Vegetables and Fruit as a Reservoir of β-Lactam and Colistin-Resistant Gram-Negative Bacteria: A Review
Abstract
:1. Introduction
2. β-Lactam Resistance and Gram-Negative Bacteria
3. Colistin Resistance in Gram-Negative Bacteria
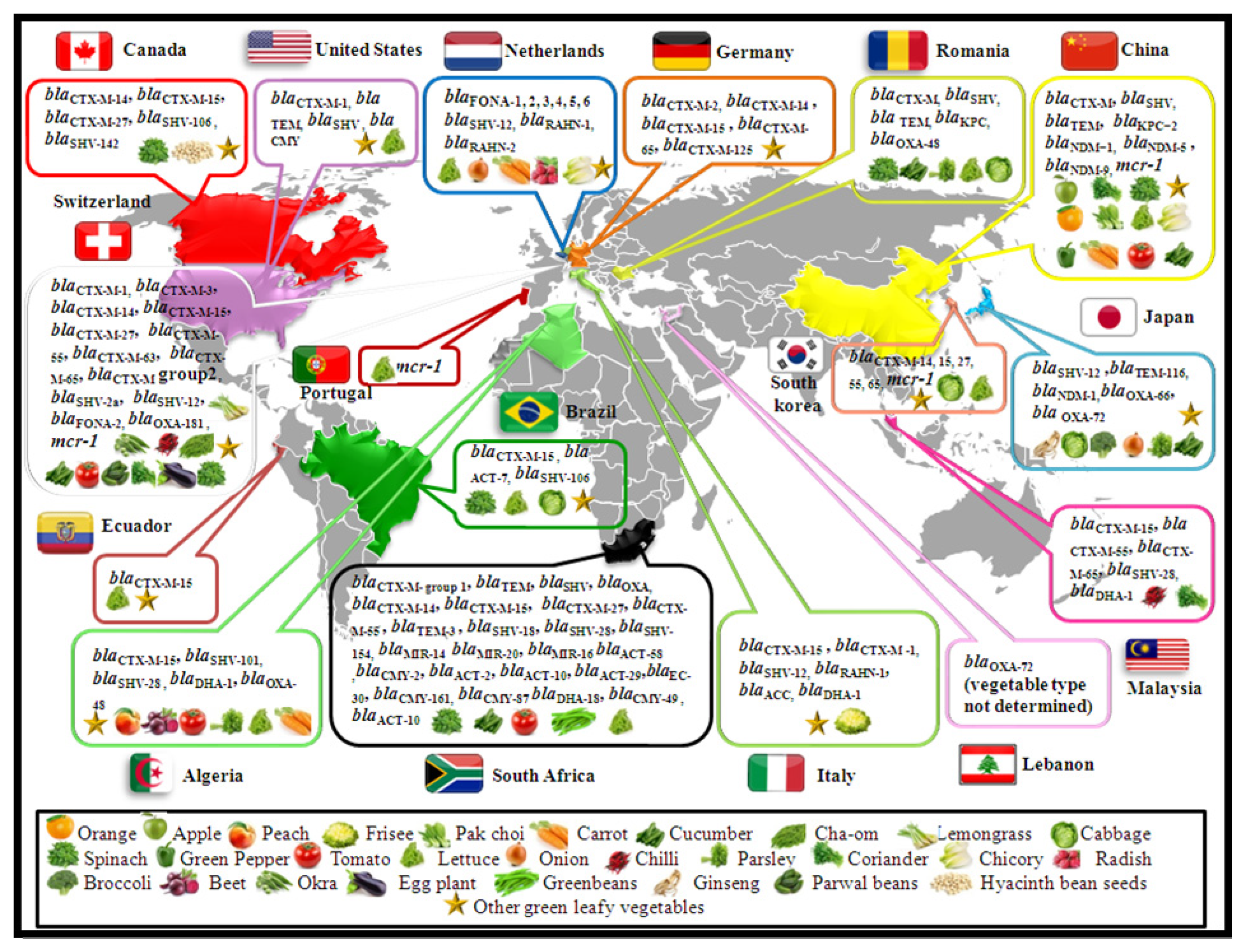
4. Literature Search Strategy and Data Collection
5. Vegetable and Fruit Isolates with ESBL and Cephalosporinase Genes
| Vegetable Type | ESBL/AmpC Gene | Isolation Period | Species | Isolates Number | Country | Other Antibiotic Resistance Genes | Sequence Type | References |
|---|---|---|---|---|---|---|---|---|
| Lettuce | blaFONA-5 | 2011 | Serratia fonticola | 1 | The Netherlands | ND | ND | [42] |
| blaRAHN-2 | Rahnella aquatilis | 1 | ||||||
| blaCTX-M-15 | 2013–2014 | Klebsiella pneumoniae | 1 | Algeria | aph(3′)-Ia, aadA2, strB, strA, qnrS1, oqxB, oqxA, fosA, mph(A), catA2, sul1, sul2, tet(A), dfrA12 | ST219 | [48] | |
| blaDHA-1 blaSHV-101 | K. pneumoniae | 1 | blaOXA-1, aac(6′)Ib-cr, aph(3′)-Ia, aac(6′)Ib-cr, qnrB4, oqxB, oqxA, fosA, mph(A) catB3, ARR-3, sul1 | ST882 | ||||
| blaSHV-28, blaCTX-M-15, | K. pneumoniae | 1 | blaOXA-1, aac(6′)Ib-cr, aac(3)-Iia, aac(6′)Ib-cr, qnrB66, oqxB, oqxA, fosA, catB3, dfrA14. | ST14 | ||||
| blaCTX-M-15 | 2015 | Escherichia coli | 1 | Ecuador | dfrA1, aadA5 | ST44 | [54] | |
| blaCTX-M-15 | 1 | None | ST44 | |||||
| blaCTX-M-14 | 2017–2018 | S. fonticola | 1 | South Africa | ND | ND | [9] | |
| blaSHV-154 | S. marcescens | 1 | ND | ND | ||||
| blaCTX-M-15 | 2018 | E. coli | 1 | South Korea | ND | ST2509 | [51] | |
| blaSHV, blaTEM | 2019 | Proteus vulgaris | 1 | Romania | ND | ND | [46] | |
| blaCTX-M-15 | ND | K. pneumoniae | 1 | Brazil | blaOXA-1, blaSHV-110, aac(3)IIa, aac(6′)-Ib-cr, opxAB, drfA14, catA1, tet(A), fosA, opxB | ST198 | [56] | |
| Butterhead lettuce | blaFONA-1 (1–6) | 2012–2013 | S. fonticola | ND | The Netherlands | ND | ND | [3] |
| Iceberg lettuce | blaRAHN-1 | 2011 | R. aquatilis | ND | The Netherlands | ND | [42] | |
| blaSHV, blaTEM | 2011–2012 | K. pneumoniae | 2 | United States | ND | ND | [52] | |
| blaCTX-M-1 | S. marcescens | 1 | ND | ND | ||||
| blaFONA-1 (1–6) | 2012–2013 | S. fonticola | ND | The Netherlands | ND | ND | [3] | |
| Tomato | blaCTX-M, blaSHV, blaTEM | 2011–2014 | E. coli | 1 | China | ND | ND | [7] |
| blaSHV-28, blaCTX-M-15 | 2013–2014 | K. pneumoniae | 1 | Algeria | aac(3)-Iia, qnrB66, oqxB, oqxA, fosA | ST14 | [48] | |
| blaSHV-28, blaCTX-M-15, | 1 | blaOXA-1, aac(6′)Ib-cr, aac(3)-Iia, aac(6′)Ib-cr, qnrB66, oqxB, oqxA, fosA, catB3, dfrA14 | ST14 | |||||
| blaCMY-2 | 2017–2018 | Citrobacter freundii | 1 | South Africa | ND | ND | [9] | |
| blaCTX-M-14 | 1 | ND | ND | |||||
| blaCTX-M-55 | E. coli | 1 | ND | ND | ||||
| blaCTX-M-14 | 1 | ND | ND | |||||
| blaCTX-M-14 | 1 | blaSHV-1, blaTEM-215 | ND | |||||
| blaSHV-18 | E. asburiae | 1 | ND | ND | ||||
| blaMIR-14 | 1 | blaSHV-26 | ND | |||||
| blaACT-29 | 1 | ND | ND | |||||
| blaCTX-M-27, blaCTX-M-15 | E. cloacae | 1 | blaSHV-26 | ND | ||||
| blaMIR-20 | 1 | ND | ND | |||||
| blaTEM-3, blaACT-2, blaSHV-18 | 1 | blaTEM-1, blaSHV-11 | ND | |||||
| blaSHV-18, blaTEM-3 | E. cowanii | 1 | ND | ND | ||||
| blaCTX-M-15 | K. pneumoniae | 1 | ND | ND | ||||
| blaACT-10 | K. oxytoca | 1 | ND | ND | ||||
| blaCTX-M-55 | Proteus mirabilis | 1 | blaTEM-215 | ND | ||||
| blaACT-10, blaDHA-18, blaCMY-49 | Pseudomonas penneri | 1 | ND | ND | ||||
| blaSHV-18 | R. aquatilis | 1 | blaTEM-215 | ND | ||||
| blaMIR-16 | R. aquatilis | 1 | ND | ND | ||||
| blaSHV-18, blaMIR-16 | 2017–2018 | E. asburiae | 1 | South Africa | blaTEM-1, blaOXA-1 | ND | [9] | |
| Diced tomato | blaCTX-MGroup2 | 2014 | Kluyvera ascorbata | 1 | Switzerland | ND | ND | [44] |
| Spinach | blaFONA-2 | 2014 | S. fonticola | 1 | Switzerland | ND | ND | [44] |
| blaCTX-M-group1, blaTEM | 2017 | E. asburiae | 1 | South Africa | ND | ND | [47] | |
| blaCTX-M-group1, blaTEM, blaSHV, blaOXA | E. coli | 2 | ND | ND | ||||
| blaCTX-M-group1, blaTEM, blaSHV, blaOXA | K. pneumoniae | 3 | ND | ND | ||||
| blaCTX-M-group1, blaTEM | E. asburiae | 1 | ND | ND | ||||
| blaCTX-M-group1, blaTEM, blaSHV, blaOXA | E. coli | 2 | ND | ND | ||||
| blaCTX-M-group1, blaTEM, blaSHV, blaOXA | K. pneumoniae | 3 | ND | ND | ||||
| blaCTX-M-group1 | R. aquatilis | 1 | ND | ND | ||||
| blaCTX-M-group1, blaTEM, blaSHV, blaOXA | R. aquatilis | 1 | ND | ND | ||||
| blaCTX-M-group1, blaTEM, blaSHV | R. aquatilis | 2 | ND | ND | ||||
| blaCIT | S. fonticola | 3 | ND | ND | ||||
| blaTEM, blaSHV | 1 | ND | ND | |||||
| blaCTX-M-group1, blaTEM, blaSHV, blaOXA | 2 | ND | ND | |||||
| blaCTX-M-group1, blaTEM, blaSHV, blaOXA, blaCIT | 1 | ND | ND | |||||
| blaCTX-M-group1, blaSHV | 1 | ND | ND | |||||
| blaCTX-M-group1, blaTEM, blaSHV, blaCIT | 2 | ND | ND | |||||
| blaCTX-M-27 | 2017–2018 | E. coli | 2 | South Africa | ND | ND | [9] | |
| blaMIR-20 | 1 | ND | ND | |||||
| blaSHV-18, blaCTX-M-15, blaTEM-3 | 1 | ND | ND | |||||
| blaCTX-M-14, blaTEM-3 | 1 | ND | ND | |||||
| blaCTX-M-14 | 1 | ND | ND | |||||
| blaCTX-M-15 | 1 | ND | ||||||
| blaCTX-M-55 | 1 | ND | ND | |||||
| blaCTX-M-14, blaACT-58 | 1 | ND | ND | |||||
| blaCTX-M-14 | 2 | ND | ND | |||||
| blaCTX-M-14 | 2 | blaTEM-215 | ND | |||||
| blaACT-58 | E. asburiae | 1 | blaTEM-215 | ND | ||||
| blaCMY-87 | E. ludwigii | 1 | ND | ND | ||||
| blaCTX-M-27, blaEC-30 | R. aquatilis | 1 | ND | ND | ||||
| blaCTX-M-15 | 1 | blaSHV-11 | ND | |||||
| blaCTX-M-15, blaSHV-28 | S. fonticola | 1 | ND | ND | ||||
| blaCTX-M-14, blaSHV-28 | 1 | ND | ND | |||||
| blaMIR-16 | 1 | blaTEM-1, blaOXA-1 | ND | |||||
| blaCTXM-15 | 1 | blaTEM-215 | ND | |||||
| blaSHV, blaTEM | 2019 | S. marcescens | 1 | Romania | ND | ND | [46] | |
| blaCTXM | E. cloacae | 1 | ND | ND | ||||
| blaCTXM-15 | ND | E. cloacae | 1 | Brazil | blaOXA-1, blaTEM-1B, blaACT-7, aac(3)-IIa, aac(6′)Ib-cr, ant(3′’)Ia, strA, strB, qnrB, sul2, tet(A), fosA. | ST927 | [56] | |
| blaCTXM-15 | ND | E. coli | 1 | blaTEM-1B, aac(3)IId, aadA5, strA, strB, tet(A) | ST14012 | |||
| Chopped Spinach | blaCTXM14, blaSHV-142 | 2017 | K. pneumoniae | 1 | Canada | ND | ST261 | [55] |
| blaCTXM-27 | E. cloacae | 1 | qnrB2, qnrS1, aac(6′)Ib cr | ND | ||||
| blaCTXM-27 | E. aerogenes | 1 | aac(6′) Ib cr | ND | ||||
| Ceylon spinach | blaCTXM-14 | 2014 | K. pneumoniae | 1 | Switzerland | ND | ST37 | [10] |
| Water spinach | blaCTXM-15 | K. pneumoniae | 1 | ND | ST16 | |||
| Cucumber | blaCTX-M, blaSHV | 2011–2014 | E. coli | 2 | China | ND | ND | [7] |
| blaCTXM-15 | 2014 | E. cloacae | 1 | Switzerland | ND | ND | [10] | |
| blaCTXM-15 | E. coli | 1 | ND | ST410 | ||||
| blaTEM-116 | 2015–2016 | P. mosselii | 1 | Japan | ND | ND | [49] | |
| blaMIR-20 | 2017–2018 | E. cloacae | 1 | South Africa | ND | ND | [9] | |
| blaSHV-18 | R. aquatilis | 1 | blaOXA-1 | ND | ||||
| blaCTXM, blaTEM | 2019 | E. coli | 1 | Romania | ND | ND | [46] | |
| blaDHA | E. cloacae | 1 | ND | |||||
| Bitter cucumber | blaCTXM-15 | 2014 | E. coli | 1 | Switzerland | ND | ST131 | [10] |
| Coriander | blaCTXM-55 | 2011–2014 | E. coli | 2 | China | ND | ST48, ST4680 | [7] |
| blaCTX-M, blaOXA | Citrobacter freundii | 1 | ND | ND | ||||
| blaCTX-M-55 | 2018 | E. coli | 1 | Malaysia | blaTEM-1B, aph(3 0)-Ia, aph(300)-Ib, aph(6)-Id, mdf(A), floR, ARR-2, sul2, tet(A), dfrA14 | ST155 | [50] | |
| blaCTX-M-65 | E. coli | 1 | aac(3)-IV, aadA5, aph(4)-Ia, oqxA, oqxB, mdf(A), floR, sul1, sul2, tet(A), dfrA17 | ST479 | ||||
| Parsley | blaSHV-28, blaCTX-M-15, blaOXA-1 | 2013–2014 | K. pneumoniae | 1 | Algeria | aac(6′)Ib-cr, aac(3)-Iia, aac(6′)Ib-cr, qnrB66, oqxB, oqxA, fosA, catB3, dfrA14 | ST14 | [48] |
| blaCTX-M-15, blaOXA-1 | 1 | aac(6′)Ib-cr, aac(3)-IIa, strB, strA, aac(6′)Ib-cr, oqxB, oqxA, fosA, catB3, sul2, tet(A), dfrA14 | ST45 | |||||
| blaSHV | 2019 | K. oxytoca | 1 | Romania | ND | ND | [46] | |
| Water parsley | blaCTX-M-55 | 2018 | E. coli | 1 | South Korea | ND | ND | [51] |
| blaCTX-M-15, blaTEM-1 | 1 | ND | ST101 | |||||
| blaCTX-M-14, blaTEM-1 | 1 | ND | ST354 | |||||
| blaCTX-M-14 | 1 | ND | ST38 | |||||
| Parsley/cilantro | blaCTX-M-15 | 2015 | E. coli | 1 | Ecuador | None | ST410 | [54] |
| 1 | dfrA1, aadA5 | ST44 | ||||||
| Soy sprouts | blaCTX-M-65 | 2011–2013 | E. coli | 1 | Germany | floR, aac(6′)-Ib3, sul2, tet(A), fosA3 | ST10 | [45] |
| blaCTX-M-125 | 1 | aph(3′)-II, tet(A), fosA3 | ST542 | |||||
| blaCTX-M-14 | 1 | catA1, floR, aac(6′)Ib-cr, aph(3′)-Ia, aadA5, sul1, sul2, tet(A), dfrA17, fosA3 | ST527 | |||||
| blaCTXM-14 | 2014 | K. pneumoniae | 1 | Switzerland | ND | ST208 | [10] | |
| Sprouts-mixture | blaCTX-M-15 | 2011–2013 | E. coli | 1 | Germany | blaTEM-1, qnrS1, strA, strB, sul2, tet(A), dfrA14 | ST847 | [45] |
| Alfalfa | blaCTX-M-15 | 2015 | E. coli | 1 | Ecuador | dfrA1, aadA5 | ST410 | [54] |
| blaCTX-M-15 | 1 | None | ST44 | |||||
| blaCTX-M-15 | 1 | None | ST44 | |||||
| Alfalfa sprouts | blaCTX-M-15 | 2011–2013 | E. coli | 1 | Germany | blaTEM-1, qnrS1, strA, strB, sul2, tet(A), dfrA14 | ST410 | [45] |
| Greenbeans | blaCTX-M-14 | 2017–2018 | E. coli | 2 | South Africa | ND | ND | [9] |
| blaCTX-M-14, blaCMY-2 | S. fonticola | 1 | blaTEM-215 | ND | ||||
| blaCTX-M-14, blaCMY-161 | 2017–2018 | S. fonticola | 1 | blaTEM-215 | ND | |||
| Curry leaves | blaCTXM-15 | 2014 | K. pneumoniae | 1 | Switzerland | ND | ST307 | [10] |
| blaCTXM-14 | E. coli | 1 | ND | ST38 | ||||
| blaCTXM-15 | K. pneumoniae | 1 | ND | ST1742 | ||||
| blaSHV-12 | E. coli | 1 | ND | ST1656 | ||||
| blaCTXM-15 | K. pneumoniae | 4 | ND | ST1739, ST1741, ST1881, ST1740 | ||||
| blaCTXM-1 | E. coli | 1 | ND | ST1555 | ||||
| blaCTXM-15 | 1 | ND | ST4681, ST152 | |||||
| blaCTXM-14 | 1 | ND | ST4679 | |||||
| blaCTXM-55 | 1 | ND | ST10 | |||||
| Mint | blaCTX-M-15, blaSHV-28 | 2013–2014 | K. pneumoniae | 1 | Algeria | blaOXA-1, aac(6′)Ib-cr, aac(3)-Iia, aac(6′)Ib-cr, qnrB66, oqxB, oqxA, fosA, catB3, dfrA14 | ST14 | [48] |
| blaCTX-M-15, blaSHV-28 | 1 | blaOXA-1, aac(6′)Ib-cr, aac(3)-Iia, aac(6′)Ib-cr, qnrB66, oqxB, oqxA, fosA, catB3, dfrA14 | ST14 | |||||
| Chili | blaCTXM-15 | 2014 | E. coli | 1 | Switzerland | ND | ST405 | [10] |
| Green chili | blaCTXM-15 | E. cloacae | 1 | ND | ND | [10] | ||
| blaCTXM-15 | K. pneumoniae | 2 | ND | ST1740, ST37 | ||||
| blaCTXM-27 | 1 | ND | ST458 | |||||
| Small chili | blaCTXM-65 | E. coli | 1 | ND | ST167 | |||
| Chili pepper | blaCTX-M-15, blaSHV-28 | 2018 | K. pneumoniae | 1 | Malaysia | blaTEM-1B, blaOXA-1, aac(3)-IIa, aac(6 0)-Ib-cr, aph(300)-Ib, aph(6)-Id, aac(6 0)-Ib-cr, oqxA, oqxB, qnrB1, fosA, catB3, sul2, tet(A), dfrA14 | ST307 | [50] |
| blaDHA-1, blaSHV-28 | K. pneumoniae | 1 | oqxA, oqxB, qnrS1, fosA, sul1, tet(A), dfrA1 | ST101 | ||||
| Hyacinth bean seeds | blaCTXM-15 | 2017 | E. coli | 1 | Canada | ND | ST189 | [55] |
| 1 | blaTEM-1 | ST226 | ||||||
| Ginseng | blaTEM-116 | 2015–2016 | Pseudomonas paralactis | 1 | Japan | ND | ND | [49] |
| blaTEM-116 | 1 | ND | ND | |||||
| blaTEM-116 | P. arsenicoxydans | 1 | ND | ND | ||||
| Beets | blaCTX-M-15 | 2013–2014 | K. pneumoniae | 1 | Algeria | aph(3′)-Ia, aadA2, strB, strA, nrS1, oqxB, oqxA, fosA, mph(A), catA2, sul1, sul2, tet(A), dfrA12 | ST219 | [48] |
| Carrot | blaCTX-M-15, blaOXA-1 | 2013–2014 | K. pneumoniae | 1 | blaTEM-1B, aac(6′)Ib-cr, aac(3)-IIa, strB, strA, ac(6′)Ib-cr, qnrB66, oqxB, oqxA, fosA, catB3, sul2, dfrA14. | ST45 | [48] | |
| blaRAHN-1 | 2011 | R. aquatilis | ND | The Netherlands | ND | ND | [42] | |
| Bunched carrot | blaFONA(1–6) | 2012–2013 | S. fonticola | ND | The Netherlands | ND | ND | [3] |
| Arugula | blaRAHN-1 | 2015–2016 | R. aquatilis | 4 | Italy | ND | ND | [43] |
| blaCTX-M-15 | C. freundii | 4 | ND | ND | ||||
| blaACC | Hafnia alvei | 2 | ND | ND | ||||
| blaCTXM-15blaSHV-106 | ND | K. pneumoniae | 1 | Brazil | blaOXA-1, blaTEM-1B, aac(6)Ib-cr, strA, strB, qnrB1, opxAB, gyrA, parC, tet(A), fosA. | ST2739 | [56] | |
| Egg plant | blaCTXM-15 | 2014 | K. pneumoniae | 1 | Switzerland | ND | ST45 | [10] |
| blaCTXM-15 | 1 | ND | ST307 | |||||
| blaCTXM-15 | E. cloacae | 1 | ND | ND | ||||
| Chinese chive | blaSHV-12 | 2015–2016 | P. parafulva | 1 | Japan | ND | ND | [49] |
| Chopped chives | blaCTX-M-15 | 2014 | E. cloacae | 1 | Switzerland | ND | ND | [44] |
| Onion | blaFONA-1 (1–6) | 2012–2013 | S. fonticola | ND | The Netherlands | ND | ND | [3] |
| blaTEM-116 | 2015–2016 | Pseudomonas beteli | 1 | Japan | ND | ND | [49] | |
| Broccoli | blaTEM-116 | 2015–2016 | P. hunanensis | 1 | Japan | ND | ND | [49] |
| Cabbage | blaTEM-116 | 2015–2016 | P. hunanensis | 1 | Japan | ND | ND | [49] |
| blaCTX-M, blaSHV | 2019 | E. cloacae | 1 | Romania | ND | ND | [46] | |
| blaCTXM-65 | 2018 | E. coli | 1 | South Korea | ND | 2847 | [51] | |
| blaCTXM-15 | ND | E. coli | 1 | Brazil | blaOXA-1, blaTEM-1B, aac(3)IIa, aac(6′)Ib-cr, aadA5, gyrA, parC, sul1. | ST648 | [56] | |
| blaCTXM-15 | ND | E. coli | 1 | blaOXA-1, aac(6′)Ib-cr, aadA5, aac(6′)Ib-cr, sul1, drfA17, tet(B), mph(A) | ST38 | |||
| Cut cabbage | blaSHV-12 | 2015–2016 | P. hunanensis | 1 | Japan | ND | ND | [49] |
| Bean sprout | blaSHV-12 | P. putida | 1 | ND | ND | |||
| Yard long beans | blaCTXM-55 | 2015–2016 | E. cloacae | 1 | Japan | ND | ND | [49] |
| blaSHV-12 | 2014 | Cronobacter sakazakii | 1 | Switzerland | ND | ST3696 | [10] | |
| blaCTXM-14 | E. coli | 1 | ND | ND | ||||
| Holy Basil | blaCTXM-15 | 2014 | K. pneumoniae | 1 | Switzerland | ND | ST36 | |
| blaCTXM-65 | E. coli | 1 | ND | ST58 | ||||
| Okra (marrow) | blaCTXM-14 | E. coli | 1 | ND | ST38 | |||
| blaCTXM-15 | E. coli | 2 | ND | ST155, ST443 | ||||
| Okra | blaCTXM-15 | K. pneumoniae | 2 | ND | ST997, ST244 | |||
| blaCTXM-15 | E. aerogenes | 1 | ND | ND | ||||
| blaCTXM-15 | E. cloacae | 2 | ND | ND | ||||
| blaCTXM-15 | E. coli | 2 | ND | ST4682, ST4684 | ||||
| Parwal beans | blaCTXM-15 | E. coli | 1 | ND | ST641 | |||
| Peppermint | blaCTXM-3 | K. pneumoniae | 1 | ND | ST15 | |||
| Cha-om (acacia) | blaSHV-12 | K. pneumoniae | 1 | ND | ND | |||
| blaCTXM-55 | E. coli | 2 | ND | ST167, ST393 | ||||
| blaCTXM-14 | E. coli | 1 | ND | ST58 | ||||
| Garlic chives | blaCTXM-63 | K. pneumoniae | 1 | ND | ST1743 | |||
| blaCTXM-55 | E. coli | 1 | ND | ST226 | ||||
| Lemongrass | blaCTXM-14 | K. pneumoniae | 1 | ND | ST1530 | |||
| Sweet basil | blaSHV-2a | K. pneumoniae | 1 | ND | ST76 | |||
| Basil leaves | blaCTXM-65 | E. coli | 1 | ND | ST4683 | |||
| Celery | blaRAHN-1 | 2011 | R. aquatilis | 34 | The Netherlands | ND | ND | [42] |
| blaSHV-60, blaDHA-1 | 2013–2014 | K. pneumoniae | 1 | Algeria | blaTEM-1D, aadA1, strB, strA, qnrB4, oqxB, oqxA, fosA, sul1,tet(A), dfrA1 | ST236 | [48] | |
| Lollo rosso leaves | blaCTX-M-14 | 2011–2013 | E. coli | 1 | Germany | strA, strB, sul1, dfrA1 | ST973 | [45] |
| Lollo rosso and Lollo bionda leaves | blaCTX-M-2 | E. coli | 1 | blaTEM-1, strA, strB, aadA5 | ST120 | |||
| Blanched celery | blaSHV-12 | 2012–2013 | E. coli | 1 | The Netherlands | ND | ND | [3] |
| blaFONA-1 | S. fonticola | ND | ND | ND | ||||
| Radish | blaRAHN-1 | 2012–2013 | R. aquatilis | 1 | The Netherlands | ND | ND | [3] |
| blaFONA(1–6) | S. fonticola | ND | ND | ND | ||||
| blaRAHN-1 | 2011 | R. aquatilis | ND | ND | ND | [42] | ||
| Chicory | blaRAHN-1 | 2011 | R. aquatilis | ND | The Netherlands | ND | ND | [42] |
| Endive | blaRAHN-1 | 2011 | R. aquatilis | ND | ND | ND | ||
| blaFONA-1 (1–6) | 2012–2013 | S. fonticola | ND | The Netherlands | ND | ND | [3] | |
| Iceberg lettuce + arugula | blaSHV-12 | 2015–2016 | E. coli | 3 | Italy | ND | ND | [43] |
| blaCTX-M-15 | E. coli | 1 | ND | ND | ||||
| Mixed green vegetables | blaCTXM-15 | 2017 | E. cloacae | 1 | Canada | blaTEM-1, qnrB1, aac(6′) Ib cr | ND | [55] |
| Sambhar vegetables | blaCTXM15, blaSHV-106 | K. pneumoniae | 1 | ND | ST101 | |||
| Aster scaber | blaCTX-14, blaTEM-1 | 2018 | E. coli | 1 | South Korea | ND | ST69 | [51] |
| Perilla leaf | blaCTX-M-27, blaTEM-1 | 1 | ND | ST349 | ||||
| Sweet potato stalk | blaCTX-M-15 | 1 | ND | ST224 | ||||
| Pepper leaf | blaCTX-M-55, blaTEM-1 | 1 | ND | ND | ||||
| Mapleleaf ainsliaea | blaCTX-M-27 | 1 | ND | ST10 | ||||
| Leafy greens | blaCTX-M | 2015–2016 | Enterobacterale | 1 | United States | ND | ND | [53] |
| bla CMY | Enterobacterale | 6 | ND | ND | ||||
| Frisee salad | blaCTX-M-1, blaDHA-1 | 2015–2016 | E. cloacae | 2 | Italy | ND | ND | [43] |
| Frisee salad + carrot | blaCTX-M-15 | Pantoea agglomerans | 6 | ND | ND | |||
| Peach | blaCTX-M-15 | 2013–2014 | K. pneumoniae | 1 | Algeria | aadA2, strB, strA, qnrS1, oqxB, oqxA, fosA, mph(A), catA2, sul1, sul2, dfrA12 | ST219 | [48] |
6. Vegetables and Fruit Isolates with Carbapenemase Genes
| Vegetables Type | Carbapenemase/mcr Gene | Isolation Period | Species | Isolates Number | Country | Other Antibiotic Resistance Genes | Sequence Type | Plasmid Type | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Coriander | blaOXA-181 | 2015 | Klebsiella variicola | 1 | Switzerland | qnrS1 | ND | IncX3 | [57] |
| Lettuce | blaKPC-2 and blaNDM-1 | 2015 | Escherichia coli | 1 | China | blaDHA-1, fosA3, floR aacA4, tet(D), sul1, armA, mph(E), msr(E), erm(B), strA, strB | ST877 | IncA/C (blaNDM-1), Untypable (blaKPC-2) | [60] |
| blaOXA-48 | 2016 | K. pneumoniae | 1 | Algeria | blaTEM-1 | ST391 | ND | [11] | |
| blaNDM-5 | 2017 | E. coli | 1 | China | blaCTX−M−1G, fosA3, floR | ST4762 | X3 | [61] | |
| 1 | blaCTX−M−1G, fosA3, floR, oqxAB. | ST4762 | X3 | ||||||
| blaNDM-1 | C. freundii | 1 | fosA3, floR, qnrB | ND | ND | ||||
| Parsley | blaNDM-1 | 2015 | K. pneumoniae | 1 | Japan | blaSHV-28, blaSHV-1, blaTEM-1A, blaCTX-M-15, blaCTX-M-14b, blaTEM-1A, blaOXA-9, fosA, oqxAB, tet(D), aac(69)-Ib, aadA1, aph(39)-VI, aph(6)-Id, aph(39)-VIb, aph(39’)-Ib, aac(69)-Ib-cr, qnrS1 | ST15 | ND | [59] |
| blaOXA-48 | 2016 | K. pneumoniae | 1 | Algeria | None | ND | ND | [11] | |
| blaOXA-48 | 2019 | E. cloacae | 1 | Romania | ND | ND | ND | [46] | |
| blaKPC | K. oxytoca | 1 | ND | ND | ND | ||||
| Baby leaf mix | blaNDM-1 | 2015 | K. pneumoniae | 1 | Japan | blaCTX-M-15, blaOXA-9, blaTEM-1A, blaSHV-28, blaCTX-M-14b, fosA, oqxAB, aac(69)-Ib, aadA1, aph(39)-VI, aac(69)-Ib-cr, qnrS1, aph(6)-Id, aph(39)-VIb, aph(39’)-Ib. | ST15 | ND | [59] |
| blaOXA-66, blaOXA-72 | A. baumannii | 1 | blaADC-25, blaOXA-66, blaOXA-72, sul2, tet(B), aac(3)-Ia aac(69)-Ip, aph(39’)-Ib, aph(6)-Id | ST2 | GR2 (blaOXA-72) | ||||
| Cucumber | blaKPC-2 | 2017 | K. pneumoniae | 1 | China | qnrB, oqxAB | ST23 | F35:A-:B1 | [61] |
| 1 | blaCTX−M−1G, qnrB, oqxAB | ST23 | F35:A-:B1 | ||||||
| blaNDM-5 | E. coli | 1 | fosA3, floR, qnrB | UT | ND | ||||
| 1 | blaCTX−M−1G, fosA3, floR,. | ST4762 | ND | ||||||
| blaNDM-5 and blaKPC-2 | E. coli | 1 | None | ND | ND | ||||
| Curly endive | blaNDM-5 | 2017 | E. coli | 1 | China | blaCTX−M−1G, fosA3, floR, rmtB | ST167 | ND | [61] |
| blaNDM-1 | C. freundii | 1 | fosA3, floR, qnrB | ND | ND | ||||
| 1 | fosA3, floR, oqxAB, qnrB, rmtB | ND | ND | ||||||
| 1 | fosA3, floR, qnrB | ND | X3 | ||||||
| Tomato | blaOXA-48 | 2016 | K. pneumoniae | 1 | Algeria | None | ST1877 | ND | [11] |
| blaNDM-1 | 2017 | C. freundii | 1 | China | blaCTX−M−1G, fosA3, floR, qnrB | ND | X3 | [61] | |
| Leaf rape | blaNDM-5 | 2017–2018 | E. coli | 1 | China | mcr-1, fosA3 | ST156 | X3 | [4] |
| Spinach | blaNDM-9 | 1 | mcr-1, fosA3 | ST2847 | Untypable | ||||
| blaKPC | 2019 | Morganella morganii | 1 | Romania | ND | ND | ND | [46] | |
| Vegetables (ND) | blaOXA-72 | ND | Acinetobacter calcoaceticus | 2 | Lebanon | ND | ND | ND | [58] |
| Lettuce | mcr-1 | 2013 | E. coli | 1 | Portugal | blaTEM-1, aadA1y, aph(4)-Ia, estX-12, floR, sat2, strA, strB, sul2, tetA | ST1716 | ND | [62] |
| 2013–2014 | E. coli | 1 | blaTEM-1, aac(3)-Iv, aadA1, aph(4)-Ia, aph(6)-Id, mdf(A)-type, tetA, sul2, floR | ST1716 | ND | [63] | |||
| 2016 | E. coli | 1 | China | blaCTX-M-14, floR, fosA3, oqxAB | ST795 | IncHI2 | [64] | ||
| 1 | blaCTX-M-55, floR | ST2505 | ncI2 | ||||||
| 2015 | E. coli | 1 | blaCTX-M-55, rmtB, floR, fosA3. | ST156 | IncI2 | ||||
| 2016 | E. coli | 1 | floR | ST48 | IncX4 | ||||
| 2015 | Raoultella ornithinolytica | 2 | blaCTX-M-14, floR, fosA3, oqxAB | NA | IncHI2 | ||||
| mcr-1 | 2018 | E. coli | 1 | South Korea | blaTEM-1 and blaCTX-M-55 | ST10 | ND | [65] | |
| mcr-1 | 2017–2018 | E. coli | 1 | China | ND | ST10 | X4 | [66] | |
| 1 | ND | ST2705 | HI2 | ||||||
| Tomato | mcr-1 | 2016 | E. coli | 1 | China | blaCTX-M-14, floR, fosA3, oqxAB | ST69 | IncHI2 | [64] |
| 2015 | E. coli | 2 | floR | ST206 | chromosome | ||||
| mcr-1 | 2017–2018 | E. coli | 1 | China | ND | ST713 | X4 | [66] | |
| 1 | China | ND | UT | I2 | |||||
| Leaf rape | mcr-1 | 2017–2018 | K. pneumoniae | 1 | China | blaNDM-5, fosA3 | ST156 | X4 | [4] |
| E. coli | 1 | China | ND | ST744 | X4 | [66] | |||
| Green Pepper | mcr-1 | 2017–2018 | E. cloacae | 1 | China | ND | ND | ND | [66] |
| mcr-1 | E. coli | 1 | ND | ST5873 | X4 | ||||
| Spinach | mcr-1 | 2017–2018 | E. coli | 1 | China | blaNDM-9, fosA3 | ST2847 | I2 | [4] |
| mcr-1 | 1 | ND | ST2253 | I2 | [66] | ||||
| Cha-om | mcr-1 | 2014 | E. coli | 1 | Switzerland | blaCTX-M-55 | ST167 | ND | [67] |
| Basil leaves | mcr-1 | 2014 | E. coli | 1 | blaCTX-M-65 | ST4683 | ND | ||
| Cucumber | mcr-1 | 2017–2018 | E. coli | 2 | China | ND | ST744 | X4 | [66] |
| 2 | ND | ST1115 | I2 | ||||||
| Carrot | mcr-1 | 2017–2018 | E. coli | 1 | ND | ST5539 | X4 | ||
| E. coli | 1 | ND | ST13 | I2 | |||||
| Curly endive | mcr-1 | 2017–2018 | E. coli | 1 | ND | ST13 | X4 | ||
| Pak choi | mcr-1 | 2017–2018 | E. coli | 1 | ND | ST648 | I2 | ||
| Apple | mcr-1 | 2016 | E. coli | 1 | China | aadA2, aadA1,floR, cmlA1, sul2, sul3, tetA, tetM, dfrA12,mdfA | ST189 | IncFIA | [68] |
| Orange | mcr-1 | K. pneumoniae | 1 | blaSHV-110, qnrS1, oqxA, oqxB, fosA6, sul1, tetA, dfrA1 | ST442 | IncHI1 |
7. Vegetables and Fruit Isolates with the mcr Gene
8. Contamination Pathways and Genetic Characteristics of β-Lactamases and mcr-Producing Gram-Negative Bacteria
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xylia, P.; Botsaris, G.; Chrysargyris, A.; Skandamis, P.; Tzortzakis, N. Variation of microbial load and biochemical activity of ready-to-eat salads in Cyprus as affected by vegetable type, season, and producer. Food Microbiol. 2019, 83, 200–210. [Google Scholar] [CrossRef]
- Holzel, C.S.; Tetens, J.L.; Schwaiger, K. Unraveling the role of vegetables in spreading antimicrobial-resistant bacteria: A need for quantitative risk assessment. Foodborne Pathog. Dis. 2018, 15, 671–688. [Google Scholar] [CrossRef]
- Van Hoek, A.H.; Veenman, C.; Van Overbeek, W.M.; Lynch, G.; De Roda Husman, A.M.; Blaak, H. Prevalence a characterization of ESBL- and AmpC-producing Enterobacteriaceae on retail vegetables. Int. J. Food Microbiol. 2015, 204, 1–8. [Google Scholar] [CrossRef]
- Liu, B.T.; Song, F.J. Emergence of two Escherichia coli strains co-harboring mcr-1 and bla NDM in fresh vegetables from China. Infect. Drug Resist. 2019, 12, 2627–2635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, Y.; Paterson, D.L. Carbapenemase-producing Enterobacteriaceae. Semin. Respir. Crit. Care Med. 2015, 36, 74–84. [Google Scholar]
- Bassetti, M.; Pecori, D.; Sibani, M.; Corcione, S.; De Rosa, F.G. Epidemiology and treatment of MDR Enterobacteriaceae. Curr. Treat. Options Infect. Dis. 2015, 7, 291–316. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, Q.; Zhang, S.; Zhang, J.; Yang, G.; Wang, J.; Xue, L.; Chen, M. Characterization of extended spectrum beta-lactamase-producing Enterobacteriaceae from retail food in China. Front. Microbiol. 2018, 9, 1709. [Google Scholar] [CrossRef] [PubMed]
- Bakthavatchalam, Y.D.; Pragasam, A.K.; Biswas, I.; Veeraraghavan, B. Polymyxin susceptibility testing, interpretative breakpoints and resistance mechanisms: An update. J. Glob. Antimicrob. Resist. 2018, 12, 124–136. [Google Scholar] [CrossRef]
- Richter, L.; Du Plessis, E.M.; Duvenage, S.; Korsten, L. Occurrence, identification, and antimicrobial resistance profiles of Extended-Spectrum and AmpC beta-Lactamase-producing Enterobacteriaceae from fresh vegetables retailed in Gauteng Province, South Africa. Foodborne Pathog. Dis. 2019, 16, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Zurfluh, K.; Nuesch-Inderbinen, M.; Morach, M.; Zihler, B.A.; Hachler, H.; Stephan, R. Extended-spectrum-beta-lactamase-producing Enterobacteriaceae isolated from vegetables imported from the Dominican Republic, India, Thailand, and Vietnam. Appl. Environ. Microbiol. 2015, 81, 3115–3120. [Google Scholar] [CrossRef] [Green Version]
- Touati, A.; Mairi, A.; Baloul, Y.; Lalaoui, R.; Bakour, S.; Thighilt, L.; Gharout, A.; Rolain, J.M. First detection of Klebsiella pneumoniae producing OXA-48 in fresh vegetables from Bejaia city, Algeria. J. Glob. Antimicrob. Resist. 2017, 9, 17–18. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Epidemiology of beta-Lactamase-producing pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P. Carbapenemase-producing Enterobacteriaceae: Overview of a major public health challenge. Med. Mal. Infect. 2014, 44, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Bush, K. Past and present perspectives on beta-Lactamases. Antimicrob. Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordmann, P.; Poirel, L. Epidemiology and diagnostics of carbapenem resistance in Gram-negative bacteria. Clin. Infect. Dis. 2019, 69, S521–S528. [Google Scholar] [CrossRef] [Green Version]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Kooguchi, K.; Moriyama, K. Molecular diversity of extended-spectrum beta-lactamases and carbapenemases, and antimicrobial resistance. J. Intensive Care 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meini, S.; Tascini, C.; Cei, M.; Sozio, E.; Rossolini, G.M. AmpC beta-lactamase-producing Enterobacterales: What a clinician should know. Infection 2019, 47, 363–375. [Google Scholar] [CrossRef]
- Hennequin, C.; Ravet, V.; Robin, F. Plasmids carrying DHA-1 beta-lactamases. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1197–1209. [Google Scholar] [CrossRef]
- Doi, Y. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin. Infect. Dis. 2019, 69, S565–S575. [Google Scholar] [CrossRef] [Green Version]
- Yigit, H.; Queenan, A.M.; Anderson, G.J.; Domenech-Sanchez, A.; Biddle, J.W.; Steward, C.D.; Alberti, S.; Bush, K.; Tenover, F.C. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1151–1161. [Google Scholar] [CrossRef] [Green Version]
- Elshamy, A.A.; Aboshanab, K.M. A review on bacterial resistance to carbapenems: Epidemiology, detection and treatment options. Future Sci. OA 2020, 6, FSO438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupo, A.; Papp-Wallace, K.M.; Sendi, P.; Bonomo, R.A.; Endimiani, A. Non-phenotypic tests to detect and characterize antibiotic resistance mechanisms in Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2013, 77, 179–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefaniuk, E.M.; Tyski, S. Colistin resistance in Enterobacterales strains—A current view. Pol. J. Microbiol. 2019, 68, 417–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, T.; Domingues, S.; Da Silva, G.J. Plasmid-mediated colistin resistance in Salmonella enterica: A review. Microorganisms 2019, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Gharaibeh, M.H.; Shatnawi, S.Q. An overview of colistin resistance, mobilized colistin resistance genes dissemination, global responses, and the alternatives to colistin: A review. Vet. World 2019, 12, 1735–1746. [Google Scholar] [CrossRef] [Green Version]
- Mendes Oliveira, V.R.; Paiva, M.C.; Lima, W.G. Plasmid-mediated colistin resistance in Latin America and Caribbean: A systematic review. Travel. Med. Infect. Dis. 2019, 31, 101459. [Google Scholar] [CrossRef]
- Kai, J.; Wang, S. Recent progress on elucidating the molecular mechanism of plasmid-mediated colistin resistance and drug design. Int. Microbiol. 2019, 23, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Anyanwu, M.U.; Jaja, I.F.; Nwobi, O.C. Occurrence and characteristics of mobile colistin resistance (mcr) gene-containing isolates from the environment: A review. Int. J. Environ. Res. Public Health 2020, 17, 1028. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Hinchliffe, P.; Yang, Q.E.; Portal, E.; Young, T.; Li, H.; Tooke, C.L.; Carvalho, M.J.; Paterson, N.G.; Brem, J.; Niumsup, P.R.; et al. Insights into the mechanistic basis of plasmid-mediated colistin resistance from crystal structures of the catalytic domain of MCR-1. Sci. Rep. 2017, 7, 39392. [Google Scholar] [CrossRef] [Green Version]
- Xavier, B.B.; Lammens, C.; Ruhal, R.; Kumar-Singh, S.; Butaye, P.; Goossens, H.; Malhotra-Kumar, S. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Eurosurveillance 2016, 21, 30280. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Li, H.; Shen, Y.; Liu, Z.; Wang, S.; Shen, Z.; Zhang, R.; Walsh, T.R.; Shen, J.; Wang, Y. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. MBio 2017, 8, e00543-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carattoli, A.; Villa, L.; Feudi, C.; Curcio, L.; Orsini, S.; Luppi, A.; Pezzotti, G.; Magistrali, C.F. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Eurosurveillance 2017, 22, 30589. [Google Scholar] [CrossRef] [Green Version]
- Borowiak, M.; Fischer, J.; Hammerl, J.A.; Hendriksen, R.S.; Szabo, I.; Malorny, B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother. 2017, 72, 3317–3324. [Google Scholar] [CrossRef] [Green Version]
- AbuOun, M.; Stubberfield, E.J.; Duggett, N.A.; Kirchner, M.; Dormer, L.; Nunez-Garcia, J.; Randall, L.P.; Lemma, F.; Crook, D.W.; Teale, C.; et al. mcr-1 and mcr-2 (mcr-6.1) variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J. Antimicrob. Chemother. 2018, 73, 2904. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Li, Y.X.; Lei, C.W.; Zhang, A.Y.; Wang, H.N. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J. Antimicrob. Chemother. 2018, 73, 1791–1795. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Y.; Zhou, Y.; Li, J.; Yin, W.; Wang, S.; Zhang, S.; Shen, J.; Shen, Z.; Wang, Y. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg. Microbes Infect. 2018, 7, 122. [Google Scholar] [CrossRef] [Green Version]
- Carroll, L.M.; Gaballa, A.; Guldimann, C.; Sullivan, G.; Henderson, L.O.; Wiedmann, M. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica Serotype Typhimurium isolate. MBio 2019, 10, e00853-19. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Feng, Y.; Liu, L.; Wei, L.; Kang, M.; Zong, Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg. Microbes Infect. 2020, 9, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Blaak, H.; Van Hoek, A.H.; Veenman, C.; Docters Van Leeuwen, A.E.; Lynch, G.; Van Overbeek, W.M.; De Roda Husman, A.M. Extended spectrum β-lactamase- and constitutively AmpC-producing Enterobacteriaceae on fresh produce and in the agricultural environment. Int. J. Food Microbiol. 2014, 168, 8–16. [Google Scholar] [CrossRef]
- Iseppi, R.; De, N.S.; Bondi, M.; Messi, P.; Sabia, C. Extended-spectrum beta-lactamase, AmpC, and MBL-producing Gram-negative bacteria on fresh vegetables and ready-to-eat salads sold in local markets. Microb. Drug Resist. 2018, 24, 1156–1164. [Google Scholar] [CrossRef]
- Nuesch-Inderbinen, M.; Zurfluh, K.; Peterhans, S.; Hachler, H.; Stephan, R. Assessment of the prevalence of Extended-Spectrum beta-Lactamase-producing Enterobacteriaceae in ready-to-eat Salads, fresh-cut fruit, and sprouts from the Swiss market. J. Food Prot. 2015, 78, 1178–1181. [Google Scholar] [CrossRef]
- Freitag, C.; Michael, G.B.; Li, J.; Kadlec, K.; Wang, Y.; Hassel, M.; Schwarz, S. Occurrence and characterisation of ESBL-encoding plasmids among Escherichia coli isolates from fresh vegetables. Vet. Microbiol. 2018, 219, 63–69. [Google Scholar] [CrossRef]
- Colosi, I.A.; Baciu, A.M.; Opris, R.V.; Peca, L.; Gudat, T.; Simon, L.M.; Colosi, H.A.; Costache, C. Prevalence of ESBL, AmpC and carbapenemase-producing Enterobacterales isolated from raw vegetables retailed in Romania. Foods 2020, 9, 1726. [Google Scholar] [CrossRef]
- Richter, L.; Du Plessis, E.M.; Duvenage, S.; Korsten, L. Occurrence, phenotypic and molecular characterization of extended-spectrum- and AmpC- β-Lactamase producing Enterobacteriaceae isolated from selected commercial spinach supply chains in South Africa. Front. Microbiol. 2020, 11, 638. [Google Scholar] [CrossRef]
- Mesbah, Z.F.; Granier, S.A.; Touati, A.; Millemann, Y. Occurrence of third-generation cephalosporins-resistant Klebsiella pneumoniae in fresh fruits and vegetables purchased at markets in Algeria. Microb. Drug Resist. 2020, 26, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.; Ozeki, K.; Komatsu, T.; Fukuda, A.; Tamura, Y. Prevalence of extended-spectrum beta-lactamase-producing bacteria on fresh vegetables in Japan. J. Food Prot. 2019, 82, 1663–1666. [Google Scholar] [CrossRef] [PubMed]
- Kurittu, P.; Khakipoor, B.; Aarnio, M.; Nykasenoja, S.; Brouwer, M.; Myllyniemi, A.L.; Vatunen, E.; Heikinheimo, A. Plasmid-borne and chromosomal ESBL/AmpC genes in Escherichia coli and Klebsiella pneumoniae in global food products. Front. Microbiol. 2021, 12, 592291. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Oh, S.S.; Kim, J.; Shin, J. Extended-spectrum β-lactamase-producing Escherichia coli isolated from raw vegetables in South Korea. Sci. Rep. 2020, 10, 19721. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, N.; Muraleedharan, C.; Talreja, D.; Rana, S.W.; Walia, S.; Kumar, A.; Walia, S.K. Occurrence of multidrug resistant extended spectrum beta-lactamase-producing bacteria on iceberg lettuce retailed for human consumption. BioMed Res. Int. 2015, 2015, 547547. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.; Albers, A.; Mollenkopf, D.; Korec, D.; Mathys, D.; Stuever, D.; Wittum, T. AmpC- and Extended-Spectrum β-Lactamase-producing Enterobacteriaceae detected in fresh produce in central Ohio. J. Food Prot. 2021, 84, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paredes, D.; Barba, P.; Mena-Lopez, S.; Espinel, N.; Zurita, J. Escherichia coli hyperepidemic clone ST410-A harboring blaCTX-M-15 isolated from fresh vegetables in a municipal market in Quito-Ecuador. Int. J. Food Microbiol. 2018, 280, 41–45. [Google Scholar] [CrossRef]
- Jung, D.; Rubin, J.E. Identification of antimicrobial resistant bacteria from plant-based food products imported into Canada. Int. J. Food Microbiol. 2020, 319, 108509. [Google Scholar] [CrossRef]
- Lopes, R.; Fuentes-Castillo, D.; Fontana, H.; Rodrigues, L.; Dantas, K.; Cerdeira, L.; Henriques, I.; Lincopan, N. Endophytic lifestyle of global clones of Extended-Spectrum β-Lactamase-producing priority pathogens in fresh vegetables: A trojan horse strategy favoring human colonization? MSystems 2021, 6, e01125-20. [Google Scholar] [CrossRef]
- Zurfluh, K.; Poirel, L.; Nordmann, P.; Klumpp, J.; Stephan, R. First detection of Klebsiella variicola producing OXA-181 carbapenemase in fresh vegetable imported from Asia to Switzerland. Antimicrob. Resist. Infect. Control 2015, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Al Atrouni, A.; Kempf, M.; Eveillard, M.; Rafei, R.; Hamze, M.; Joly-Guillou, M.L. First report of Oxa-72-producing Acinetobacter calcoaceticus in Lebanon. New Microbes New Infect. 2016, 9, 11–12. [Google Scholar] [CrossRef] [Green Version]
- Soliman, A.M.; Nariya, H.; Tanaka, D.; Yu, L.; Hisatsune, J.; Kayama, S.; Kondo, K.; Sugai, M.; Shimamoto, T.; Shimamoto, T. Vegetable-derived carbapenemase-producing high-risk Klebsiella pneumoniae ST15 and Acinetobacter baumannii ST2 clones in Japan: Coexistence of bla (NDM-1), bla (OXA-66), bla (OXA-72), and an AbaR4-Like Resistance Island in the Same Sample. Appl. Environ. Microbiol. 2021, 87, e02166-20. [Google Scholar] [CrossRef]
- Wang, J.; Yao, X.; Luo, J.; Lv, L.; Zeng, Z.; Liu, J.H. Emergence of Escherichia coli co-producing NDM-1 and KPC-2 carbapenemases from a retail vegetable, China. J. Antimicrob. Chemother. 2018, 73, 252–254. [Google Scholar] [CrossRef]
- Liu, B.T.; Zhang, X.Y.; Wan, S.W.; Hao, J.J.; Jiang, R.D.; Song, F.J. Characteristics of carbapenem-resistant Enterobacteriaceae in ready-to-eat vegetables in China. Front. Microbiol. 2018, 9, 1147. [Google Scholar] [CrossRef] [Green Version]
- Jones-Dias, D.; Manageiro, V.; Ferreira, E.; Barreiro, P.; Vieira, L.; Moura, I.B.; Canica, M. Architecture of class 1, 2, and 3 integrons from Gram negative bacteria recovered among fruits and vegetables. Front. Microbiol. 2016, 7, 1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manageiro, V.; Jones-Dias, D.; Ferreira, E.; Canica, M. Plasmid-mediated colistin resistance (mcr-1) in Escherichia coli from non-imported fresh vegetables for human consumption in Portugal. Microorganisms 2020, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Yao, X.; Lv, L.; Doi, Y.; Huang, X.; Huang, S.; Liu, J.H. Emergence of mcr-1 in Raoultella ornithinolytica and Escherichia coli Isolates from retail vegetables in China. Antimicrob. Agents Chemother. 2017, 61, e01139-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.S.; Song, J.; Kim, J.; Shin, J. Increasing prevalence of multidrug-resistant mcr-1-positive Escherichia coli isolates from fresh vegetables and healthy food animals in South Korea. Int. J. Infect. Dis. 2020, 92, 53–55. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.T.; Li, X.; Zhang, Q.; Shan, H.; Zou, M.; Song, F.J. Colistin-resistant mcr-positive Enterobacteriaceae in fresh vegetables, an increasing infectious threat in China. Int. J. Antimicrob. Agents 2019, 54, 89–94. [Google Scholar] [CrossRef]
- Zurfuh, K.; Poirel, L.; Nordmann, P.; Nuesch-Inderbinen, M.; Hachler, H.; Stephan, R. Occurrence of the plasmid-borne mcr-1 colistin resistance gene in Extended-Spectrum-beta-Lactamase-producing Enterobacteriaceae in river water and imported vegetable samples in Switzerland. Antimicrob. Agents Chemother. 2016, 60, 2594–2595. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Shen, C.; Zheng, X.; Liu, Y.; El-Sayed Ahmed, M.A.E.; Zhao, Z.; Liao, K.; Shi, Y.; Guo, X.; Zhong, R.; et al. Plasmid-mediated colistin resistance gene mcr-1 in Escherichia coli and Klebsiella pneumoniae isolated from market retail fruits in Guangzhou, China. Infect. Drug Resist. 2019, 12, 385–389. [Google Scholar] [CrossRef] [Green Version]
- Chong, Y.; Shimoda, S.; Shimono, N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 2018, 61, 185–188. [Google Scholar] [CrossRef]
- Wu, W.; Feng, Y.; Tang, G.; Qiao, F.; McNally, A.; Zong, Z. NDM Metallo-β-lactamases and their bacterial producers in health care settings. Clin. Microbiol. Rev. 2019, 32, e00115-18. [Google Scholar] [CrossRef] [Green Version]
- Pu, C.; Yu, Y.; Diao, J.; Gong, X.; Li, J.; Sun, Y. Exploring the persistence and spreading of antibiotic resistance from manure to biocompost, soils and vegetables. Sci. Total Environ. 2019, 688, 262–269. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Hu, H.W.; Chen, Q.L.; Singh, B.K.; Yan, H.; Chen, D.; He, J.Z. Transfer of antibiotic resistance from manure-amended soils to vegetable microbiomes. Environ. Int. 2019, 130, 104912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Hu, H.W.; Gou, M.; Wang, J.T.; Chen, D.; He, J.Z. Temporal succession of soil antibiotic resistance genes following application of swine, cattle and poultry manures spiked with or without antibiotics. Environ. Pollut. 2017, 231, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Chen, Q.; Chen, S.; Zhu, Y.G. Does organically produced lettuce harbor higher abundance of antibiotic resistance genes than conventionally produced? Environ. Int. 2017, 98, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qiu, T.; Gao, M.; Shi, M.; Zhang, H.; Wang, X. Inorganic and organic fertilizers application enhanced antibiotic resistome in greenhouse soils growing vegetables. Ecotoxicol. Environ. Saf. 2019, 179, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; He, T.; Zhang, S.; Zhu, L.; Shang, B.; Li, Z.; Wang, R. Occurrence of seventeen veterinary antibiotics and resistant bacterias in manure-fertilized vegetable farm soil in four provinces of China. Chemosphere 2019, 215, 234–240. [Google Scholar] [CrossRef]
- Peng, S.; Feng, Y.; Wang, Y.; Guo, X.; Chu, H.; Lin, X. Prevalence of antibiotic resistance genes in soils after continually applied with different manure for 30 years. J. Hazard Mater. 2017, 340, 16–25. [Google Scholar] [CrossRef]
- Adegoke, A.A.; Amoah, I.D.; Stenstrom, T.A.; Verbyla, M.E.; Mihelcic, J.R. Epidemiological evidence and health risks associated xith agricultural reuse of partially treated and untreated wastewater: A review. Front. Public Health 2018, 6, 337. [Google Scholar] [CrossRef] [Green Version]
- Araujo, S.; Silva, A.T.; Tacao, M.; Patinha, C.; Alves, A.; Henriques, I. Characterization of antibiotic resistant and pathogenic Escherichia coli in irrigation water and vegetables in household farms. Int. J. Food Microbiol. 2017, 257, 192–200. [Google Scholar] [CrossRef]
- Makkaew, P.; Miller, M.; Fallowfield, H.J.; Cromar, N.J. Microbial risk in wastewater irrigated lettuce: Comparing Escherichia coli contamination from an experimental site with a laboratory approach. Water Sci. Technol. 2016, 74, 749–755. [Google Scholar] [CrossRef]
- Antwi-Agyei, P.; Cairncross, S.; Peasey, A.; Price, V.; Bruce, J.; Baker, K.; Moe, C.; Ampofo, J.; Armah, G.; Ensink, J. A farm to fork risk assessment for the use of wastewater in agriculture in Accra, Ghana. PLoS ONE 2015, 10, e0142346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelaghma, W.; Loucif, L.; Bendahou, M.; Rolain, J.-M. Vegetables and Fruit as a Reservoir of β-Lactam and Colistin-Resistant Gram-Negative Bacteria: A Review. Microorganisms 2021, 9, 2534. https://doi.org/10.3390/microorganisms9122534
Chelaghma W, Loucif L, Bendahou M, Rolain J-M. Vegetables and Fruit as a Reservoir of β-Lactam and Colistin-Resistant Gram-Negative Bacteria: A Review. Microorganisms. 2021; 9(12):2534. https://doi.org/10.3390/microorganisms9122534
Chicago/Turabian StyleChelaghma, Widad, Lotfi Loucif, Mourad Bendahou, and Jean-Marc Rolain. 2021. "Vegetables and Fruit as a Reservoir of β-Lactam and Colistin-Resistant Gram-Negative Bacteria: A Review" Microorganisms 9, no. 12: 2534. https://doi.org/10.3390/microorganisms9122534






