Factors Influencing the Fungal Diversity on Audio–Visual Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Archives and Audio–Visual Materials Stored Inside
2.2. Sampling
2.3. Extraction of Total Genomic DNA
2.4. DNA Library Preparing and Sequencing
2.5. Data Processing and Multivariate Statistical Analysis
3. Results and Discussion
3.1. Fungal Diversity on Audio–Visual Materials
3.2. Factors Influencing the Structure of Fungal Communities
3.3. Relative Abundance of Fungi on Audio–Visual Materials
3.4. Fungal Relative Abundance in the Air of Archives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bingley, G.; Verran, J. Counts of fungal spores released during inspection of mouldy cinematographic film and determination of the gelatinolytic activity of predominant isolates. Int. Biodeterior. Biodegrad. 2013, 84, 381–387. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, Y.H.; Zhang, F.Y.; Hu, C.T.; Liu, G.L.; Pan, J. Microbial Community Analyses of the Deteriorated Storeroom Objects in the Tianjin Museum Using Culture-Independent and Culture-Dependent Approaches. Front. Microbiol. 2018, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Cappitelli, F.; Sorlini, C. From papyrus to compact disc: The microbial deterioration of documentary heritage. Crit. Rev. Microbiol. 2005, 31, 1–10. [Google Scholar] [CrossRef]
- Sclocchi, M.C.; Krakova, L.; Pinzari, F.; Colaizzi, P.; Bicchieri, M.; Sakova, N.; Pangallo, D. Microbial Life and Death in a Foxing Stain: A Suggested Mechanism of Photographic Prints Defacement. Microb. Ecol. 2017, 73, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Borrego, S.; Guiamet, P.; de Saravia, S.G.; Batistini, P.; Garcia, M.; Lavin, P.; Perdomo, I. The quality of air at archives and the biodeterioration of photographs. Int. Biodeterior. Biodegrad. 2010, 64, 139–145. [Google Scholar] [CrossRef]
- Borrego, S.; Lavin, P.; Perdomo, I.; de Saravia, S.G.; Guiamet, P. Determination of Indoor Air Quality in Archives and Biodeterioration of the Documentary Heritage. ISRN Microbiol. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Ciesielski, S.; Pokoj, T.; Klimiuk, E. Cultivation-Dependent and -Independent Characterization of Microbial Community Producing Polyhydroxyalkanoates from Raw Glycerol. J. Microbiol. Biotechnol. 2010, 20, 853–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepla, B.; Demnerova, K.; Stiborova, H. History and microbial biodeterioration of audiovisual materials. J. Cult. Herit. 2020, 44, 218–228. [Google Scholar] [CrossRef]
- Otlewska, A.; Adamiak, J.; Gutarowska, B. Application of molecular techniques for the assessment of microorganism diversity on cultural heritage objects. Acta Biochim. Pol. 2014, 61, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Bodor, A.; Bounedjoum, N.; Vincze, G.E.; Kis, A.E.; Laczi, K.; Bende, G.; Szilagyi, A.; Kovacs, T.; Perei, K.; Rakhely, G. Challenges of unculturable bacteria: Environmental perspectives. Rev. Environ. Sci. Biotechnol. 2020, 19, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Branysova, T.; Tepla, B.; Demnerova, K.; Stiborova, H.; Durovic, M. Biodeterioration of Audio-Visual Materials. Chem. Listy 2021, 115, 260–265. [Google Scholar]
- Antonelli, F.; Esposito, A.; Galotta, G.; Petriaggi, B.D.; Piazza, S.; Romagnoli, M.; Guerrieri, F. Microbiota in Waterlogged Archaeological Wood: Use of Next-Generation Sequencing to Evaluate the Risk of Biodegradation. Appl. Sci. 2020, 10, 4636. [Google Scholar] [CrossRef]
- Torralba, M.G.; Kuelbs, C.; Moncera, K.J.; Roby, R.; Nelson, K.E. Characterizing Microbial Signatures on Sculptures and Paintings of Similar Provenance. Microb. Ecol. 2020, 81, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.Y.; Chen, X.P.; Huang, J.Z.; Lu, Y.S.; Dong, H.Y.; Wu, Y.H.; Song, S.L.; Yu, J.; Bai, S.; Chen, Z.; et al. Microbial biofilms on a giant monolithic statue of Buddha: The symbiosis of microorganisms and mosses and implications for bioweathering. Int. Biodeterior. Biodegrad. 2021, 156. [Google Scholar] [CrossRef]
- Migliore, L.; Perini, N.; Mercuri, F.; Orlanducci, S.; Rubechini, A.; Thaller, M.C. Three ancient documents solve the jigsaw of the parchment purple spot deterioration and validate the microbial succession model. Sci. Rep. 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szulc, J.; Ruman, T.; Karbowska-Berent, J.; Kozielec, T.; Gutarowska, B. Analyses of microorganisms and metabolites diversity on historic photographs using innovative methods. J. Cult. Herit. 2020, 45, 101–113. [Google Scholar] [CrossRef]
- Kracmarova, M.; Karpiskova, J.; Uhlik, O.; Strejcek, M.; Szakova, J.; Balik, J.; Demnerova, K.; Stiborova, H. Microbial Communities in Soils and Endosphere of Solanum tuberosum L. and their Response to Long-Term Fertilization. Microorganisms 2020, 8, 9. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing in R Foundation for Statistical Computing; R Core Team R: Vienna, Austria, 2017. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- UNITE Community. UNITE General FASTA Release for Fungi 2. Version 18.11.2018. UNITE Community. 2019. Available online: https://unite.ut.ee/repository.php (accessed on 4 September 2020).
- Oksanen, J.; Blanchet, F.G.; Kindlt, R.; Legendre, P.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R-Package Version 2.5-7. 2019. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 26 October 2021).
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Vivar, I.; Borrego, S.; Ellis, G.; Moreno, D.A.; Garcia, A.M. Fungal biodeterioration of color cinematographic films of the cultural heritage of Cuba. Int. Biodeterior. Biodegrad. 2013, 84, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Buckova, M.; Puskarova, A.; Sclocchi, M.C.; Bicchieri, M.; Colaizzi, P.; Pinzari, F.; Pangallo, D. Co-occurrence of bacteria and fungi and spatial partitioning during photographic materials biodeterioration. Polym. Degrad. Stab. 2014, 108, 1–11. [Google Scholar] [CrossRef]
- Mazzoli, R.; Giuffrida, M.G.; Pessione, E. Back to the past: “Find the guilty bug-microorganisms involved in the biodeterioration of archeological and historical artifacts”. Appl. Microbiol. Biotechnol. 2018, 102, 6393–6407. [Google Scholar] [CrossRef]
- Lourenco, M.J.L.; Sampaio, J.P. Microbial deterioration of gelatin emulsion photographs: Differences of susceptibility between black and white and colour materials. Int. Biodeterior. Biodegrad. 2009, 63, 496–502. [Google Scholar] [CrossRef]
- Kwasna, H.; Karbowska-Berent, J.; Behnke-Borowczyk, J. Effect of Fungi on the Destruction of Historical Parchment and Paper Documents. Pol. J. Environ. Stud. 2020, 29, 2679–2695. [Google Scholar] [CrossRef]
- Cattaneo, B.; Chelazzi, D.; Giorgi, R.; Serena, T.; Merlo, C.; Baglioni, P. Physico-chemical characterization and conservation issues of photographs dated between 1890 and 1910. J. Cult. Herit. 2008, 9, 277–284. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation of three-layer laminate films based on gelatin under indoor soil conditions. Polym. Degrad. Stab. 2009, 94, 1307–1313. [Google Scholar] [CrossRef]
- Khoramnejadian, S. Microbial Degradation of Starch Based Polypropylene. J. Pure Appl. Microbiol. 2013, 7, 2857–2860. [Google Scholar]
- Savkovic, Z.; Stupar, M.; Unkovic, N.; Ivanovic, Z.; Blagojevic, J.; Vukojevic, J.; Grbic, M.L. In vitro biodegradation potential of airborne Aspergilli and Penicillia. Sci. Nat. 2019, 106, 8. [Google Scholar] [CrossRef]
- Okpalanozie, O.E.; Adebusoye, S.A.; Troiano, F.; Catto, C.; Ilori, M.O.; Cappitelli, F. Assessment of indoor air environment of a Nigerian museum library and its biodeteriorated books using culture-dependent and -independent techniques. Int. Biodeterior. Biodegrad. 2018, 132, 139–149. [Google Scholar] [CrossRef]
- Dannemiller, K.C.; Weschler, C.J.; Peccia, J. Fungal and bacterial growth in floor dust at elevated relative humidity levels. Indoor Air 2017, 27, 354–363. [Google Scholar] [CrossRef]
- Puskarova, A.; Buckova, M.; Habalova, B.; Krakova, L.; Makova, A.; Pangallo, D. Microbial communities affecting albumen photography heritage: A methodological survey. Sci. Rep. 2016, 6, 20810. [Google Scholar] [CrossRef] [Green Version]
- Grbic, M.L.; Stupar, M.; Vukojevic, J.; Maricic, I.; Bungur, N. Molds in Museum Environments: Biodeterioration of Art Photographs and Wooden Sculptures. Arch. Biol. Sci. 2013, 65, 955–962. [Google Scholar] [CrossRef]
- Soffritti, I.; D’Accolti, M.; Lanzoni, L.; Volta, A.; Bisi, M.; Mazzacane, S.; Caselli, E. The Potential Use of Microorganisms as Restorative Agents: An Update. Sustainability 2019, 11, 3853. [Google Scholar] [CrossRef] [Green Version]
- Ciferri, O. Microbial degradation of paintings. Appl. Environ. Microbiol. 1999, 65, 879–885. [Google Scholar] [CrossRef] [Green Version]
- Pyzik, A.; Ciuchcinski, K.; Dziurzynski, M.; Dziewit, L. The Bad and the Good-Microorganisms in Cultural Heritage Environments-An Update on Biodeterioration and Biotreatment Approaches. Materials 2021, 14, 177. [Google Scholar] [CrossRef]
- Borrego, S.; Molina, A.; Santana, A. Fungi in Archive Repositories Environments and the Deterioration of the Graphics Documents. EC Microbiol. 2017, 11, 205–226. [Google Scholar]
- Pietrzak, K.; Puchalski, M.; Otlewska, A.; Wrzosek, H.; Guiamet, P.; Piotrowska, M.; Gutarowska, B. Microbial diversity of pre-Columbian archaeological textiles and the effect of silver nanoparticles misting disinfection. J. Cult. Herit. 2017, 23, 138–147. [Google Scholar] [CrossRef]
- Rick, E.M.; Woolnough, K.F.; Seear, P.J.; Fairs, A.; Satchwell, J.; Richardson, M.; Monteiro, W.R.; Craner, M.; Bourne, M.; Wardlaw, A.J.; et al. The airway fungal microbiome in asthma. Clin. Exp. Allergy 2020, 50, 1325–1341. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Suto, H.; Unno, T.; Tsuboi, R.; Ogawa, H.; Shinoda, T.; Nishikawa, A. Molecular Analysis of Malassezia Microflora on the Skin of Atopic Dermatitis Patients and Healthy Subjects. J. Clin. Microbiol. 2001, 39, 3486–3490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Ji, W.; Zhao, B. Size-dependent efficiencies of ultrafine particle removal of various filter media. Build. Environ. 2019, 160. [Google Scholar] [CrossRef]
- Eckmanns, T.; Rüden, H.; Gastmeier, P. The Influence of High-Efficiency Particulate Air Filtration on Mortality and Fungal Infection among Highly Immunosuppressed Patients: A Systematic Review. J. Infect. Dis. 2006, 193, 1408–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Xiao, M.; Zhang, X.; Gal, C.; Chen, X.; Liu, L.; Pan, S.; Wu, J.; Tang, L.; Clements-Croome, D. A review of air filtration technologies for sustainable and healthy building ventilation. Sustain. Cities Soc. 2017, 32, 375–396. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Toxicological interactions between mycotoxins from ubiquitous fungi: Impact on hepatic and intestinal human epithelial cells. Chemosphere 2018, 202, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Rojas, T.I.; Aira, M.J.; Batista, A.; Cruz, I.L.; Gonzalez, S. Fungal biodeterioration in historic buildings of Havana (Cuba). Grana 2012, 51, 44–51. [Google Scholar] [CrossRef]
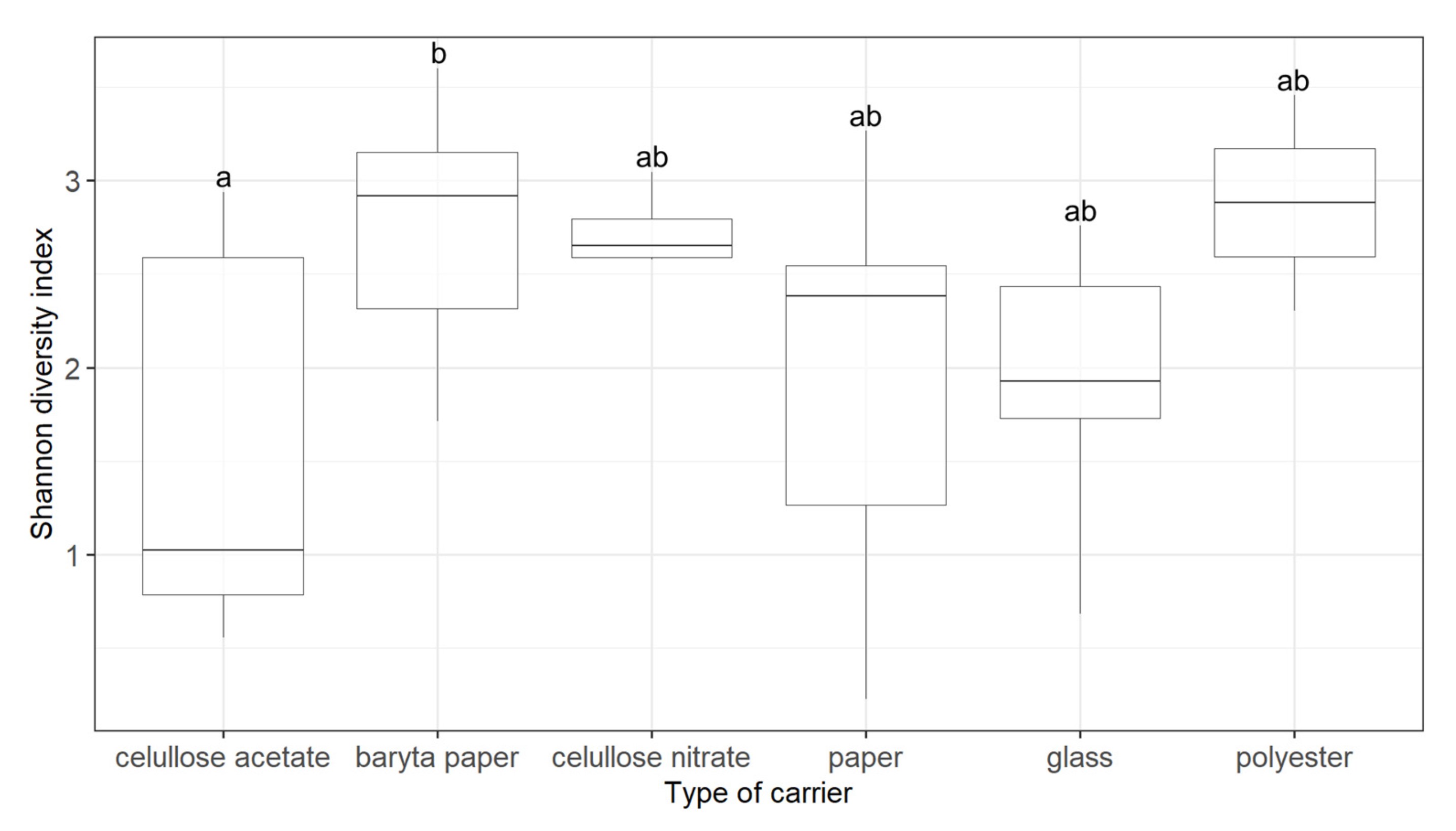

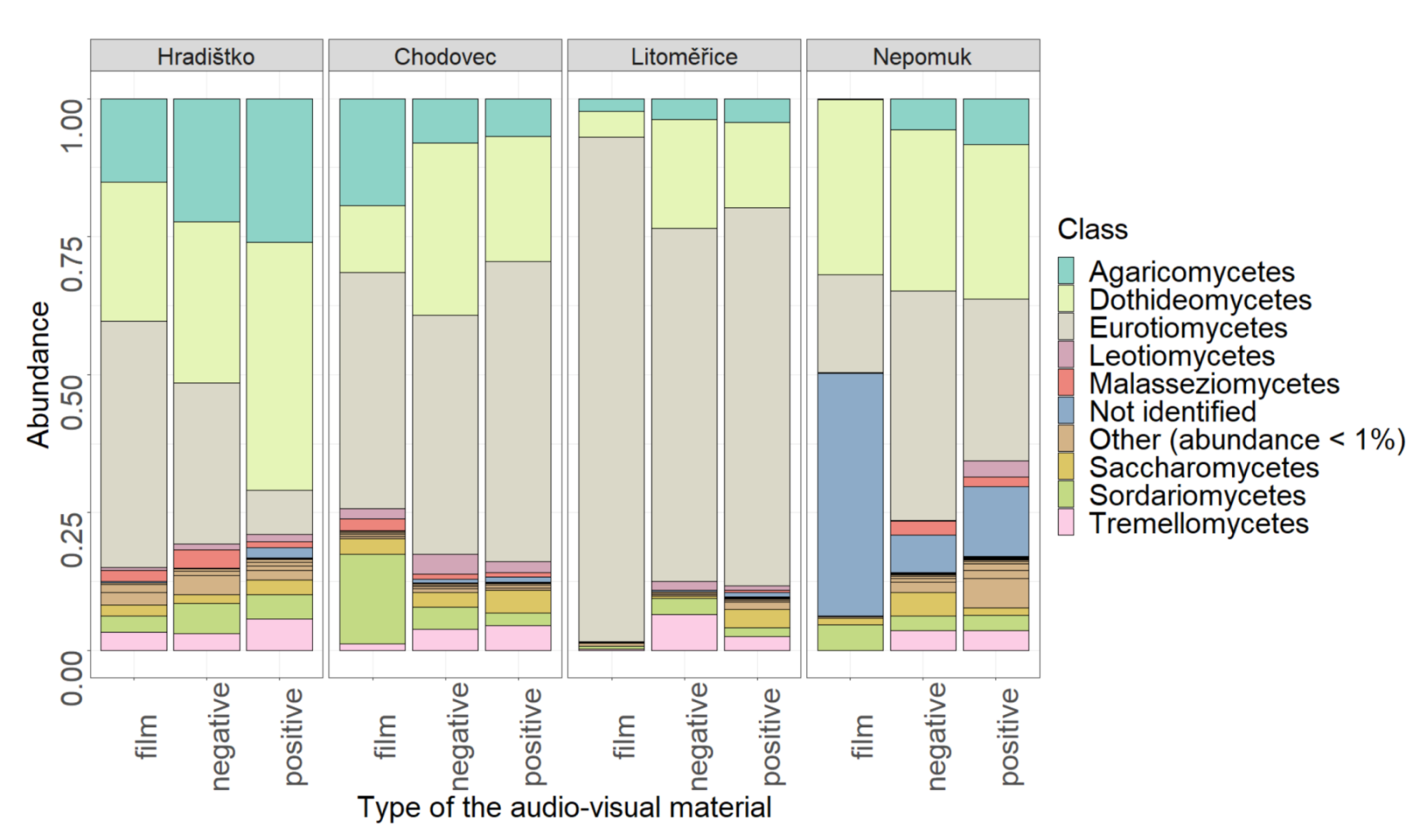
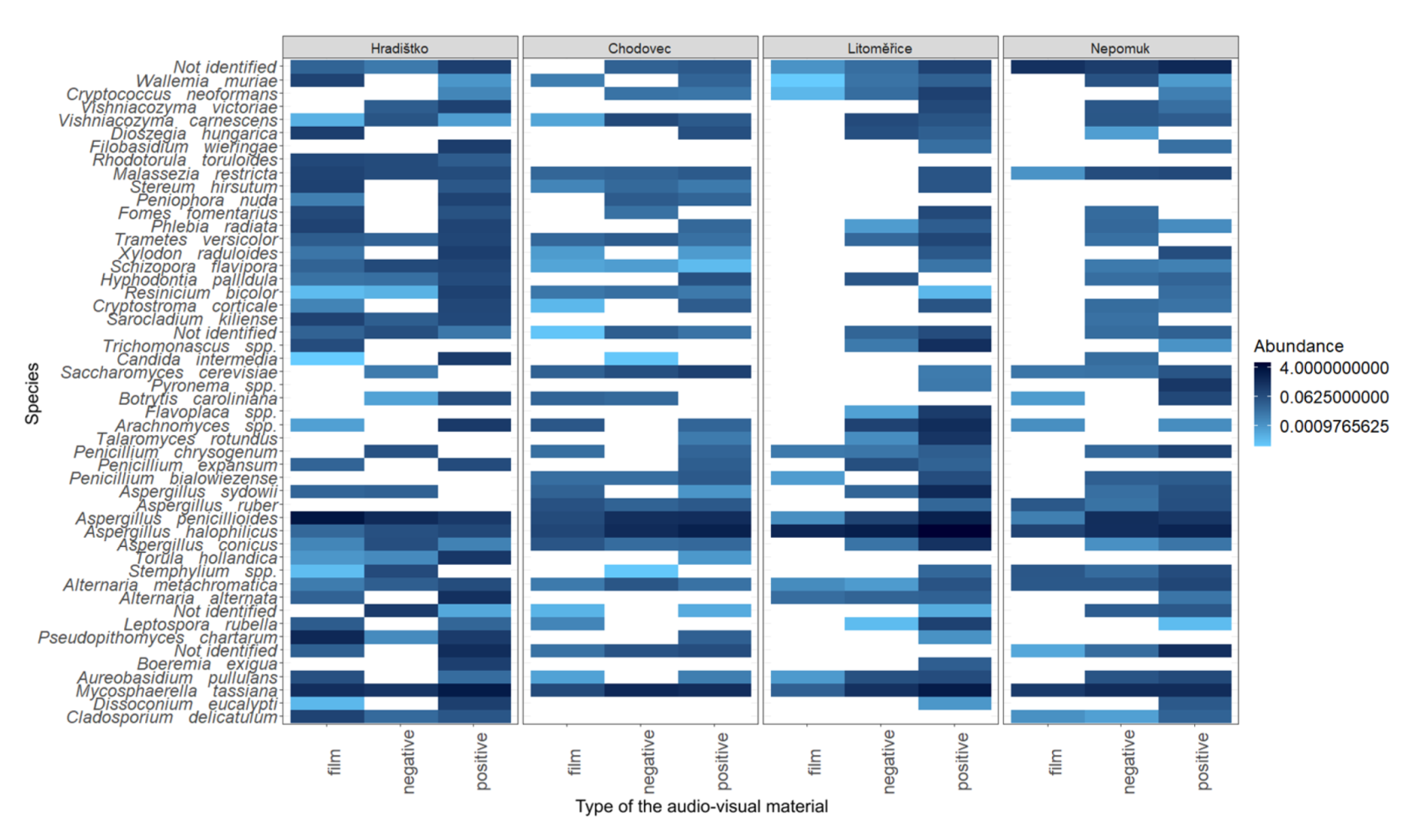
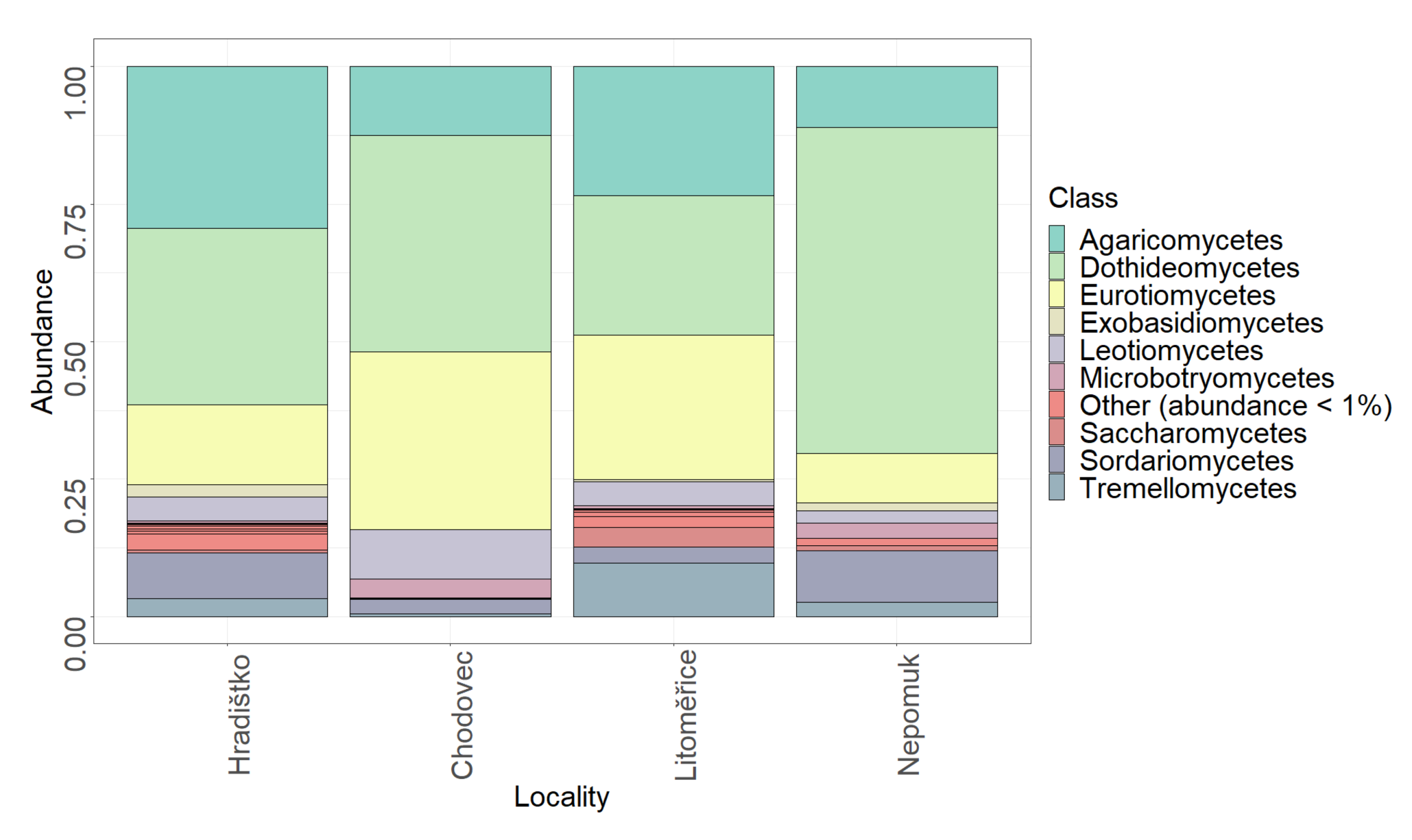


| Archive | Date | Temperature (°C) | Relative Humidity (%) | Air Filters |
|---|---|---|---|---|
| Litoměřice | 11 December 2019 | 16.7 | 47.9 | none |
| Hradištko | 26 August 2019 | 24.7 | 54.6 | none |
| Chodovec | 16 July 2020 | 21.3 | 50.7 | Class H12 |
| Nepomuk | 22 September 2020 | 26.0 | 45.5 | Class M5 |
| Archive | Photographs | Cinematographic Films | |||
|---|---|---|---|---|---|
| Positive | Negative | Gelatine | |||
| Gelatine | Albumen | Collodion | Gelatine | ||
| Litoměřice | 7 | 5 | 6 | 3 | 1 |
| Hradištko | 7 | 2 | 1 | 3 | 7 |
| Chodovec | 2 | 1 | 1 | 3 | 1 |
| Nepomuk | 2 | 3 | 1 | 3 | 1 |
| Factor | R2 | p-Value |
|---|---|---|
| Locality | 0.1884 | 0.001 * |
| Carrier | 0.0837 | 0.039 * |
| Binder | 0.0285 | 0.362 |
| Type of audio–visual material | 0.0214 | 0.010 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branysova, T.; Kracmarova, M.; Durovic, M.; Demnerova, K.; Stiborova, H. Factors Influencing the Fungal Diversity on Audio–Visual Materials. Microorganisms 2021, 9, 2497. https://doi.org/10.3390/microorganisms9122497
Branysova T, Kracmarova M, Durovic M, Demnerova K, Stiborova H. Factors Influencing the Fungal Diversity on Audio–Visual Materials. Microorganisms. 2021; 9(12):2497. https://doi.org/10.3390/microorganisms9122497
Chicago/Turabian StyleBranysova, Tereza, Martina Kracmarova, Michal Durovic, Katerina Demnerova, and Hana Stiborova. 2021. "Factors Influencing the Fungal Diversity on Audio–Visual Materials" Microorganisms 9, no. 12: 2497. https://doi.org/10.3390/microorganisms9122497






