Effects of Bacteroides-Based Microecologics against Antibiotic-Associated Diarrhea in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture
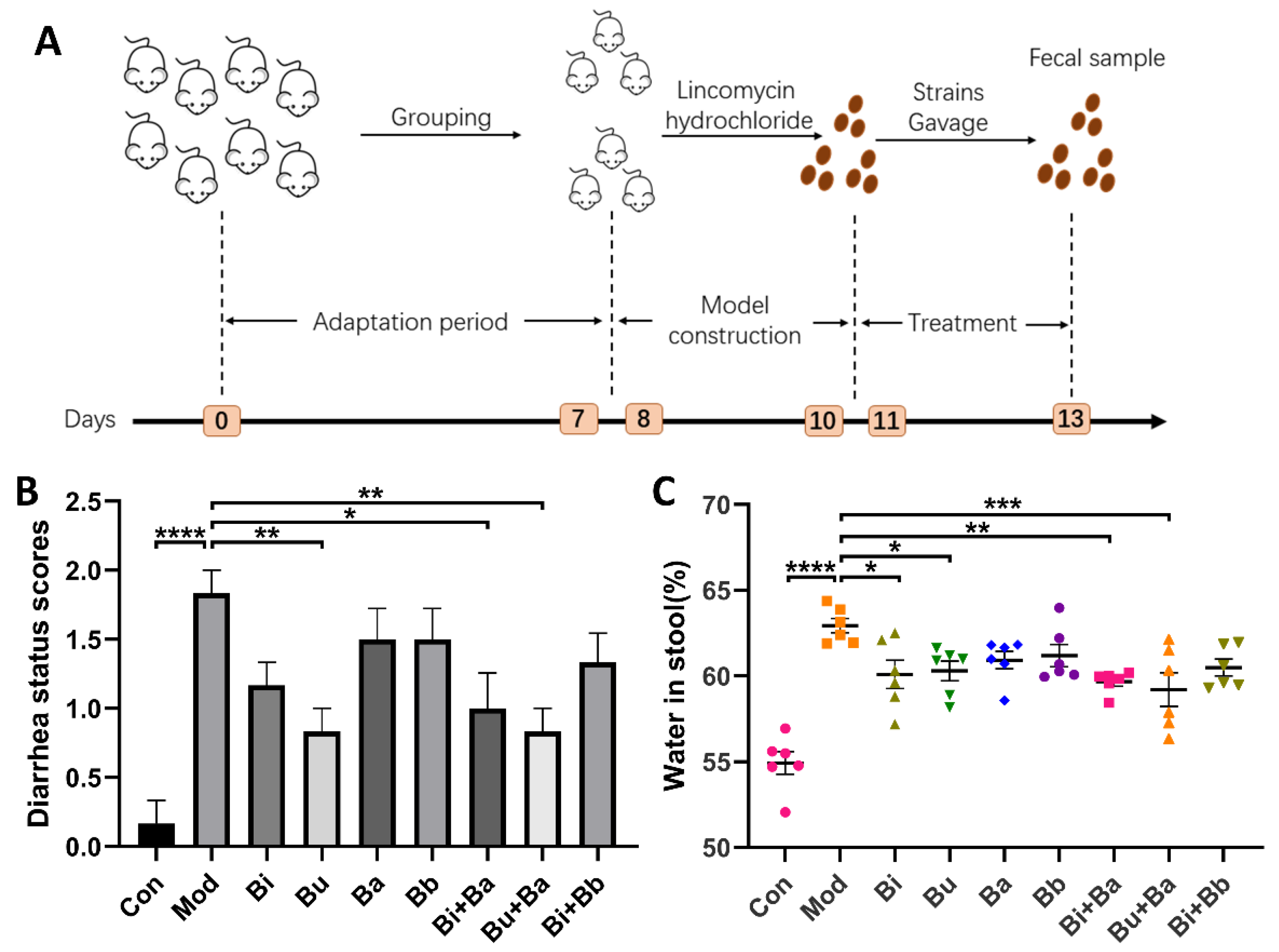
2.2. Animal Experimental Design
2.3. Ethics Statement
2.4. Histological Colon Observations
2.5. Biochemical Analyses of the Colon and Serum
2.6. Analysis of SCFAs
2.7. Real-Time PCR Analysis
2.8. Preparation of Total DNAs and HIGH throughput Sequencing Analysis
2.9. Statistical Analysis
3. Results
3.1. Effects of Different Treatments on Diarrhea Status Scores and Water Content in Feces
3.2. Effect of Different Treatments on Histopathological Structure of Colon Tissues
3.3. Effects of Different Treatments on Proinflammatory Cytokine Expression
3.4. Effects of Different Treatments on Intestinal Barrier Integrity
3.5. Effects of Different Treatments on SCFA Production
3.6. Effect of Different Treatments on the Composition and Diversity of Gut Microbiota
3.7. Spearman’s Correlation Analysis of Physiological and Anti-Inflammatory Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandes, P.; Martens, E. Antibiotics in late clinical development. Biochem. Pharmacol. 2017, 133, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Silverman, M.A.; Konnikova, Y.; Gerber, J.S. Impact of antibiotics on necrotizing enterocolitis and antibiotic-associated diarrhea. Gastroenterol. Clin. N. Am. 2017, 46, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, M. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 385, 117171. [Google Scholar]
- Gillespie, D.; Hood, K.; Bayer, A.; Carter, B.; Duncan, D.; Espinasse, A.; Evans, M.; Nuttall, J.; Stanton, H.; Acharjya, A.; et al. Antibiotic prescribing and associated diarrhoea: A prospective cohort study of care home residents. Age Ageing 2015, 44, 853–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Zhang, L.; Zhang, Y.; Liu, Y.; He, Y.; Guo, L. Combined administration of antibiotics increases the incidence of antibiotic-associated diarrhea in critically ill patients. Infect. Drug Resist. 2019, 12, 1047–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogenauer, C.; Hammer, H.F.; Krejs, G.J.; Reisinger, E.C. Mechanisms and management of antibiotic-associated diarrhea. Clin. Infect. Dis. 1998, 27, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Sammons, J.S.; Toltzis, P.; Zaoutis, T.E. Clostridium difficile infection in children. JAMA Pediatrics 2013, 167, 567–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szajewska, H.; Mrukowicz, J.Z. Probiotics in prevention of antibiotic-associated diarrhea: Meta-analysis. J. Pediatr. 2003, 142, 85. [Google Scholar]
- Lee, W.-J.; Hase, K. Gut microbiota–generated metabolites in animal health and disease. Nat. Chem. Biol. 2014, 10, 416–424. [Google Scholar] [CrossRef]
- Arpaia, N.; Rudensky, A.Y. Microbial metabolites control gut inflammatory responses. Proc. Natl. Acad. Sci. USA 2014, 111, 2058–2059. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.-M.; Xie, J.-J.; Peng, C.-G.; Wang, B.-H.; Wang, K.-C.; Li, L.-J. Enhancing clinical efficacy through the gut microbiota: A new field of traditional Chinese medicine. Engineering 2018, 5, 40–49. [Google Scholar] [CrossRef]
- Antunes, L.C.M.; Han, J.; Ferreira, R.; Lolić, P.; Borchers, C.H.; Finlay, B.B. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob. Agents Chemother. 2011, 55, 1494–1503. [Google Scholar] [CrossRef] [Green Version]
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015, 2, 1–58. [Google Scholar] [CrossRef]
- Lange, K.W.; Nakamura, Y.; Guo, J.; Chen, N. Diet and medical foods in Parkinson’s disease. Food Sci. Hum. Wellness 2019, 8, 83–95. [Google Scholar] [CrossRef]
- Rolfe, R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000, 130, 396S–402S. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gong, W.; Li, Z.; Gao, D.; Gao, Y. Research progress of gut flora in improving human wellness. Food Sci. Hum. Wellness 2019, 8, 102–105. [Google Scholar] [CrossRef]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.V.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [PubMed] [Green Version]
- Xu, H.-B.; Jiang, R.-H.; Sheng, H.-B. Meta-analysis of the effects of Bifidobacterium preparations for the prevention and treatment of pediatric antibiotic-associated diarrhea in China. Complement. Ther. Med. 2017, 33, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Yuan, L.; Li, L.; Xiang, C. Clostridium butyricum combined with bifidobacterium infantis probiotic mixture restores fecal microbiota and attenuates systemic inflammation in mice with antibiotic-associated diarrhea. BioMed Res. Int. 2015, 2015, 582048. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Deng, H.; Zhou, Y.; Tan, Y.; Wang, X.; Han, Y.; Liu, Y.; Wang, Y.; Yang, R.; Bi, Y.; et al. Bioluminescence imaging to track bacteroides fragilis inhibition of vibrio parahaemolyticus infection in mice. Front. Cell. Infect. Microbiol. 2017, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Hudcovic, T.; Kozáková, H.; Kolínská, J.; Štěpánková, R.; Hrncir, T.; Tlaskalová-Hogenová, H. Monocolonization with Bacteroides ovatus protects immunodeficient SCID mice from mortality in chronic intestinal inflammation caused by long-lasting dextran sodium sulfate treatment. Physiol. Res. 2009, 58, 101–110. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, B.; Xu, J.; Liu, Y.; Qiu, E.; Li, Z.; Li, Z.; He, Y.; Zhou, H.; Bai, Y.; et al. Bacteroides fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses. Front. Immunol. 2018, 9, 1040. [Google Scholar] [CrossRef] [Green Version]
- Chng, K.R.; Ghosh, T.; Tan, Y.H.; Nandi, T.; Lee, I.R.; Ng, A.H.Q.; Li, C.; Ravikrishnan, A.; Lim, K.M.; Lye, D.; et al. Metagenome-wide association analysis identifies microbial determinants of post-antibiotic ecological recovery in the gut. Nat. Ecol. Evol. 2020, 4, 1256–1267. [Google Scholar] [CrossRef]
- Masoudi, A.; Raetz, C.R.H.; Zhou, P.; Iv, C.W.P.; Pemble, C.W. Chasing acyl carrier protein through a catalytic cycle of lipid A production. Nature 2013, 505, 422–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, M.; Zhou, R.; Wang, Y.; Zhang, M.; Liu, K.; Ma, C.-C. Beneficial effects of sulfated polysaccharides from the red seaweed Gelidium pacificum Okamura on mice with antibiotic-associated diarrhea. Food Funct. 2020, 11, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gao, Y.; Wang, X.; Hu, G.; Wang, Y.; Feng, B.; Hu, Y.; Mu, X.; Zhang, Y.; Dong, H. Escherichia coli O101-induced diarrhea develops gut microbial dysbiosis in rats. Exp. Ther. Med. 2018, 17, 824–834. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, L.; Xu, Q.; Jiang, T.; Fang, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacteria exert species-specific effects on constipation in BALB/c mice. Food Funct. 2017, 8, 3587–3600. [Google Scholar] [CrossRef]
- Wang, G.; Tang, H.; Zhang, Y.; Xiao, X.; Xia, Y.; Ai, L. The intervention effects of Lactobacillus casei LC2W on Escherichia coli O157:H7 -induced mouse colitis. Food Sci. Hum. Wellness 2020, 9, 289–294. [Google Scholar] [CrossRef]
- Yue, Y.; He, Z.; Zhou, Y.; Ross, R.P.; Stanton, C.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Lactobacillus plantarum relieves diarrhea caused by enterotoxin-producing Escherichia coli through inflammation modulation and gut microbiota regulation. Food Funct. 2020, 11, 10362–10374. [Google Scholar] [CrossRef]
- Lin, J.-N.; Lee, P.-S.; Mei, N.-W.; Cheng, A.-C.; Yu, R.C.; Pan, M.-H. Effects of ginseng dietary supplementation on a high-Fat diet-induced obesity in C57BL/6 Mice. Food Sci. Hum. Wellness 2019, 8, 344–350. [Google Scholar] [CrossRef]
- Sicard, J.-F.; Le Bihan, G.; Vogeleer, P.; Jacques, M.; Harel, J. Interactions of intestinal bacteria with components of the intestinal mucus. Front. Cell. Infect. Microbiol. 2017, 7, 387. [Google Scholar] [CrossRef] [PubMed]
- Tailford, L.E.; Crost, E.H.; Kavanaugh, D.; Juge, N. Mucin glycan foraging in the human gut microbiome. Front. Genet. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, H.J.; Scott, K.P.; Duncan, S.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willemsen, L.E.M.; Koetsier, M.A.; van Deventer, S.J.H.; van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Reyes-Díaz, A.; Mata-Haro, V.; Hernández, J.; González-Córdova, A.F.; Hernández-Mendoza, A.; Reyes-Díaz, R.; Torres-Llanez, M.J.; Beltrán-Barrientos, L.M.; Vallejo-Cordoba, B. Milk fermented by specific lactobacillus strains regulates the serum levels of IL-6, TNF-α and IL-10 cytokines in a LPS-stimulated murine model. Nutrients 2018, 10, 691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Schultheis, P.J.; Clarke, L.L.; Meneton, P.; Miller, M.L.; Soleimani, M.; Gawenis, L.R.; Riddle, T.M.; Duffy, J.J.; Doetschman, T.; Wang, T.; et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat. Genet. 1998, 19, 282–285. [Google Scholar] [CrossRef]
- Urdaci, M.C.; Lefevre, M.; Lafforgue, G.; Cartier, C.; Rodriguez, B.; Fioramonti, J. Antidiarrheal action of bacillus subtilis CU1 CNCM I-2745 and lactobacillus plantarum CNCM I-4547 in mice. Front. Microbiol. 2018, 9, 1537. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Huang, Y.; Zhou, Y.; Buckley, T.; Wang, H.H. Antibiotic administration routes significantly influence the levels of antibiotic resistance in gut microbiota. Antimicrob. Agents Chemother. 2013, 57, 3659–3666. [Google Scholar] [CrossRef] [Green Version]
- Mekonnen, S.A.; Merenstein, D.; Fraser, C.M.; Marco, M.L. Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr. Opin. Biotechnol. 2020, 61, 226–234. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.-H.; Park, J.H.; Jeon, W.-M.; Han, K.-S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr. Res. Pr. 2015, 9, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wu, J.; Li, J.; Zhou, N.-Y.; Tang, H.; Wang, Y. Gut microbiota composition modifies fecal metabolic profiles in mice. J. Proteome Res. 2013, 12, 2987–2999. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Xu, J.; Leip, D.D.; Chen, C.-H.; Westover, B.P.; Weatherford, J.; Buhler, J.D.; Gordon, J.I. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 2005, 307, 1955–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arike, L.; Hansson, G.C. The densely o-glycosylated MUC2 mucin protects the intestine and provides food for the commensal bacteria. J. Mol. Biol. 2016, 428, 3221–3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinolo, M.A.; Rodrigues, H.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Chen, L.; Gao, K.; Shao, Z.; Huo, X.; Hua, M.; Liu, S.; Sun, Y.; Li, S. Effects of Schisandra chinensis polysaccharides on rats with antibiotic-associated diarrhea. Int. J. Biol. Macromol. 2018, 124, 627–634. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Yim, S.; Gwon, S.-Y.; Hwang, S.; Kim, N.H.; Jung, B.D.; Rhee, K.-J. Enterotoxigenic bacteroides fragilis causes lethal colitis in Mongolian gerbils. Anaerobe 2013, 21, 64–66. [Google Scholar] [CrossRef]
- Chen, Y.; Kinouchi, T.; Kataoka, K.; Akimoto, S.; Ohnishi, Y. Purification and characterization of a fibrinogen-degrading protease in bacteroides fragilis strain YCH46. Microbiol. Immunol. 1995, 39, 967–977. [Google Scholar] [CrossRef]
- Brodmann, T.; Endo, A.; Gueimonde, M.; Vinderola, G.; Kneifel, W.; de Vos, W.M.; Salminen, S.; Gómez-Gallego, C. Safety of novel microbes for human consumption: Practical examples of assessment in the European Union. Front. Microbiol. 2017, 8, 1725. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Jiang, P.; Liu, Y.; Duan, M. The specific use of alginate from Laminaria japonica by Bacteroides species determined its modulation of the Bacteroides community. Food Funct. 2019, 10, 4304–4314. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Zheng, H.; Joglekar, P.; Higginbottom, S.K.; Firbank, S.J.; Bolam, D.N.; Sonnenburg, J.L. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell 2010, 141, 1241–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Strain | Origin | Region |
|---|---|---|
| FJSWX61K18 | Human feces | Jiangsu Province, China |
| FGDLZ48B1 | Human feces | Guangdong Province, China |
| FHNFQ48M5 | Human feces | Henan Province, China |
| FGZ30MM3 | Human feces | Guizhou Province, China |
| Groups | Modeling Period (3 Days) | Recovery Period (3 Days) |
|---|---|---|
| Con | PBS | PBS |
| Mod | lincomycin hydrochloride (3 g/kg) twice daily | PBS |
| Bi | lincomycin hydrochloride (3 g/kg) twice daily | 5 × 108 CFUs of B. intestinalis FJSWX61K18 |
| Bu | lincomycin hydrochloride (3 g/kg) twice daily | 5 × 108 CFUs of B. uniformis FGDLZ48B1 |
| Ba | lincomycin hydrochloride (3 g/kg) twice daily | 5 × 108 CFUs of B. adolescentis FHNFQ48M5 |
| Bb | lincomycin hydrochloride (3 g/kg) twice daily | 5 × 108 CFUs of B. bifidum FGZ30MM3 |
| Bi + Ba | lincomycin hydrochloride (3 g/kg) twice daily | 5 × 108 CFUs of B. intestinalis FJSWX61K18 + 5 × 108 CFUs of B. adolescentis FHNFQ48M5 |
| Bu + Ba | lincomycin hydrochloride (3 g/kg) twice daily | 5 × 108 CFUs of B. uniformis FGDLZ48B1 + 5 × 108 CFUs of B. adolescentis FHNFQ48M5 |
| Bi + Bb | lincomycin hydrochloride (3 g/kg) twice daily | 5 × 108 CFUs of B. intestinalis FJSWX61K18 + 5 × 108 CFUs of B. bifidum FGZ30MM3 |
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| Mucin-2 | CAACAAGCTTCACCACAATCTC | CAGACCAAAAGCAGCAAGGTA |
| Occludin | CACACTTGCTTGGGACAGAG | TAGCCATAGCCTCCATAGCC |
| NHE3 | TGGCCGGGCTTTCGACCACA | GGGACCCACGGCGCTCTCCCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Effects of Bacteroides-Based Microecologics against Antibiotic-Associated Diarrhea in Mice. Microorganisms 2021, 9, 2492. https://doi.org/10.3390/microorganisms9122492
Guo H, Yu L, Tian F, Zhao J, Zhang H, Chen W, Zhai Q. Effects of Bacteroides-Based Microecologics against Antibiotic-Associated Diarrhea in Mice. Microorganisms. 2021; 9(12):2492. https://doi.org/10.3390/microorganisms9122492
Chicago/Turabian StyleGuo, Hang, Leilei Yu, Fengwei Tian, Jianxin Zhao, Hao Zhang, Wei Chen, and Qixiao Zhai. 2021. "Effects of Bacteroides-Based Microecologics against Antibiotic-Associated Diarrhea in Mice" Microorganisms 9, no. 12: 2492. https://doi.org/10.3390/microorganisms9122492
APA StyleGuo, H., Yu, L., Tian, F., Zhao, J., Zhang, H., Chen, W., & Zhai, Q. (2021). Effects of Bacteroides-Based Microecologics against Antibiotic-Associated Diarrhea in Mice. Microorganisms, 9(12), 2492. https://doi.org/10.3390/microorganisms9122492






