Hide-and-Seek: A Game Played between Parasitic Protists and Their Hosts
Abstract
:1. Introduction
2. Neither Naked nor Clothed: A Smart Strategy for Epicellular Parasitism in Cryptosporidia
2.1. Formation of Parasitophorous Sac
2.2. Parasite Invasive Apparatus and Its Role in Modulation of Host Cell Actin
2.3. Modulation of Host Cell Apoptosis
2.4. Immune Evasion
3. Chicken-and-Egg Dilemma: Protists on the Way in or out?
4. The Darkest Place Is under the Candle: Taming of the Immune Cells by Leishmania
4.1. Early Survival in Neutrophils
4.2. Silent Entry into Monocytes and Macrophages
4.3. Parasite Internalisation into Monocytes and Macrophages and Modulation of Host Cell Actin
4.4. Formation of Parasitophorous Vacuole
4.5. Immune Evasion
4.6. Apoptosis as a Dissemination Strategy
4.7. Other Cells That Can Host Leishmania
5. Conclusions
- (i)
- What is the real invasive potential of epicellular parasites and what drives them to invade deeper tissues of some hosts (parasite virulence differing among species, host fitness/immunity status, or other non-considered factors)?
- (ii)
- What is the reason for the limited efficacy of tested chemotherapeutics if cryptosporidiosis is an acute self-limiting infection in immunocompetent hosts?
- (iii)
- What determines whether Leishmania promastigotes transform into the amastigote stage? Why can it not transform within dermal tissue-resident macrophages or neutrophils?
- (iv)
- What is the exact composition of parasitophorous envelopes (parasitophorous sac or parasitophorous vacuole) in Cryptosporidium, Leishmania, and, consequently, in other parasites with similar localisation? How does the parasite contribute to its composition?
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| H+ ATPase | Hydrogen-exporting Adenosine Triphosphate Phosphohydrolase |
| GIPLs | Glycosylinositolphospholipids |
| GP63 | Glycoprotein 63, syn. Leishmania metalloproteinase, leishmanolysin |
| IFN-γ | Interferon gamma |
| IL | Interleukin |
| iNOS | Inducible Nitric Oxide Synthase |
| LAMP | Lysosome-Associated Membrane glycoproteins |
| LMO7 | LIM domain Only 7 |
| LPG | Lipophosphoglycan |
| NK | Natural Killer cells |
| NO | Nitric Oxide |
| PKC | Protein Kinase C |
| PS | Parasitophorous Sac |
| PV | Parasitophorous Vacuole |
| RON | Rhoptry Neck Protein |
| ROP | Rhoptry (bulb) Protein |
| ROS | Reactive Oxygen Species |
| STAT-1 | Signal Transducer and Activator of Transcription 1 |
| TNF-α | Tumor Necrosis Factor alpha, syn. cachexin |
References
- Ratcliffe, M.J.H. Encyclopedia of Immunobiology, 1st ed.; Academic Press: Oxford, UK, 2016; p. 3126. [Google Scholar]
- Briceño, A.L.; Contreras, Z.P.; Vera, D.D.; Briceño, R.M.; Pru, E.P. Tissue culture to assess bacterial enteropathogenicity. In Biomedical Tissue Culture; Ceccherini-Nelli, L., Matteoli, B., Eds.; IntechOpen: Rijeka, Croatia, 2012; pp. 203–220. [Google Scholar]
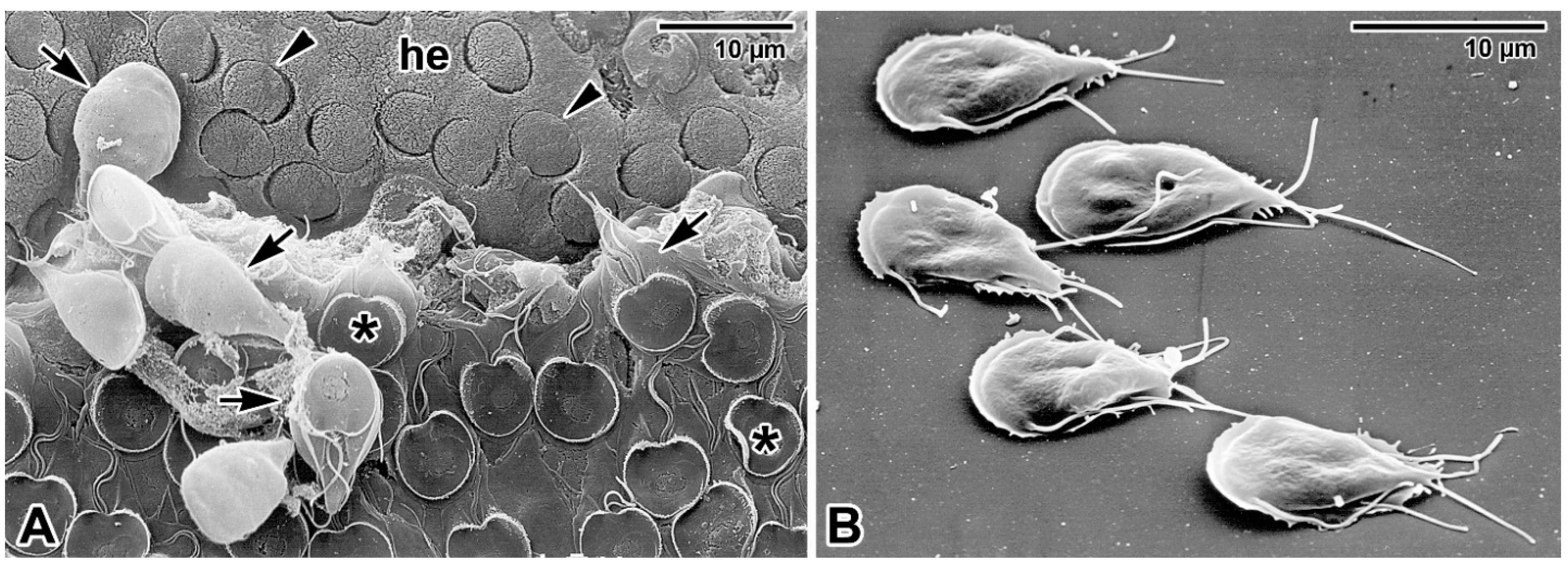
- Valigurová, A.; Jirků, M.; Koudela, B.; Gelnar, M.; Modrý, D.; Šlapeta, J. Cryptosporidia: Epicellular parasites embraced by the host cell membrane. Int. J. Parasitol. 2008, 38, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Valigurová, A.; Hofmannová, L.; Koudela, B.; Vávra, J. An ultrastructural comparison of the attachment sites between Gregarina steini and Cryptosporidium muris. J. Eukaryot. Microbiol. 2007, 54, 495–510. [Google Scholar] [CrossRef]
- Valigurová, A.; Paskerova, G.G.; Diakin, A.; Kováčiková, M.; Simdyanov, T.G. Protococcidian Eleutheroschizon duboscqi, an unusual apicomplexan interconnecting gregarines and cryptosporidia. PLoS ONE 2015, 10, e0125063. [Google Scholar] [CrossRef] [Green Version]
- Dubremetz, J.F.; Garcia-Reguet, N.; Conseil, V.; Fourmaux, M.N. Apical organelles and host-cell invasion by Apicomplexa. Int. J. Parasitol. 1998, 28, 1007–1013. [Google Scholar] [CrossRef]
- Bargieri, D.; Lagal, V.; Andenmatten, N.; Tardieux, I.; Meissner, M.; Ménard, R. Host cell invasion by apicomplexan parasites: The junction conundrum. PLoS Pathog. 2014, 10, e1004273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umemiya, R.; Fukuda, M.; Fujisaki, K.; Matsui, T. Electron microscopic observation of the invasion process of Cryptosporidium parvum in severe combined immunodeficiency mice. J. Parasitol. 2005, 91, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Borowski, H.; Thompson, R.C.; Armstrong, T.; Clode, P.L. Morphological characterization of Cryptosporidium parvum life-cycle stages in an in vitro model system. Parasitology 2010, 137, 13–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcial, M.A.; Madara, J.L. Cryptosporidium: Cellular localization, structural analysis of absorptive cell-parasite membrane-membrane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology 1986, 90, 583–594. [Google Scholar] [CrossRef]
- Barta, J.R.; Thompson, R.C.A. What is Cryptosporidium? Reappraising its biology and phylogenetic affinities. Trends Parasitol. 2006, 22, 463–468. [Google Scholar] [CrossRef]
- Carreno, R.A.; Martin, D.S.; Barta, J.R. Cryptosporidium is more closely related to the gregarines than to coccidia as shown by phylogenetic analysis of apicomplexan parasites inferred using small-subunit ribosomal RNA gene sequences. Parasitol. Res. 1999, 85, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Clode, P.L.; Koh, W.H.; Thompson, R.C.A. Life without a host cell: What is Cryptosporidium? Trends Parasitol. 2015, 31, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Melicherová, J.; Hofmannová, L.; Valigurová, A. Response of cell lines to actual and simulated inoculation with Cryptosporidium proliferans. Eur. J. Protistol. 2018, 62, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Paparini, A.; Monis, P.; Hijjawi, N. It’s official-Cryptosporidium is a gregarine: What are the implications for the water industry? Water Res. 2016, 105, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumb, R.; Smith, K.; Odonoghue, P.J.; Lanser, J.A. Ultrastructure of the attachment of Cryptosporidium sporozoites to tissue-culture cells. Parasitol. Res. 1988, 74, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Koudela, B.; Vitovec, J.; Sterba, J.; Milacek, P. An unusual localization of developmental stages of Cryptosporidium parvum Tyzzer, 1912 in the cells of small intestine of a gnotobiotic piglet. Folia Parasitol. 1989, 36, 219–222. [Google Scholar]
- Valentini, E.; Cherchi, S.; Possenti, A.; Dubremetz, J.F.; Pozio, E.; Spano, F. Molecular characterisation of a Cryptosporidium parvum rhoptry protein candidate related to the rhoptry neck proteins TgRON1 of Toxoplasma gondii and PfASP of Plasmodium falciparum. Mol. Biochem. Parasitol. 2012, 183, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mirdha, B.R.; Srinivasan, A.; Rukmangadachar, L.A.; Singh, S.; Sharma, P.; Gururao, H.; Luthra, K. Identification of invasion proteins of Cryptosporidium parvum. World J. Microbiol. Biotechnol. 2015, 31, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Guérin, A.; Roy, N.H.; Kugler, E.M.; Berry, L.; Burkhardt, J.K.; Shin, J.-B.; Striepen, B. Cryptosporidium rhoptry effector protein ROP1 injected during invasion targets the host cytoskeletal modulator LMO7. Cell Host Microbe 2021, 29, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.V.; Svezhova, N.V.; Sidorenko, N.V.; Khokhlov, S.E. Cryptosporidium parvum (Coccidia, Apicomplexa): Some new ultrastructural observations on its endogenous development. Eur. J. Protistol. 2000, 36, 151–159. [Google Scholar] [CrossRef]
- Forney, J.R.; DeWald, D.B.; Yang, S.G.; Speer, C.A.; Healey, M.C. A role for host phosphoinositide 3-kinase and cytoskeletal remodeling during Cryptosporidium parvum infection. Infect. Immun. 1999, 67, 844–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landsberg, J.H.; Paperna, I. Ultrastructural study of the coccidian Cryptosporidium sp. from stomachs of juvenile cichlid fish. Dis. Aquat. Organ. 1986, 2, 13–20. [Google Scholar] [CrossRef]
- O’Hara, S.P.; Small, A.J.; Chen, X.M.; LaRusso, N.F. Host cell actin remodeling in response to Cryptosporidium. Subcell. Biochem. 2008, 47, 92–100. [Google Scholar]
- Perkins, M.E.; Riojas, Y.A.; Wu, T.W.; Le Blancq, S.M. CpABC, a Cryptosporidium parvum ATP-binding cassette protein at the host-parasite boundary in intracellular stages. Proc. Natl. Acad. Sci. USA 1999, 96, 5734–5739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzipori, S.; Griffiths, J.K. Natural history and biology of Cryptosporidium parvum. Adv. Parasitol. 1998, 40, 5–36. [Google Scholar] [CrossRef]
- Dyková, I.; Lom, J. Fish coccidia: Critical notes on life cycles, classification and pathogenicity. J. Fish. Dis. 1981, 4, 487–505. [Google Scholar] [CrossRef]
- Lukes, J. Life cycle of Goussia pannonica (Molnar, 1989) (Apicomplexa, Eimeriorina), an extracytoplasmic coccidium from the white bream Blicca bjoerkna. J. Protozool. 1992, 39, 484–494. [Google Scholar] [CrossRef]
- Molnar, K.; Baska, F. Light and electron microscopic studies on Epieimeria anguillae (Léger & Hollande, 1922), a coccidium parasitizing the European eel, Anguilla anguilla L. J. Fish Dis. 1986, 9, 99–110. [Google Scholar]
- Valigurová, A.; Florent, I. Nutrient acquisition and attachment strategies in basal lineages: A tough nut to crack in the evolutionary puzzle of Apicomplexa. Microorganisms 2021, 9, 1430. [Google Scholar] [CrossRef]
- Bartošová-Sojková, P.; Oppenheim, R.D.; Soldati-Favre, D.; Lukeš, J. Epicellular apicomplexans: Parasites “on the way in”. PLoS Pathog. 2015, 11, e1005080. [Google Scholar] [CrossRef] [Green Version]
- Lovy, J.; Friend, S.E. Intestinal coccidiosis of anadromous and landlocked alewives, Alosa pseudoharengus, caused by Goussia ameliae n. sp. and G. alosii n. sp. (Apicomplexa: Eimeriidae). Int. J. Parasitol. Parasites Wildl. 2015, 4, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Carruthers, V.B.; Tomley, F.M. Microneme proteins in apicomplexans. Subcell. Biochem. 2008, 47, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Ben Chaabene, R.; Lentini, G.; Soldati-Favre, D. Biogenesis and discharge of the rhoptries: Key organelles for entry and hijack of host cells by the Apicomplexa. Mol. Microbiol. 2021, 115, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Dogga, S.K.; Bartošová-Sojková, P.; Lukeš, J.; Soldati-Favre, D. Phylogeny, Morphology, and metabolic and invasive capabilities of epicellular fish coccidium Goussia janae. Protist 2015, 166, 659–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnin, A.; Dubremetz, J.F.; Camerlynck, P. Characterization of microneme antigens of Cryptosporidium parvum (Protozoa, Apicomplexa). Infect. Immun. 1991, 59, 1703–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melicherová, J.; Ilgová, J.; Kváč, M.; Sak, B.; Koudela, B.; Valigurová, A. Life cycle of Cryptosporidium muris in two rodents with different responses to parasitization. Parasitology 2014, 141, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.Q.; Chen, X.M.; LaRusso, N.F. Cryptosporidium parvum attachment to and internalization by human biliary epithelia in vitro: A morphologic study. J. Parasitol. 2004, 90, 212–221. [Google Scholar] [CrossRef]
- Mele, R.; Morales, M.A.G.; Tosini, F.; Pozio, E. Cryptosporidium parvum at different developmental stages modulates host cell apoptosis in vitro. Infect. Immun. 2004, 72, 6061–6067. [Google Scholar] [CrossRef] [Green Version]
- McCole, D.F.; Eckmann, L.; Laurent, F.; Kagnoff, M.F. Intestinal epithelial cell apoptosis following Cryptosporidium parvum infection. Infect. Immun. 2000, 68, 1710–1713. [Google Scholar] [CrossRef] [Green Version]
- Sasahara, T.; Maruyama, H.; Aoki, M.; Kikuno, R.; Sekiguchi, T.; Takahashi, A.; Satoh, Y.; Kitasato, H.; Takayama, Y.; Inoue, M. Apoptosis of intestinal crypt epithelium after Cryptosporidium parvum infection. J. Infect. Chemother. 2003, 9, 278–281. [Google Scholar] [CrossRef]
- Widmer, G.; Yang, Y.L.; Bonilla, R.; Tanriverdi, S.; Ciociola, K.M. Preferential infection of dividing cells by Cryptosporidium parvum. Parasitology 2006, 133, 131–138. [Google Scholar] [CrossRef]
- Ojcius, D.M.; Perfettini, J.L.; Bonnin, A.; Laurent, F. Caspase-dependent apoptosis during infection with Cryptosporidium parvum. Microbes Infect. 1999, 1, 1163–1168. [Google Scholar] [CrossRef]
- Chen, X.M.; Levine, S.A.; Tietz, P.; Krueger, E.; McNiven, M.A.; Jefferson, D.M.; Mahle, M.; LaRusso, N.F. Cryptosporidium parvum is cytopathic for cultured human biliary epithelia via an apoptotic mechanism. Hepatology 1998, 28, 906–913. [Google Scholar] [CrossRef]
- Widmer, G.; Corey, E.A.; Stein, B.; Griffiths, J.K.; Tzipori, S. Host cell apoptosis impairs Cryptosporidium parvum development in vitro. J. Parasitol. 2000, 86, 922–928. [Google Scholar] [CrossRef]
- Chen, X.M.; Gores, G.J.; Paya, C.V.; LaRusso, N.F. Cryptosporidium parvum induces apoptosis in biliary epithelia by a Fas/Fas ligand-dependent mechanism. Am. J. Physiol. 1999, 277, G599–G608. [Google Scholar] [CrossRef]
- Crawford, C.K.; Kol, A. The mucosal innate immune response to Cryptosporidium parvum, a global one health issue. Front. Cell. Infect. Microbiol. 2021, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Petry, F.; Jakobi, V.; Tessema, T.S. Host immune response to Cryptosporidium parvum infection. Exp. Parasitol. 2010, 126, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Quach, J.; Chadee, K.; Mead, J.R.; Singer, S.M. Immunity to intestinal protozoa: Entamoeba, Cryptosporidium, and Giardia. In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Academic Press: Oxford, UK, 2016; pp. 133–141. [Google Scholar]
- Barakat, F.M.; McDonald, V.; Di Santo, J.P.; Korbel, D.S. Roles for NK cells and an NK cell-independent source of intestinal gamma interferon for innate immunity to Cryptosporidium parvum infection. Infect. Immun. 2009, 77, 5044–5049. [Google Scholar] [CrossRef] [Green Version]
- Choudhry, N.; Korbel, D.S.; Edwards, L.A.; Bajaj-Elliott, M.; McDonald, V. Dysregulation of interferon-γ-mediated signalling pathway in intestinal epithelial cells by Cryptosporidium parvum infection. Cell. Microbiol. 2009, 11, 1354–1364. [Google Scholar] [CrossRef]
- Zaalouk, T.K.; Bajaj-Elliott, M.; George, J.T.; McDonald, V. Differential regulation of defensin gene expression during Cryptosporidium parvum infection. Infect. Immun. 2004, 72, 2772–2779. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gong, A.-Y.; Ma, S.; Chen, X.; Li, Y.; Su, C.-J.; Norall, D.; Chen, J.; Strauss-Soukup, J.K.; Chen, X.-M. Delivery of parasite RNA transcripts into infected epithelial cells during Cryptosporidium infection and its potential impact on host gene transcription. J. Infect. Dis. 2016, 215, 636–643. [Google Scholar] [CrossRef] [Green Version]
- Ashigbie, P.G.; Shepherd, S.; Steiner, K.L.; Amadi, B.; Aziz, N.; Manjunatha, U.H.; Spector, J.M.; Diagana, T.T.; Kelly, P. Use-case scenarios for an anti-Cryptosporidium therapeutic. PLoS Negl. Trop. Dis. 2021, 15, e0009057. [Google Scholar] [CrossRef]
- Alvarez-Pellitero, P.; Quiroga, M.I.; Sitjà-Bobadilla, A.; Redondo, M.J.; Palenzuela, O.; Padrós, F.; Vázquez, S.; Nieto, J.M. Cryptosporidium scophthalmi n. sp. (Apicomplexa: Cryptosporidiidae) from cultured turbot Scophthalmus maximus. Light and electron microscope description and histopathological study. Dis. Aquat. Organ. 2004, 62, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Pellitero, P.; Sitja-Bobadilla, A. Cryptosporidium molnari n. sp. (Apicomplexa: Cryptosporidiidae) infecting two marine fish species, Sparus aurata L. and Dicentrarchus labrax L. Int. J. Parasitol. 2002, 32, 1007–1021. [Google Scholar] [CrossRef]
- Liebler, E.M.; Pohlenz, J.F.; Woodmansee, D.B. Experimental intrauterine infection of adult BALB/c mice with Cryptosporidium sp. Infect. Immun. 1986, 54, 255–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, G.A.; Kreitner, G.L.; Strafuss, A.C. Cryptosporidiosis in three pigs. J. Am. Vet. Med. Assoc. 1977, 170, 348–350. [Google Scholar]
- Dillon, A.; Lo, D.D. M Cells: Intelligent engineering of mucosal immune surveillance. Front. Immunol. 2019, 10, 1499. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Z.; Zhang, Z.; Pan, L.; Zhang, Y. Roles of M cells in infection and mucosal vaccines. Hum. Vaccin Immunother. 2014, 10, 3544–3551. [Google Scholar] [CrossRef] [Green Version]
- De Sablet, T.; Potiron, L.; Marquis, M.; Bussière, F.I.; Lacroix-Lamandé, S.; Laurent, F. Cryptosporidium parvum increases intestinal permeability through interaction with epithelial cells and IL-1β and TNFα released by inflammatory monocytes. Cell. Microbiol. 2016, 18, 1871–1880. [Google Scholar] [CrossRef] [Green Version]
- Allain, T.; Amat, C.B.; Motta, J.-P.; Manko, A.; Buret, A.G. Interactions of Giardia sp. with the intestinal barrier: Epithelium, mucus, and microbiota. Tissue Barriers 2017, 5, e1274354. [Google Scholar] [CrossRef] [Green Version]
- Koh, W.H.; Geurden, T.; Paget, T.; O’Handley, R.; Steuart, R.F.; Thompson, R.C.; Buret, A.G. Giardia duodenalis assemblage-specific induction of apoptosis and tight junction disruption in human intestinal epithelial cells: Effects of mixed infections. J. Parasitol. 2013, 99, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Sánchez, J.; Liñan, R.F.; del Rosario Salinas-Tobón, M.; Ortega-Pierres, G. Giardia duodenalis: Adhesion-deficient clones have reduced ability to establish infection in Mongolian gerbils. Exp. Parasitol. 2008, 119, 364–372. [Google Scholar] [CrossRef]
- Sousa, M.C.; Gonçalves, C.A.; Bairos, V.A.; Poiares-Da-Silva, J. Adherence of Giardia lamblia trophozoites to Int-407 human intestinal cells. Clin. Diagn. Lab. Immunol. 2001, 8, 258–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandborg, L.L.; Tankersley, C.B.; Gottieb, S.; Barancik, M.; Sartor, V.E. Histological demonstration of mucosal invasion by Giardia lamblia in man. Gastroenterology 1967, 52, 143–150. [Google Scholar] [CrossRef]
- Reynoso-Robles, R.; Ponce-Macotela, M.; Rosas-López, L.E.; Ramos-Morales, A.; Martínez–Gordillo, M.N.; González-Maciel, A. The invasive potential of Giardia intestinalis in an in vivo model. Sci. Rep. 2015, 5, 15168. [Google Scholar] [CrossRef]
- Saha, T.K.; Ghosh, T.K. Invasion of small intestinal mucosa by Giardia lamblia in man. Gastroenterology 1977, 72, 402–405. [Google Scholar] [CrossRef]
- Owen, R.L.; Nemanic, P.C.; Stevens, D.P. Ultrastructural observations on giardiasis in a murine model. I. Intestinal distribution, attachment, and relationship to the immune system of Giardia muris. Gastroenterology 1979, 76, 757–769. [Google Scholar] [CrossRef]
- Bannister, L.H. The interactions of intracellular Protista and their host cells, with special reference to heterotrophic organisms. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1979, 204, 141–163. [Google Scholar] [CrossRef]
- Mathur, V.; Kolísko, M.; Hehenberger, E.; Irwin, N.A.T.; Leander, B.S.; Kristmundsson, A.; Freeman, M.A.; Keeling, P.J. Multiple independent origins of apicomplexan-like parasites. Curr. Biol. 2019, 29, 2936–2941.e2935. [Google Scholar] [CrossRef] [PubMed]
- Janouskovec, J.; Paskerova, G.G.; Miroliubova, T.S.; Mikhailov, K.V.; Birley, T.; Aleoshin, V.V.; Simdyanov, T.G. Apicomplexan-like parasites are polyphyletic and widely but selectively dependent on cryptic plastid organelles. eLife 2019, 8, e49662. [Google Scholar] [CrossRef]
- Benajiba, M.H.; Marques, A.; Lom, J.; Bouix, G. Ultrastructure and sporogony of Eimeria (syn. Epieimeria) anguillae (Apicomplexa) in the Eel (Anguilla anguilla). J. Eukaryot. Microbiol. 1994, 41, 215–222. [Google Scholar] [CrossRef]
- Eli, A.; Briyai, O.F.; Abowei, J.F.N. A review of some parasite diseases of African fish gut lumen Protozoa, coccidioses, Cryptosporidium infections, Haemoprotozoa, Haemosporidia. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 1438–1447. [Google Scholar]
- Chaves, M.M.; Lee, S.H.; Kamenyeva, O.; Ghosh, K.; Peters, N.C.; Sacks, D. The role of dermis resident macrophages and their interaction with neutrophils in the early establishment of Leishmania major infection transmitted by sand fly bite. PLoS Pathog. 2020, 16, e1008674. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.C.; Egen, J.G.; Secundino, N.; Debrabant, A.; Kimblin, N.; Kamhawi, S.; Lawyer, P.; Fay, M.P.; Germain, R.N.; Sacks, D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 2008, 321, 970–974. [Google Scholar] [CrossRef] [Green Version]
- Regli, I.B.; Passelli, K.; Hurrell, B.P.; Tacchini-Cottier, F. Survival Mechanisms Used by Some Leishmania Species to Escape Neutrophil Killing. Front. Immunol. 2017, 8, 1558. [Google Scholar] [CrossRef] [Green Version]
- Gueirard, P.; Laplante, A.; Rondeau, C.; Milon, G.; Desjardins, M. Trafficking of Leishmania donovani promastigotes in non-lytic compartments in neutrophils enables the subsequent transfer of parasites to macrophages. Cell. Microbiol. 2008, 10, 100–111. [Google Scholar] [CrossRef]
- Cecílio, P.; Pérez-Cabezas, B.; Santarém, N.; Maciel, J.; Rodrigues, V.; Cordeiro da Silva, A. Deception and manipulation: The arms of Leishmania, a successful parasite. Front. Immunol. 2014, 5, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Real, F.; Florentino, P.T.; Reis, L.C.; Ramos-Sanchez, E.M.; Veras, P.S.; Goto, H.; Mortara, R.A. Cell-to-cell transfer of Leishmania amazonensis amastigotes is mediated by immunomodulatory LAMP-rich parasitophorous extrusions. Cell. Microbiol. 2014, 16, 1549–1564. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.A.; Nandan, D.; Kass, J.; Reiner, N.E. Countervailing, time-dependent effects on host autophagy promote intracellular survival of Leishmania. J. Biol. Chem. 2018, 293, 2617–2630. [Google Scholar] [CrossRef] [Green Version]
- Getti, G.T.; Cheke, R.A.; Humber, D.P. Induction of apoptosis in host cells: A survival mechanism for Leishmania parasites? Parasitology 2008, 135, 1391–1399. [Google Scholar] [CrossRef] [Green Version]
- Salei, N.; Hellberg, L.; Köhl, J.; Laskay, T. Enhanced survival of Leishmania major in neutrophil granulocytes in the presence of apoptotic cells. PLoS ONE 2017, 12, e0171850. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Fernandez, T.; Volpedo, G.; Verma, C.; Satoskar, A.R. Understanding the immune responses involved in mediating protection or immunopathology during leishmaniasis. Biochem. Soc. Trans. 2021, 49, 297–311. [Google Scholar] [CrossRef]
- Sacks, D.L. The null hypothesis of IFN-γ and monocyte function in leishmaniasis. Cell Host Microbe 2020, 27, 683–684. [Google Scholar] [CrossRef]
- Carneiro, M.B.; Lopes, M.E.; Hohman, L.S.; Romano, A.; David, B.A.; Kratofil, R.; Kubes, P.; Workentine, M.L.; Campos, A.C.; Vieira, L.Q.; et al. Th1-Th2 cross-regulation controls early Leishmania infection in the skin by modulating the size of the permissive monocytic host cell reservoir. Cell Host Microbe 2020, 27, 752–768. [Google Scholar] [CrossRef] [PubMed]
- Laskay, T.; van Zandbergen, G.; Solbach, W. Neutrophil granulocytes as host cells and transport vehicles for intracellular pathogens: Apoptosis as infection-promoting factor. Immunobiology 2008, 213, 183–191. [Google Scholar] [CrossRef]
- van Zandbergen, G.; Klinger, M.; Mueller, A.; Dannenberg, S.; Gebert, A.; Solbach, W.; Laskay, T. Cutting Edge: Neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J. Immunol. 2004, 173, 6521–6525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritter, U.; Frischknecht, F.; van Zandbergen, G. Are neutrophils important host cells for Leishmania parasites? Trends Parasitol. 2009, 25, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, J.L.M.; Pinto da Silva, L.H.; Deolindo, P.; Soong, L.; Borges, V.M.; Prates, D.B.; de Souza, A.P.A.; Barral, A.; Balanco, J.M.d.F.; do Nascimento, M.T.C.; et al. Cooperation between apoptotic and viable metacyclics enhances the pathogenesis of Leishmaniasis. PLoS ONE 2009, 4, e5733. [Google Scholar] [CrossRef] [Green Version]
- Van Zandbergen, G.; Bollinger, A.; Wenzel, A.; Kamhawi, S.; Voll, R.; Klinger, M.; Müller, A.; Hölscher, C.; Herrmann, M.; Sacks, D.; et al. Leishmania disease development depends on the presence of apoptotic promastigotes in the virulent inoculum. Proc. Natl. Acad. Sci. USA 2006, 103, 13837–13842. [Google Scholar] [CrossRef] [Green Version]
- El-Hani, C.; Borges, V.; Wanderley, J.L.; Barcinski, M. Apoptosis and apoptotic mimicry in Leishmania: An evolutionary perspective. Front. Cell. Infect. Microbiol. 2012, 2, 96. [Google Scholar] [CrossRef] [Green Version]
- Wanderley, J.L.M.; DaMatta, R.A.; Barcinski, M.A. Apoptotic mimicry as a strategy for the establishment of parasitic infections: Parasite- and host-derived phosphatidylserine as key molecule. Cell Commun. Signal. 2020, 18, 10. [Google Scholar] [CrossRef]
- Terrazas, C.; Oghumu, S.; Jha, B.K.; Natarajan, G.; Drew, M.; Denkers, E.Y.; Satoskar, A.R.; McGwire, B.S. Subverting immunity from the inside: Strategies of intracellular survival–protozoans. In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Academic Press: Oxford, UK, 2016; pp. 83–93. [Google Scholar]
- Ueno, N.; Wilson, M.E. Receptor-mediated phagocytosis of Leishmania: Implications for intracellular survival. Trends Parasitol. 2012, 28, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, M.B.; Peters, N.C. The paradox of a phagosomal lifestyle: How innate host cell-Leishmania amazonensis interactions lead to a progressive chronic disease. Front. Immunol. 2021, 12, 3468. [Google Scholar] [CrossRef] [PubMed]
- Courret, N.; Fréhel, C.; Gouhier, N.; Pouchelet, M.; Prina, E.; Roux, P.; Antoine, J.C. Biogenesis of Leishmania-harbouring parasitophorous vacuoles following phagocytosis of the metacyclic promastigote or amastigote stages of the parasites. J. Cell Sci. 2002, 115, 2303–2316. [Google Scholar] [CrossRef]
- Lerm, M.; Holm, Å.; Seiron, Å.; Särndahl, E.; Magnusson, K.-E.; Rasmusson, B. Leishmania donovani requires functional Cdc42 and Rac1 to prevent phagosomal maturation. Infect. Immun. 2006, 74, 2613–2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winberg, M.E.; Rasmusson, B.; Sundqvist, T. Leishmania donovani: Inhibition of phagosomal maturation is rescued by nitric oxide in macrophages. Exp. Parasitol. 2007, 117, 165–170. [Google Scholar] [CrossRef]
- Holm, Å.; Tejle, K.; Magnusson, K.-E.; Descoteaux, A.; Rasmusson, B. Leishmania donovani lipophosphoglycan causes periphagosomal actin accumulation: Correlation with impaired translocation of PKCalpha and defective phagosome maturation. Cell. Microbiol. 2001, 3, 439–447. [Google Scholar] [CrossRef]
- Kumar, G.A.; Karmakar, J.; Mandal, C.; Chattopadhyay, A. Leishmania donovani internalizes into host cells via caveolin-mediated endocytosis. Sci. Rep. 2019, 9, 12636. [Google Scholar] [CrossRef] [Green Version]
- Matte, C.; Casgrain, P.-A.; Séguin, O.; Moradin, N.; Hong, W.J.; Descoteaux, A. Leishmania major promastigotes evade LC3-associated phagocytosis through the action of GP63. PLoS Pathog. 2016, 12, e1005690. [Google Scholar] [CrossRef]
- Paixão, A.R.; Dias, B.R.S.; Palma, L.C.; Tavares, N.M.; Brodskyn, C.I.; de Menezes, J.P.B.; Veras, P.S.T. Investigating the phagocytosis of Leishmania using confocal microscopy. J. Vis. Exp. 2021, 173, e62459. [Google Scholar] [CrossRef]
- Azevedo, E.; Oliveira, L.T.; Castro Lima, A.K.; Terra, R.; Dutra, P.M.L.; Salerno, V.P. Interactions between Leishmania braziliensis and macrophages are dependent on the cytoskeleton and myosin Va. J. Parasitol. Res. 2012, 2012, 275436. [Google Scholar] [CrossRef] [Green Version]
- Young, J.; Kima, P.E. The Leishmania parasitophorous vacuole membrane at the parasite-host interface. Yale. J. Biol. Med. 2019, 92, 511–521. [Google Scholar]
- Canton, J.; Ndjamen, B.; Hatsuzawa, K.; Kima, P.E. Disruption of the fusion of Leishmania parasitophorous vacuoles with ER vesicles results in the control of the infection. Cell. Microbiol. 2012, 14, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Antoine, J.C.; Lang, T.; Prina, E.; Courret, N.; Hellio, R. H-2M molecules, like MHC class II molecules, are targeted to parasitophorous vacuoles of Leishmania-infected macrophages and internalized by amastigotes of L. amazonensis and L. mexicana. J. Cell Sci. 1999, 112 Pt 15, 2559–2570. [Google Scholar] [CrossRef]
- Ndjamen, B.; Kang, B.-H.; Hatsuzawa, K.; Kima, P.E. Leishmania parasitophorous vacuoles interact continuously with the host cell’s endoplasmic reticulum; parasitophorous vacuoles are hybrid compartments. Cell. Microbiol. 2010, 12, 1480–1494. [Google Scholar] [CrossRef] [Green Version]
- Antoine, J.C.; Prina, E.; Lang, T.; Courret, N. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 1998, 6, 392–401. [Google Scholar] [CrossRef]
- Okuda, K.; Tong, M.; Dempsey, B.; Moore, K.J.; Gazzinelli, R.T.; Silverman, N. Leishmania amazonensis engages CD36 to drive parasitophorous vacuole maturation. PLoS Pathog. 2016, 12, e1005669. [Google Scholar] [CrossRef] [Green Version]
- Batista, M.F.; Nájera, C.A.; Meneghelli, I.; Bahia, D. The parasitic intracellular lifestyle of trypanosomatids: Parasitophorous vacuole development and survival. Front. Cell Dev. Biol. 2020, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, M.; Soto, M.; Iborra, S.; Sancho, D. Leishmania hijacks myeloid cells for immune escape. Front. Microbiol. 2018, 9, 883. [Google Scholar] [CrossRef] [Green Version]
- Matte, C.; Arango Duque, G.; Descoteaux, A. Leishmania donovani metacyclic promastigotes impair phagosome properties in inflammatory monocytes. Infect. Immun. 2021, 89, e0000921. [Google Scholar] [CrossRef]
- da Silva, M.F.L.; Zampieri, R.A.; Muxel, S.M.; Beverley, S.M.; Floeter-Winter, L.M. Leishmania amazonensis arginase compartmentalization in the glycosome is important for parasite infectivity. PLoS ONE 2012, 7, e34022. [Google Scholar] [CrossRef] [Green Version]
- Boitz, J.M.; Gilroy, C.A.; Olenyik, T.D.; Paradis, D.; Perdeh, J.; Dearman, K.; Davis, M.J.; Yates, P.A.; Li, Y.; Riscoe, M.K.; et al. Arginase is essential for survival of Leishmania donovani promastigotes but not intracellular amastigotes. Infect. Immun. 2017, 85, e00554-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bichiou, H.; Bouabid, C.; Rabhi, I.; Guizani-Tabbane, L. Transcription factors interplay orchestrates the immune-metabolic response of Leishmania infected macrophages. Front. Cell. Infect. Microbiol. 2021, 11, 660415. [Google Scholar] [CrossRef]
- Torrecilhas, A.C.; Soares, R.P.; Schenkman, S.; Fernández-Prada, C.; Olivier, M. Extracellular vesicles in trypanosomatids: Host cell communication. Front. Cell. Infect. Microbiol. 2020, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Liévin-Le Moal, V.; Loiseau, P.M. Leishmania hijacking of the macrophage intracellular compartments. FEBS J. 2016, 283, 598–607. [Google Scholar] [CrossRef]
- Wanderley, J.L.; Moreira, M.E.; Benjamin, A.; Bonomo, A.C.; Barcinski, M.A. Mimicry of apoptotic cells by exposing phosphatidylserine participates in the establishment of amastigotes of Leishmania (L) amazonensis in mammalian hosts. J. Immunol. 2006, 176, 1834–1839. [Google Scholar] [CrossRef] [Green Version]
- Wanderley, J.L.M.; Deolindo, P.; Carlsen, E.; Portugal, A.B.; DaMatta, R.A.; Barcinski, M.A.; Soong, L. CD4+ T cell-dependent macrophage activation modulates sustained PS exposure on intracellular amastigotes of Leishmania amazonensis. Front. Cell. Infect. Microbiol. 2019, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Rittig, M.G.; Bogdan, C. Leishmania-host-cell interaction: Complexities and alternative views. Parasitol. Today 2000, 16, 292–297. [Google Scholar] [CrossRef]
- Bidri, M.; Vouldoukis, I.; Mossalayi, M.D.; Debré, P.; Guillosson, J.J.; Mazier, D.; Arock, M. Evidence for direct interaction between mast cells and Leishmania parasites. Parasite Immunol. 1997, 19, 475–483. [Google Scholar] [CrossRef]
- Naqvi, N.; Srivastava, R.; Selvapandiyan, A.; Puri, N. Host mast cells in leishmaniasis: Friend or foe? Trends Parasitol. 2020, 36, 952–956. [Google Scholar] [CrossRef]
- Rodríguez, N.E.; Wilson, M.E. Eosinophils and mast cells in leishmaniasis. Immunol. Res. 2014, 59, 129–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurrell, B.P.; Beaumann, M.; Heyde, S.; Regli, I.B.; Müller, A.J.; Tacchini-Cottier, F. Frontline Science: Leishmania mexicana amastigotes can replicate within neutrophils. J. Leukoc. Biol. 2017, 102, 1187–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passelli, K.; Billion, O.; Tacchini-Cottier, F. The impact of neutrophil recruitment to the skin on the pathology induced by Leishmania infection. Front. Immunol. 2021, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, M.; Descoteaux, A. Phagocytosis of Leishmania: Interaction with the host and intracellular trafficking. In Advances in Cellular and Molecular Biology of Membranes and Organelles; Gordon, S., Ed.; JAI Elsevier: Amsterdam, The Netherlands, 1999; Volume 6, pp. 297–316. [Google Scholar]
- Cavalcante-Costa, V.S.; Costa-Reginaldo, M.; Queiroz-Oliveira, T.; Oliveira, A.C.S.; Couto, N.F.; Dos Anjos, D.O.; Lima-Santos, J.; Andrade, L.O.; Horta, M.F.; Castro-Gomes, T. Leishmania amazonensis hijacks host cell lysosomes involved in plasma membrane repair to induce invasion in fibroblasts. J. Cell Sci. 2019, 132, jcs226183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M. Classical labeling of bacterial pathogens according to their lifestyle in the host: Inconsistencies and alternatives. Front. Microbiol. 2012, 3, 71. [Google Scholar] [CrossRef] [Green Version]
- Pacakova, L.; Harant, K.; Volf, P.; Lestinova, T. Three types of Leishmania mexicana amastigotes: Proteome comparison by quantitative proteomic analysis. 2021, unpublished work. [Google Scholar]
- Holzer, T.R.; McMaster, W.R.; Forney, J.D. Expression profiling by whole-genome interspecies microarray hybridization reveals differential gene expression in procyclic promastigotes, lesion-derived amastigotes, and axenic amastigotes in Leishmania mexicana. Mol. Biochem. Parasitol. 2006, 146, 198–218. [Google Scholar] [CrossRef] [PubMed]




| Adaptation/Evasion | Cryptosporidium | Leishmania |
|---|---|---|
| Preferred cells for parasite development/multiplication | Microvillous surface of the epithelial cells (mostly gastrointestinal tract) | Monocytes/macrophages |
| Parasite localization in respect to the host cell | Epicellular (Above plasma membrane) | Intracellular (Below plasma membrane) |
| Parasite compartment | Parasitophorous sac | Parasitophorous vacuole |
| Parasite invasive apparatus | present | absent |
| Modulation of host signalling pathways | yes | yes |
| Modulation of host cell actin | yes | yes |
| Modulation of host cell apoptosis | yes | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolářová, I.; Valigurová, A. Hide-and-Seek: A Game Played between Parasitic Protists and Their Hosts. Microorganisms 2021, 9, 2434. https://doi.org/10.3390/microorganisms9122434
Kolářová I, Valigurová A. Hide-and-Seek: A Game Played between Parasitic Protists and Their Hosts. Microorganisms. 2021; 9(12):2434. https://doi.org/10.3390/microorganisms9122434
Chicago/Turabian StyleKolářová, Iva, and Andrea Valigurová. 2021. "Hide-and-Seek: A Game Played between Parasitic Protists and Their Hosts" Microorganisms 9, no. 12: 2434. https://doi.org/10.3390/microorganisms9122434
APA StyleKolářová, I., & Valigurová, A. (2021). Hide-and-Seek: A Game Played between Parasitic Protists and Their Hosts. Microorganisms, 9(12), 2434. https://doi.org/10.3390/microorganisms9122434