation. Curr. Biol. 2005, 15, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Vizcaino, M.I.; Guo, X.; Crawford, J.M. Merging chemical ecology with bacterial genome mining for secondary metabolite discovery. J. Ind. Microbiol. Biotechnol. 2014, 41, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Klassen, J.L. Microbial secondary metabolites and their impacts on insect symbioses. Curr. Opin. Insect Sci. 2014, 4, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hurst, G.D.D.; Frost, C.L. Reproductive parasitism: Maternally inherited symbionts in a biparental world. Cold Spring Harb. Perspect. Biol. 2015, 7, a017699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiggins, F.M.; Hurst, G.D.D.; Majerus, M.E.N. Sex-ratio-distorting Wolbachia causes sex-role reversal in its butterfly host. Proc. R. Soc. B Biol. Sci. 2000, 267, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Abe, J.; Kamimura, Y.; Kondo, N.; Shimada, M. Extremely female-biased sex ratio and lethal male-male combat in a parasitoid wasp, Melittobia australica (Eulophidae). Behav. Ecol. 2003, 14, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Stouthamer, R.; Breeuwer, J.A.J.; Hurst, G.D.D. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef]
- Mateos, M.; Castrezana, S.J.; Nankivell, B.J.; Estes, A.M.; Markow, T.A.; Moran, N.A. Heritable endosymbionts of Drosophila. Genetics 2006, 174, 363–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodacre, S.L.; Martin, O.Y.; Thomas, C.F.G.; Hewitt, G.M. Wolbachia and other endosymbiont infections in spiders. Mol. Ecol. 2006, 15, 517–527. [Google Scholar] [CrossRef]
- Konecka, E.; Olszanowski, Z. A screen of maternally inherited microbial endosymbionts in oribatid mites (Acari: Oribatida). Microbiology 2015, 161, 1561–1571. [Google Scholar] [CrossRef]
- Kenyon, S.G.; Hunter, M.S. Manipulation of oviposition choice of the parasitoid wasp, Encarsia pergandiella, by the endosymbiotic bacterium Cardinium. J. Evol. Biol. 2007, 20, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.P.; Andersen, S.B.; Hywel-Jones, N.L.; Himaman, W.; Billen, J.; Boomsma, J.J. Behavioral mechanisms and morphological symptoms of zombie ants dying from fungal infection. BMC Ecol. 2011, 11, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurek, L.; Wes Watson, D.; Krasnoff, S.B.; Schal, C. Effect of the entomopathogenic fungus, Entomophthora muscae (Zygomycetes: Entomophthoraceae), on sex pheromone and other cuticular hydrocarbons of the house fly, Musca domestica. J. Invertebr. Pathol. 2002, 80, 171–176. [Google Scholar] [CrossRef]
- Adamo, S.A.; Kovalko, I.; Easy, R.H.; Stoltz, D. A viral aphrodisiac in the cricket Gryllus texensis. J. Exp. Biol. 2014, 217, 1970–1976. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Chen, S.; Huang, Y.; Pierce, N.E.; Riegler, M.; Yang, F.; Zeng, L.; Lu, Y.; Liang, G.; Xu, Y. Symbiotic microbiota may reflect host adaptation by resident to invasive ant species. PLOS Pathog. 2019, 15, e1007942. [Google Scholar] [CrossRef] [Green Version]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 2020, 25, 2905–2918. [Google Scholar] [CrossRef]
- Wada-Katsumata, A.; Zurek, L.; Nalyanya, G.; Roelofs, W.L.; Zhang, A.; Schal, C. Gut bacteria mediate aggregation in the German cockroach. Proc. Natl. Acad. Sci. USA 2015, 112, 15678–15683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.C.N.; Wang, Q.P.; Morimoto, J.; Senior, A.M.; Lihoreau, M.; Neely, G.G.; Simpson, S.J.; Ponton, F. Gut microbiota modifies olfactory-guided microbial preferences and foraging decisions in Drosophila. Curr. Biol. 2017, 27, 2397–2404.e4. [Google Scholar] [CrossRef]
- Carthey, A.J.R.; Gillings, M.R.; Blumstein, D.T. The Extended Genotype: Microbially mediated olfactory communication. Trends Ecol. Evol. 2018, 33, 885–894. [Google Scholar] [CrossRef] [PubMed]
- DeNieu, M.; Mounts, K.; Manier, M. Two gut microbes are necessary and sufficient for normal cognition in Drosophila melanogaster. bioRxiv 2019, 593723. [Google Scholar] [CrossRef]
- Teseo, S.; van Zweden, J.S.; Pontieri, L.; Kooij, P.W.; Sørensen, S.J.; Wenseleers, T.; Poulsen, M.; Boomsma, J.J.; Sapountzis, P. The scent of symbiosis: Gut bacteria may affect social interactions in leaf-cutting ants. Anim. Behav. 2019, 150, 239–254. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Prakash, S. A novel synbiotic delays Alzheimer’s disease onset via combinatorial gut-brain-axis signaling in Drosophila melanogaster. PLoS ONE 2019, 14, e0214985. [Google Scholar] [CrossRef]
- Chen, K.; Luan, X.; Liu, Q.; Wang, J.; Chang, X.; Snijders, A.M.; Mao, J.H.; Secombe, J.; Dan, Z.; Chen, J.H.; et al. Drosophila histone demethylase KDM5 regulates social behavior through immune control and gut microbiota maintenance. Cell Host Microbe 2019, 25, 537–552.e8. [Google Scholar] [CrossRef] [Green Version]
- Van Moll, L.; De Smet, J.; Cos, P.; Van Campenhout, L. Microbial symbionts of insects as a source of new antimicrobials: A review. Crit. Rev. Microbiol. 2021, 47, 562–579. [Google Scholar] [CrossRef]
- Xu, L.; Xu, S.; Sun, L.; Zhang, Y.; Luo, J.; Bock, R.; Zhang, J. Synergistic action of the gut microbiota in environmental RNA interference in a leaf beetle. Microbiome 2021, 9, 98. [Google Scholar] [CrossRef]
- Whitten, M.; Dyson, P. Gene silencing in non-model insects: Overcoming hurdles using symbiotic bacteria for trauma-free sustainable delivery of RNA interference: Sustained RNA interference in insects mediated by symbiotic bacteria: Applications as a genetic tool and as a biocid. BioEssays 2017, 39, 1600247. [Google Scholar] [CrossRef] [PubMed]
 . Beneficial or commensal bacteria
. Beneficial or commensal bacteria  .
.
 . Beneficial or commensal bacteria
. Beneficial or commensal bacteria  .
.

| Bacterial Symbionts | Insect Host | Niche Location within Host | Transmission Mode | Interaction Benefits | References |
|---|---|---|---|---|---|
| Ishikawaella capsulate (Obligate mutualist) | Megacopta punctatissima (Plataspid stinkbugs) | Extracellular midgut | Inheritable and transmitted through a capsule | Enhance pest status of the insect host. Microbe compensates for nutritional deficiency of host diet by supplying essential amino acids. | [16,17] |
| Regiella insecticola (Facultative commensal) | Acyrthosiphon pisum (Aphid) | Bacteriocytes, Haemolymph | Inheritable and transmitted via Transovarial | Influence host plant range; survival, and reproduction on clover of insect host. | [18] |
| Wolbachia sp. (Facultative parasite) | Diabrotica virgifera | Bacteriocytes, extracellularly scattered | Inheritable and transmitted via Transovarial | Silencing of maize (host plant) defence induction via insect host. | [19] |
| Regiella insecticola (Facultative commensal) | Myzus persicae (peach-potato aphid) | Bacteriocytes, Haemolymph | Inheritable and transmitted via Transovarial | Protection against parasitoids. | [20] |
| Wolbachia sp. (Facultative parasite) | Cimex lectularius | Bacteriocytes, extracellularly scattered | Inheritable and transmitted via Transovarial | Provisioning of B vitamins. | [21] |
| Candidatus liberibacter Psyllaurous (Facultative) | Bactericera cockerelli (Tomato psyllid) | Extracellular | Acquired during feeding and vectored by the insect host | Reduced expression of plant defensive gene in tomato probably for psyllid success. | [22,23] |
| Wolbachia sp. (Facultative parasite) | Phyllonorycter blancardella (Leaf mining moth) | Bacteriocytes, extracellularly scattered | Inheritable and transmitted via Transovarial | To increase host insect fitness, the maintenance of chlorophyll and nutrient-rich “green island” (insect feeding site) in senescent leaves of the host plant. | [24] |
| Buchnera spp. (Obligate mutualists) | Bemisia tabaci (Whitefly) | Mycetocytes | Inheritable and transmitted via Transovarial | Produces GroEL chaperone protein that binds to plant viruses and makes virus transmission efficient. | [25] |
| Hamiltonella (Facultative Commensal) | Bemisia tabaci (Whitefly) | Sheath Cells, Secondary Myocetocytes, Haemolymph | Acquired and Inheritable; Horizontal and Maternal | GroEL protein produced by Hamiltonella facilitates transmission of tomato yellow leaf curl virus vectored by whitefly. | [26] |
| Candidatus Westeberhardia cardiocondylae | Cardiocondyla obscurior (Invasive ant) | Gut-associated bacteriomes | Transmitted to late-stage oocytes; Vertical transmission | Contributes to cuticle formation and is responsible for host invasive success. | [27] |
| Hamiltonella (Facultative Commensal) | Acyrthosiphon pisum (Pea aphid) | Sheath Cells, Secondary Myocetocytes, Haemolymph | Acquired and Inheritable; Horizontal and Maternal | It confers resistance to host insects from a parasitoid attack. | [28] |
| Regiella insecticola(Facultative commensal) | Acyrthosiphon pisum | Bacteriocytes, Haemolymph | Inheritable and transmitted via Transovarial | Resistance to host insect from fungal pathogens | [29] |
| Burkholderia sp. | Riptortus pedestris | Crypts at posterior midgut region | Acquired from environment and undergo horizontal transmission | Symbiont-mediated fenitrothion (insecticide) resistance to insect host | [30] |
| Baumannia cicadellinicola (obligate mutualist) | Sharpshooters | Bacteriocytes | Inheritable and transmitted via Transovarial | Baumannia contributes several B vitamins to its host insect. | [31] |
| Buchnera spp. (Obligate mutualists) | Acyrthosiphon pisum | Bacteriocytes | Inheritable and transmitted via Transovarial | Buchnera contributes several B vitamins to its host insect. | [32] |
| Wigglesworthia glossinidia(Obligate mutualist) | Tsetse flies | Bacteriocytes | Inheritable and transmitted via Transovarial | Wigglesworthia presence during the development of larval stages is vital for Tsetse flies’ immune system development and function. | [33] |
| Sodalis glossinidius (Secondary facultative) | Tsetse flies | Numerous tissues | Both inheritable and acquired; Transmitted via milk gland, mating and transovarial | Sodalis impacts tsetse flies vector competence and longevity | [34] |
| Serratia symbiotica (Facultative symbiont) | Aphids | NA | Acquired from the environment; Horizontal transmission | In the Lachninae subfamily, Serratia supplements Buchnera aphidicola ability of tryptophan biosynthesis. In Acyrthosiphon pisum, S. symbiotica is involved in heat stress tolerance and parasitoid resistance to host insect. | [35] |
| Serratia marcescens (Facultative symbiont) | hematophagous insects | midgut | Adhere to eggs surface, colonize oviposition site | Serratia marcescens have an anti-Plasmodium function in Anopheles mosquito midgut | [36] |
| Fungal Symbionts | Insect Host | Niche location within the host | Transmission mode | Interaction Benefits | References |
| Grosmannia clavigera (Obligate mutualist) | Dendroctonus ponderosae (Bark beetle) | Mycangia, exoskeleton | Acquire spores in the pupal chamber just before emergence | Increased success of host insect on jack pines (host plant) reduces food quality for interspecific competitors | [37] |
| Oxygenated monoterpenes produced by microbial activity is used as host (beetle) location cues by parasitoids. | [38] | ||||
| Grosmannia clavigera can detoxify oleoresin terpenoids (conifer-defence chemicals) and utilize them as carbon sources. It allows host insects to tolerate terpenoids and grow successfully on pine hosts | [39] | ||||
| Raffaelea lauricola (obligate mutualist) | Xyleborus glabratus (Redbay ambrosia beetle) | Mycangia, exoskeleton | Larvae and adults feed on the conidia | Volatile cues from fungal symbionts may function as a mechanism to locate established fungal gardens of conspecific beetles (suitable microhabitat) but also as an orientation cue within a gallery | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Singh, A.; Baweja, V.; Roy, A.; Chakraborty, A.; Singh, I.K. Molecular Rationale of Insect-Microbes Symbiosis—From Insect Behaviour to Mechanism. Microorganisms 2021, 9, 2422. https://doi.org/10.3390/microorganisms9122422
Singh S, Singh A, Baweja V, Roy A, Chakraborty A, Singh IK. Molecular Rationale of Insect-Microbes Symbiosis—From Insect Behaviour to Mechanism. Microorganisms. 2021; 9(12):2422. https://doi.org/10.3390/microorganisms9122422
Chicago/Turabian StyleSingh, Sujata, Archana Singh, Varsha Baweja, Amit Roy, Amrita Chakraborty, and Indrakant Kumar Singh. 2021. "Molecular Rationale of Insect-Microbes Symbiosis—From Insect Behaviour to Mechanism" Microorganisms 9, no. 12: 2422. https://doi.org/10.3390/microorganisms9122422
APA StyleSingh, S., Singh, A., Baweja, V., Roy, A., Chakraborty, A., & Singh, I. K. (2021). Molecular Rationale of Insect-Microbes Symbiosis—From Insect Behaviour to Mechanism. Microorganisms, 9(12), 2422. https://doi.org/10.3390/microorganisms9122422








