Isolation of Lactococcus lactis from Whole Crop Rice and Determining Its Probiotic and Antimicrobial Properties towards Gastrointestinal Associated Bacteria
Abstract
:1. Introduction
2. Methods and Materials
2.1. Isolation and Characterization of LAB
2.2. Probiotic Potential of Selected LAB
2.3. Antibacterial Activity Well Diffusion
2.4. Lyophilization of Cell Free Metabolites (CFS)
2.5. Time and Dose-Dependent Killing Assay and Minimum Bactericidal (MIC) and Minimum Inhibitory Concentration (MBC)
2.6. Antagonistic Activity by Co-Culture Method
2.7. Statistical Analysis
3. Results
3.1. Isolation and Characterization
3.2. RWP-3 and RWP-7 in Simulated Gastrointestinal Tract (GIT)
3.3. Hydrophobicity and Aggregation Properties of RP3-3 and RP3-7
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christiansen, J. Infections by the Numbers. Sci. Am. 2018, 318, 48–49. [Google Scholar]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug Resistance: An Emerging Crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet. Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Hidron, A.I.; Edwards, J.R.; Patel, J.; Horan, T.C.; Sievert, D.M.; Pollock, D.A.; Fridkin, S.K. NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control. Hosp. Epidemiol. 2008, 29, 996–1011. [Google Scholar] [CrossRef] [Green Version]
- WHO. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 17 November 2021).
- Salminen, S.; Nybom, S.; Meriluoto, J.; Collado, M.C.; Vesterlund, S.; El-Nezami, H. Interaction of probiotics and pathogens—Benefits to human health? Curr. Opin. Biotechnol. 2010, 21, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska-Turak, E.; Hornowska, Ł.; Pobiega, K.; Gniewosz, M.; Witrowa-Rajchert, D. The influence of Lactobacillus bacteria type and kind of carrier on the properties of spray-dried microencapsules of fermented beetroot powders. Int. J. Food Sci. Technol. 2021, 56, 2166–2174. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Kołakowska, W.; Pobiega, K.; Gramza-Michałowska, A. Influence of Drying Type of Selected Fermented Vegetables Pomace on the Natural Colorants and Concentration of Lactic Acid Bacteria. Appl. Sci. 2021, 11, 7864. [Google Scholar] [CrossRef]
- Blaženka, K.; Jasna, B.; Andreja Leboš, P.; Ksenija, H.; Srećko, M.; Jagoda, Š. Antimicrobial Activity—The Most Important Property of Probiotic and Starter Lactic Acid Bacteria. Food Technol. Biotechnol. 2010, 48, 296–307. [Google Scholar]
- Maragkoudakis, P.A.; Mountzouris, K.C.; Psyrras, D.; Cremonese, S.; Fischer, J.; Cantor, M.D.; Tsakalidou, E. Functional properties of novel protective lactic acid bacteria and application in raw chicken meat against Listeria monocytogenes and Salmonella enteritidis. Int. J. Food Microbiol. 2009, 130, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Arasu, M.V.; Kim, D.H.; Kim, P.I.; Jung, M.W.; Ilavenil, S.; Jane, M.; Lee, K.D.; Al-Dhabi, N.A.; Choi, K.C. In vitro antifungal, probiotic and antioxidant properties of novel Lactobacillus plantarum K46 isolated from fermented sesame leaf. Ann. Microbiol. 2014, 64, 1333–1346. [Google Scholar] [CrossRef]
- Kang, H.J.; Im, S.H. Probiotics as an Immune Modulator. J. Nutr. Sci. Vitaminol. 2015, 61, S103–S105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garriga, M.; Rubio, R.; Aymerich, T.; Ruas-Madiedo, P. Potentially probiotic and bioprotective lactic acid bacteria starter cultures antagonise the Listeria monocytogenes adhesion to HT29 colonocyte-like cells. Benef. Microbes 2015, 6, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Daim, A.; Hassouna, N.; Hafez, M.; Ashor, M.S.; Aboulwafa, M.M. Antagonistic activity of Lactobacillus isolates against Salmonella typhi in vitro. Biomed Res. Int. 2013, 2013, 680605. [Google Scholar] [CrossRef] [Green Version]
- Nuryshev, M.Z.; Stoyanova, L.; Netrusov, A.I. New Probiotic Culture of Lactococcus lactis ssp. lactis: Effective Opportunities and Prospects. J. Microb. Biochem. Technol. 2016, 8, 290–295. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Savadogo, A.; Ouattara, C.; Bassolé, I.; Traore, A. Antimicrobial Activities of Lactic Acid Bacteria Strains Isolated from Burkina Faso Fermented Milk. Pak. J. Nutr. 2004, 3, 174–179. [Google Scholar]
- Soundharrajan, I.; Kim, D.; Kuppusamy, P.; Muthusamy, K.; Lee, H.J.; Choi, K.C. Probiotic and Triticale Silage Fermentation Potential of Pediococcus pentosaceus and Lactobacillus brevis and Their Impacts on Pathogenic Bacteria. Microorganisms 2019, 7, 318. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Casarotti, S.N.; Penna, A.L.B. Acidification profile, probiotic in vitro gastrointestinal tolerance and viability in fermented milk with fruit flours. Int. Dairy J. 2015, 41, 1–6. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Suzuki, C.; Sasaki, K.; Kobayashi, M.; Mizumachi, K. Survival of a Lactococcus lactis strain varies with its carbohydrate preference under in vitro conditions simulated gastrointestinal tract. Int. J. Food Microbiol. 2010, 143, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Del Re, B.; Sgorbati, B.; Miglioli, M.; Palenzona, D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 2000, 31, 438–442. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimirobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Annapolis Junction, MD, USA, 2017. [Google Scholar]
- Soleimani, N.; Kermanshahi, R.; Yakhchali, B.; Sattari, T. Antagonistic activity of probiotic lactobacilli against Staphylococcus aureus isolated from bovine mastitis. Afr. J. Microbiol. Res. 2010, 420, 2169–2173. [Google Scholar]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Singh, P.; Singh, B.R. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS ONE 2018, 13, e0192978. [Google Scholar] [CrossRef]
- Suissa, R.; Oved, R.; Jankelowitz, G.; Turjeman, S.; Koren, O.; Kolodkin-Gal, I. Molecular genetics for probiotic engineering: Dissecting lactic acid bacteria. Trends Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Singh, B.P.; Thakur, N.; Gulati, S.; Gupta, S.; Mishra, S.K.; Panwar, H. Antibacterial effects of Lactobacillus isolates of curd and human milk origin against food-borne and human pathogens. 3 Biotech 2017, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.; Kim, S.H.; Worobo, R.W. Characterization and Purification of a Bacteriocin Produced by a Potential Probiotic Culture, Lactobacillus acidophilus 30SC. J. Dairy Sci. 2000, 83, 2747–2752. [Google Scholar] [CrossRef]
- Mourad, K.; Karam, N.E. Microbiological study of naturally fermented Algerian green olives: Isolation and identification of lactic acid bacteria and yeasts along with the effects of brine solutions obtained at the end of olive fermentation on Lactobacillus plantarum growth. Grasas y Aceites 2006, 57, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Buriti, F.C.; Castro, I.A.; Saad, S.M. Viability of Lactobacillus acidophilus in synbiotic guava mousses and its survival under in vitro simulated gastrointestinal conditions. Int. J. Food Microbiol. 2010, 137, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Soundharrajan, I.; Kuppusamy, P.; Srisesharam, S.; Lee, J.C.; Sivanesan, R.; Kim, D.; Choi, K.C. Positive metabolic effects of selected probiotic bacteria on diet-induced obesity in mice are associated with improvement of dysbiotic gut microbiota. FASEB J. 2020, 34, 12289–12307. [Google Scholar] [CrossRef] [PubMed]
- Kondrotiene, K.; Lauciene, L.; Andruleviciute, V.; Kasetiene, N.; Serniene, L.; Sekmokiene, D.; Malakauskas, M. Safety Assessment and Preliminary In Vitro Evaluation of Probiotic Potential of Lactococcus lactis Strains Naturally Present in Raw and Fermented Milk. Curr. Microbiol. 2020, 77, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Varum, F.; Bravo, R.; Furrer, E.; Bojic, D.; Basit, A.W. Inflammatory bowel disease: Exploring gut pathophysiology for novel therapeutic targets. Transl. Res. J. Lab. Clin. Med. 2016, 176, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Puniya, M.; Kumar, M.R.; Panwar, H.; Kumar, N.; Ramneek, A.K.P. Screening of lactic acid bacteria of different origin for their probiotic potential. J. Food Process. Technol. 2016, 7, 545. [Google Scholar]
- Yerlikaya, O. Probiotic potential and biochemical and technological properties of Lactococcus lactis ssp. lactis strains isolated from raw milk and kefir grains. J. Dairy Sci. 2019, 102, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Vinderola, C.G.; Reinheimer, J.A. Lactic acid starter and probiotic bacteria: A comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res. Int. 2003, 36, 895–904. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Cortés-Zavaleta, O.; López-Malo, A.; Hernández-Mendoza, A.; García, H.S. Antifungal activity of lactobacilli and its relationship with 3-phenyllactic acid production. Int. J. Food Microbiol. 2014, 173, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Inglin, R.C.; Stevens, M.J.; Meile, L.; Lacroix, C.; Meile, L. High-throughput screening assays for antibacterial and antifungal activities of Lactobacillus species. J. Microbiol. Methods 2015, 114, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, L.; Li, Q.; Long, Y.; Lin, Y.; Yin, J.; Zeng, Y.; Huang, L.; Yao, T.; Abbasi, M.N.; et al. Functional probiotics of lactic acid bacteria from Hu sheep milk. BMC Microbiol. 2020, 20, 228. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Sadiq, M.B.; Ali, I.; Anwar, M.; Muhammad, N.; Muhammad, J.; Shafee, M.; Ullah, S.; Gul, Z.; Qasim, S.; et al. Lactococcus lactis subsp. lactis isolated from fermented milk products and its antimicrobial potential. CyTA J. Food 2019, 17, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, S.; Martínez-Blanco, H.; Rodríguez-Aparicio, L.B.; Ferrero, M.A. Effect of fermented broth from lactic acid bacteria on pathogenic bacteria proliferation. J. Dairy Sci. 2016, 99, 2654–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]


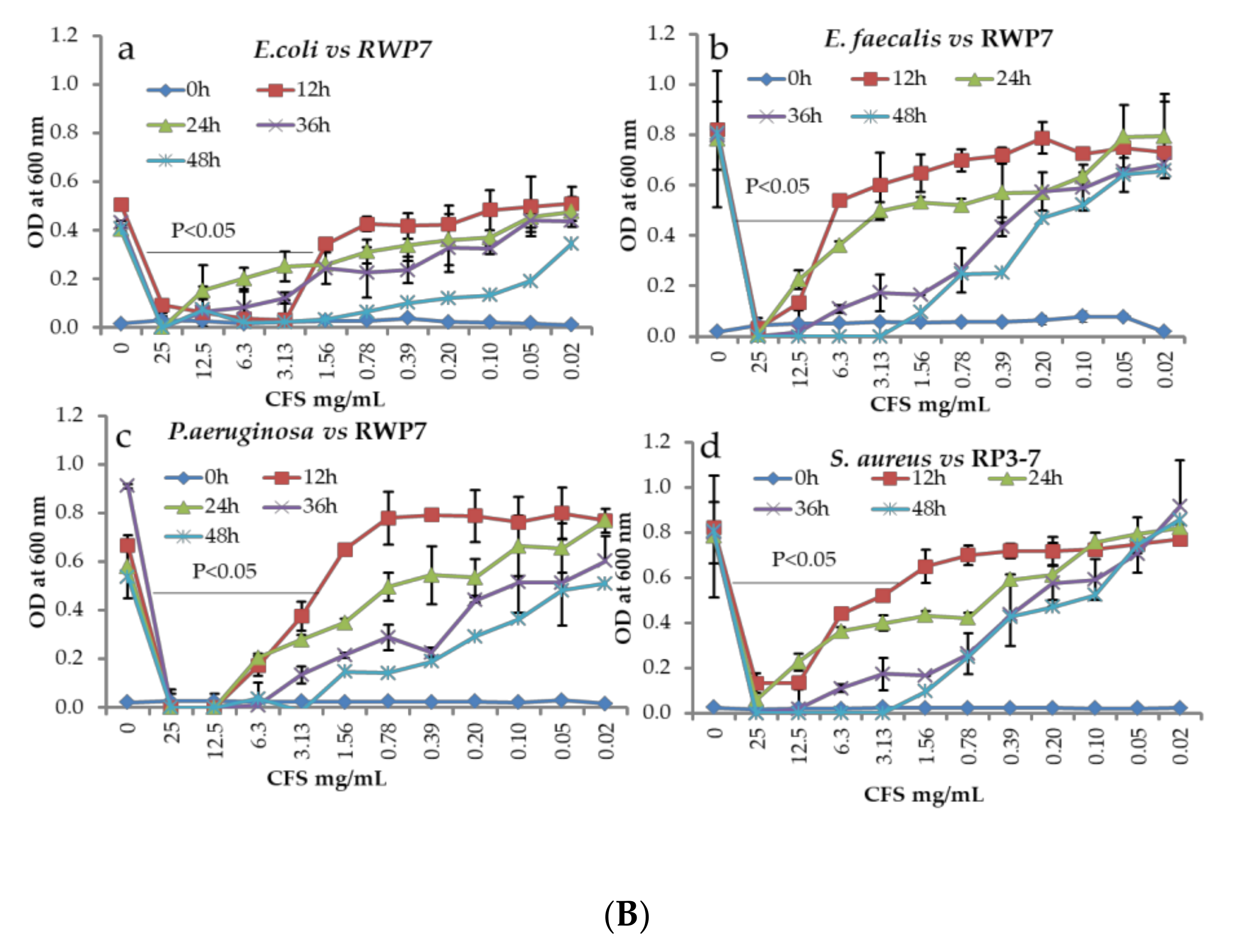
| Species Name | Zone of Inhibitions (mm) | |||
|---|---|---|---|---|
| E. coli | S. aureus | P. aeruginosa | E. faecalis | |
| RWP-3 | 23.6 ± 1.2 b | 28.60 ± 2.8 a | 17.8 ± 0.56 c | 18.3 ± 0.49 c |
| RWP-7 | 42.0 ± 1.4 a | 22.05 ± 2.1 b | 15.6 ± 0.28 c | 18.5 ± 1.06 c |
| Pathogens | CFS of RWP3 | CFS of RWP7 | ||
|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| E. coli | 12.5 | - | 25 | 12.5 |
| E. faecalis | 6.25 | 12.5 | 12.5 | 25 |
| P. aeruginosa | 12.5 | 25 | 6.25 | 12.5 |
| S. aureus | 12.5 | 25 | 12.5 | 25 |
| Groups | Growth on MRS Agar | Growth on NA | Groups | Growth on MRS Agar | Growth on NA | Groups | Growth on NA (Mono) |
|---|---|---|---|---|---|---|---|
| Bacterial growth (107 CFU/mL) after 12 h | |||||||
| RWP3 alone | 4.28 ± 0.22 # | RWP7 alone | 6.08 ± 0.45 # | ||||
| RWP3 ± SA | 3.93 ± 0.15 # | 0.236 ± 0.03 c | RWP7 ± SA | 5.84 ± 1.20 # | 0.70 ± 0.03 b | SA alone | 28.0 ± 0.56 a |
| RWP3 ± PA | 5.16 ± 0.65 # | 0.548 ± 0.06b | RWP7 ± PA | 6.08 ± 0.11 # | 0.50 ± 0.07 b | PA alone | 28.4 ± 1.98 a |
| RWP3 ± EC | 1.51 ± 0.14 * | 0.124 ± 0.02 c | RWP7 ± EC | 1.36 ± 0.14 * | 0.03 ± 0.01 b | EC alone | 29.5 ± 2.80 a |
| RWP3 ± EF | 5.06 ± 0.14 # | 0.212 ± 0.01 c | RWP7 ± EF | 2.97 ± 0.04 # | 0.27 ± 0.03 c | EF alone | 23.4 ± 0.07 a |
| Bacterial growth (107 CFU/mL) after 24 h | |||||||
| RWP3 alone | 5.85 ± 0.11 # | RWP7 alone | 7.81 ± 0.30 # | ||||
| RWP3 ± SA | 1.21 ± 0.08 * | 0.236 ± 0.03 d | RWP7 ± SA | 1.12 ± 0.06 * | 0.74 ± 0.03 c | SA alone | 54.0 ± 6.29 a |
| RWP3 ± PA | 1.26 ± 0.32 * | 0.822 ± 0.09b | RWP7 ± PA | 0.92 ± 0.29 * | 0.95 ± 0.28 b | PA alone | 47.3 ± 1.81 a |
| RWP3 ± EC | 5.50 ± 0.07 # | 0.036 ± 0.00 e | RWP7 ± EC | 1.30 ± 0.21 * | 0.17 ± 0.00 d | EC alone | 35.6 ± 2.26 a |
| RWP3 ± EF | 1.40 ± 0.14 * | 0.680 ± 0.00 c | RWP7 ± EF | 1.50 ± 0.78 * | 0.17 ± 0.00 d | EF alone | 38.7 ± 3.40 a |
| Bacterial growth (107 CFU/mL) after 36 h | |||||||
| RWP3 alone | 7.64 ± 0.53 # | RWP7 alone | 8.28 ± 0.48 # | ||||
| RWP3 ± SA | 1.68 ± 0.17 * | 0.108 ± 0.01d | RWP7 ± SA | 1.64 ± 0.48 * | 0.192 ± 0.01 c | SA alone | 48.8 ± 2.95 a |
| RWP3 ± PA | 7.44 ± 0.05 # | 0.216 ± 0.01 c | RWP7 ± PA | 8.14 ± 0.14# | 0.156 ± 0.03 c | PA alone | 33.8 ± 2.07 a |
| RWP3 ± EC | 2.80 ± 0.21 * | 0.846 ± 0.29 b | RWP7 ± EC | 2.71 ± 0.15 * | 0.590 ± 0.07 b | EC alone | 37.7 ± 2.76 a |
| RWP3 ± EF | 3.20 ± 0.28 * | 0.16 ± 0.022 d | RWP7 ± EF | 7.35 ± 0.81# | 0.148 ± 0.14 c | EF alone | 38.5 ± 5.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soundharrajan, I.; Yoon, Y.H.; Muthusamy, K.; Jung, J.-S.; Lee, H.J.; Han, O.-K.; Choi, K.C. Isolation of Lactococcus lactis from Whole Crop Rice and Determining Its Probiotic and Antimicrobial Properties towards Gastrointestinal Associated Bacteria. Microorganisms 2021, 9, 2513. https://doi.org/10.3390/microorganisms9122513
Soundharrajan I, Yoon YH, Muthusamy K, Jung J-S, Lee HJ, Han O-K, Choi KC. Isolation of Lactococcus lactis from Whole Crop Rice and Determining Its Probiotic and Antimicrobial Properties towards Gastrointestinal Associated Bacteria. Microorganisms. 2021; 9(12):2513. https://doi.org/10.3390/microorganisms9122513
Chicago/Turabian StyleSoundharrajan, Ilavenil, Yong Hee Yoon, Karnan Muthusamy, Jeong-Sung Jung, Hyun Jeong Lee, Ouk-Kyu Han, and Ki Choon Choi. 2021. "Isolation of Lactococcus lactis from Whole Crop Rice and Determining Its Probiotic and Antimicrobial Properties towards Gastrointestinal Associated Bacteria" Microorganisms 9, no. 12: 2513. https://doi.org/10.3390/microorganisms9122513







