Immunocompetent Mice Infected by Two Lineages of Dengue Virus Type 2: Observations on the Pathology of the Lung, Heart and Skeletal Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Viral Strains
2.3. Study Design
2.4. Histopathology
2.5. Immunohistochemistry
2.6. Transmission Electron Microscopy (TEM)
2.7. Histomorphometry
2.8. CK Levels Analysis
2.9. Viral Genome Extraction and Quantitation
2.10. Statistical Analisys
3. Results
3.1. Organ Weight
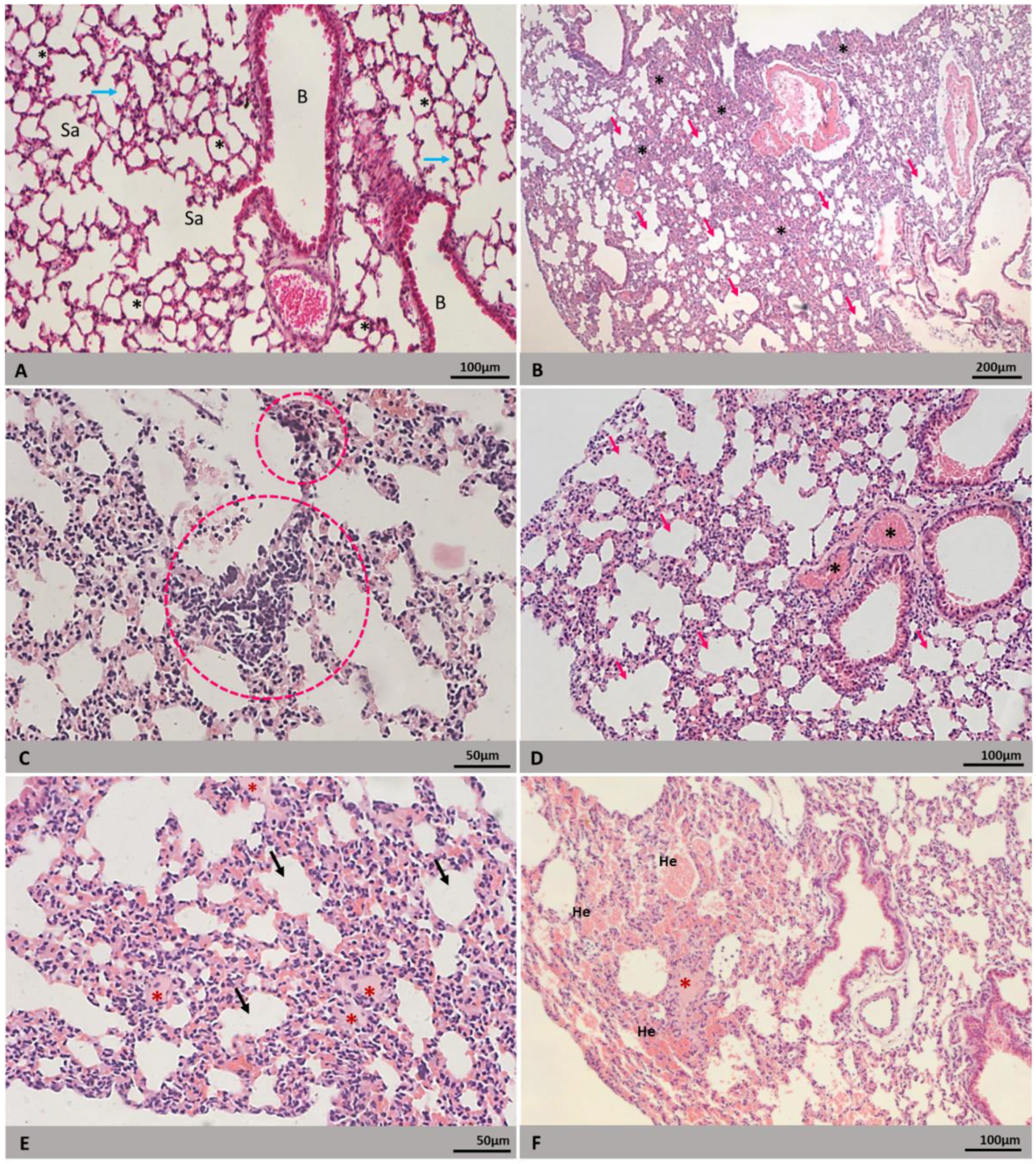
3.2. Morphology
3.3. Morphometry
3.4. Viral Genome and Antigen Detection
3.5. Creatine Kinase (CK) Levels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dengue and Severe Dengue. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 10 August 2021).
- Castro, M.C.; Wilson, M.E.; Bloom, D.E. Disease and economic burdens of dengue. Lance Infect. Dis. 2017, 17, e70–e78. [Google Scholar] [CrossRef]
- Gubler, D.J. Perspectives on the prevention and control of dengue hemorrhagic fever. Gaoxiong Yi Xue Ke Xue Za Zhi = Kaohsiung J. Med. Sci. 1994, 10, S15–S18. [Google Scholar]
- Edelman, R.; Hombach, J. “Guidelines for the clinical evaluation of dengue vaccines in endemic areas”: Summary of a World Health Organization Technical Consultation. Vaccine 2008, 26, 4113–4119. [Google Scholar] [CrossRef]
- Martina, B.E.; Koraka, P.; Osterhaus, A.D. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef]
- Póvoa, T.F.; Alves, A.M.; Oliveira, C.A.; Nuovo, G.J.; Chagas, V.L.; Paes, M.V. The pathology of severe dengue in multi-ple organs of human fatal cases: Histopathology, ultrastructure and virus replication. PLoS ONE 2014, 9, e83386. [Google Scholar] [CrossRef]
- Jessie, K.; Fong, M.Y.; Devi, S.; Lam, S.K.; Wong, K.T. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 2004, 189, 1411–1418. [Google Scholar] [CrossRef]
- Gulati, S.; Maheshwari, A. Atypical manifestations of dengue. Trop. Med. Int. Health 2007, 12, 1087–1095. [Google Scholar] [CrossRef]
- Estofolete, C.F.; de Oliveira Mota, M.T.; Bernardes Terzian, A.C.; de Aguiar Milhim, B.; Ribeiro, M.R.; Nunes, D.V.; Mourão, M.P.; Rossi, S.L.; Nogueira, M.L.; Vasilakis, N. Unusual clinical manifestations of dengue disease—Real or imagined? Acta Trop. 2019, 199, 105134. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.S.; Brum, A.L.; Paes, M.V.; Póvoa, T.F.; Basilio-de-Oliveira, C.A.; Marchiori, E.; Borghi, D.P.; Ramos, G.V.; Bozza, F.A. Lung in dengue: Computed tomography findings. PLoS ONE 2014, 9, e96313. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, E.; Hochhegger, B.; Zanetti, G. Pulmonary manifestations of dengue. J. Bras. Pneumol. 2020, 46, e20190246. [Google Scholar] [CrossRef] [PubMed]
- Hitchens, A.P.; Siler, J.F.; Hall, M.W. Dengue: Its history, epidemiology, mechanism of transmission, etiology, clinical mani-festations, immunity, and prevention. Manila Bur. Print. 1926, 29, 1–304. [Google Scholar]
- Obeyesekere, I.; Hermon, Y. Myocarditis and cardiomyopathy after arbovirus infections (dengue and chikungunya fever). Br. Heart J. 1972, 34, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Salgado, D.M.; Eltit, J.M.; Mansfield, K.; Panqueba, C.; Castro, D.; Vega, M.R.; Xhaja, K.; Schmidt, D.; Martin, K.J.; Allen, P.D.; et al. Heart and skeletal muscle are targets of dengue virus infection. J. Pediatr. Infect. Dis. 2010, 29, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Pereda, M.G.; López, M.; Mariluz, M. Dengue complicado y miocarditis: Comunicación de un caso [Myocarditis and compli-cated dengue: A case report]. Rev. Chilena Infectol. 2015, 32, 238–239. [Google Scholar] [CrossRef]
- Tahir, H.; Daruwalla, V.; Hayat, S. Myocarditis leading to severe dilated cardiomyopathy in a patient with dengue Fever. Case Rep. Cardiol. 2015, 2015, 319312. [Google Scholar] [CrossRef]
- Bhatt, M.; Soneja, M.; Farooqui, F.A.; Singla, P.; Vikram, N.K.; Biswas, A.; Roy, A.; Wig, N. Myocarditis in admitted patients with dengue fever. Infection 2020, 48, 899–903. [Google Scholar] [CrossRef]
- Abhinayaa, J.; James, S.; Jebaraj, R.; Vinoth, P.N. Incidence of Cardiac Manifestations in Children with Dengue Fever: A Cross-sectional Study. Rambam Maimonides Med. J. 2021, 12, e0014. [Google Scholar] [CrossRef]
- Filippone, C.; Legros, V.; Jeannin, P.; Choumet, V.; Butler-Browne, G.; Zoladek, J.; Mouly, V.; Gessain, A.; Ceccaldi, P.E. Arboviruses and Muscle Disorders: From Disease to Cell Biology. Viruses 2020, 12, 616. [Google Scholar] [CrossRef]
- Seet, R.C.S.; Quek, A.M.L.; Lim, E.C.H. Post-infectious fatigue syndrome in dengue infection. J. Clin. Virol. 2007, 38, 1–6. [Google Scholar] [CrossRef]
- Huerta-Alardín, A.L.; Varon, J.; Marik, P.E. Bench-to-bedside review: Rhabdomyolysis—An overview for clinicians. Crit. Care 2005, 9, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Misra, U.K.; Kalita, J.; Maurya, P.K.; Kumar, P.; Shankar, S.K.; Mahadevan, A. Dengue-associated transient muscle dys-function: Clinical, electromyography and histopathological changes. Infection 2012, 40, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Malheiros, S.M.F.; Oliveira, A.S.B.; Schmidt, B.; Camargo Lima, J.G.; Gabbai, A.A. Dengue: Muscle biopsy findings in 15 pa-tients. Arq. Neuro-Psiquiatr. 1993, 51, 159–164. [Google Scholar] [CrossRef]
- Davis, J.S.; Bourke, P. Rhabdomyolysis associated with dengue virus infection. Clin. Infect. Dis. 2004, 38, e109–e111. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Shukla, S.; Mahajan, S.N.; Diwan, S.K. Acute dengue myositis with rhabdomyolysis and acute renal failure. Ann. Indian Acad. Neurol. 2010, 13, 221–222. [Google Scholar] [CrossRef]
- Tansir, G.; Gupta, C.; Mehta, S.; Kumar, P.; Soneja, M.; Biswas, A. Expanded dengue syndrome in secondary dengue infection: A case of biopsy proven rhabdomyolysis induced acute kidney injury with intracranial and intraorbital bleeds. Intractable Rare Dis. Res. 2017, 6, 314–318. [Google Scholar] [CrossRef]
- Gulati, K.; Pasi, R.; Gupta, A.; Ravi, K.S. Dengue fever presenting with severe myositis—An unusual presentation. J. Family Med. Prim. Care 2020, 9, 6285–6287. [Google Scholar] [CrossRef]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Halstead, S.B. Pathogenesis of Dengue: Dawn of a New Era. F1000Research 2015, 4, 1353. [Google Scholar] [CrossRef]
- Nunes, P.; de Filippis, A.; Lima, M.; Faria, N.; de Bruycker-Nogueira, F.; Santos, J.B.; Heringer, M.; Chouin-Carneiro, T.; Couto-Lima, D.; de Santis Gonçalves, B.; et al. 30 years of dengue fatal cases in Brazil: A laboratorial-based investigation of 1047 cases. BMC Infect. Dis. 2018, 18, 346. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.F.; Galvão Araujo, J.M.; Ferreira, O.C., Jr.; Ferreira, D.F.; Lima, D.B.; Santos, F.B.; Schatzmayr, H.G.; Tanuri, A.; Ribeiro Nogueira, R.M. Two lineages of dengue virus type 2, Brazil. Emerg. Infect. Dis. 2010, 16, 576–578. [Google Scholar] [CrossRef]
- Faria, N.R.; Nogueira, R.M.; de Filippis, A.M.; Simões, J.B.; Nogueira, F.; da Rocha Queiroz Lima, M.; dos Santos, F.B. Twenty years of DENV-2 activity in Brazil: Molecular characterization and phylogeny of strains isolated from 1990 to 2010. PLoS Negl. Trop. Dis. 2013, 7, e2095. [Google Scholar] [CrossRef]
- Mir, D.; Romero, H.; Fagundes de Carvalho, L.M.; Bello, G. Spatiotemporal dynamics of DENV-2 Asian-American genotype lineages in the Americas. PLoS ONE 2014, 9, e98519. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.C.; Nogueira, F.B.; Fernandes, C.A.; Meira, G.L.S.; Aguiar, S.F.; Chieppe, A.O.; de Filippis, A.M.B. Re-introduction of Dengue Virus Serotype 2 in the State of Rio De Janeiro After Almost a Decade of Epidemiological Silence. PLoS ONE 2019, 11, e0225879. [Google Scholar] [CrossRef]
- Cologna, R.; Armstrong, P.M.; Rico-Hesse, R. Selection for virulent dengue viruses occurs in humans and mosquitoes. J. Virol. 2005, 79, 853–859. [Google Scholar] [CrossRef]
- Nogueira, R.M.R.; Araújo, J.M.G.; Schatzmayr, H.G. Dengue viroses in Brazil, 1986–2006. Rev. Panam. Salud Publica 2007, 22, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Macedo, G.A.; de Araújo, J.M.; Schatzmayr, H.G.; Costa, F.A.; de Filippis, A.M.; Santos, F.B.; Nogueira, R.M. Virological surveillance for early warning of dengue epidemics in the State of Rio de Janeiro, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 141–146. [Google Scholar] [CrossRef]
- Barreto, D.F.; Takiya, C.M.; Paes, M.V.; Farias-Filho, J.; Pinhão, A.T.; Alves, A.M.; Costa, S.M.; Barth, O.M. Histopathological aspects of Dengue-2 virus infected mice tissues and complementary virus isolation. J. Submicrosc. Cytol. Pathol. 2004, 36, 121–130. [Google Scholar] [PubMed]
- Barreto, D.F.; Takiya, C.M.; Schatzmayr, H.G.; Nogueira, R.M.; Farias-Filho, J.; Barth, O.M. Histopathological and ultras-tructural aspects of mice lungs experimentally infected with dengue virus serotype 2. Mem. Inst. Oswaldo Cruz 2007, 102, 175–182. [Google Scholar] [CrossRef]
- Barth, O.M.; Barreto, D.F.; Paes, M.V.; Takiya, C.M.; Pinhão, A.T.; Schatzmayr, H.G. Morphological studies in a model for dengue-2 virus infection in mice. Mem. Inst. Oswaldo Cruz 2006, 101, 905–915. [Google Scholar] [CrossRef][Green Version]
- Paes, M.V.; Pinhão, A.T.; Barreto, D.F.; Costa, S.M.; Oliveira, M.P.; Nogueira, A.C.; Takiya, C.M.; Farias-Filho, J.C.; Schatz-mayr, H.G.; Alves, A.M.; et al. Liver injury and viremia in mice infected with dengue-2 virus. Virology 2005, 338, 236–246. [Google Scholar] [CrossRef]
- Paes, M.V.; Lenzi, H.L.; Nogueira, A.C.; Nuovo, G.J.; Pinhão, A.T.; Mota, E.M.; Basílio-de-Oliveira, C.A.; Schatzmayr, H.; Barth, O.M.; Alves, A.M. Hepatic damage associated with dengue-2 virus replication in liver cells of BALB/c mice. Lab. Investig. 2009, 89, 1140–1151. [Google Scholar] [CrossRef]
- Velandia-Romero, M.L.; Acosta-Losada, O.; Castellanos, J.E. In vivo infection by a neuroinvasive neurovirulent dengue virus. J. Neurovirol. 2012, 18, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Rasinhas, A.C. Estudo do Tropismo do Vírus Dengue Tipo 4 em Modelo BALB/c: Infecção Experimental, Análises Morfológicas e de Viremia. Master’s Thesis, Instituto Oswaldo Cruz-Fiocruz, Rio de Janeiro, Brazil, 2017. Available online: https://www.arca.fiocruz.br/handle/icict/27440 (accessed on 2 August 2021).
- Rasinhas, A.D.C.; Silva, M.A.N.D.; Caldas, G.C.; Jácome, F.C.; Leonardo, R.; Santos, F.B.D.; Nunes, P.C.G.; Barth, O.M.; Barreto-Vieira, D.F. First detection of dengue virus in the saliva of immunocompetent murine model. Mem. Inst. Oswaldo Cruz 2018, 113, e170208. [Google Scholar] [CrossRef]
- Sakinah, S.; Priya, S.P.; Kumari, S.; Amira, F.; Alsaeedy, H.; Ling, M.P.; Chee, H.Y.; Higuchi, A.; Alarfaj, A.A.; Munusamy, M.A.; et al. Impact of dengue virus (serotype DENV-2) infection on liver of BALB/c mice: A histopathological analysis. Tissue Cell 2017, 49, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Caldas, G.C. Modelo Murino Imunocompetente Para Estudo Da Infecção Pelo Vírus Dengue 3: Aspectos Morfológicos, Viremia E Tropismo. Master’s thesis, Instituto Oswaldo Cruz–Fiocruz, Rio de Janeiro, Brazil, 2019. Available online: https://www.arca.fiocruz.br/handle/icict/37732 (accessed on 2 August 2021).
- Salomão, N.G.; Rabelo, K.; Póvoa, T.F.; Alves, A.; da Costa, S.M.; Gonçalves, A.; Amorim, J.F.; Azevedo, A.S.; Nunes, P.; Basílio-de-Oliveira, C.A.; et al. BALB/c mice infected with DENV-2 strain 66985 by the intravenous route display in-jury in the central nervous system. Sci. Rep. 2018, 8, 9754. [Google Scholar] [CrossRef]
- Jácome, F.C.; Caldas, G.C.; Rasinhas, A.; de Almeida, A.; de Souza, D.; Paulino, A.C.; Leonardo, R.; Barth, O.M.; Dos Santos, F.B.; Barreto-Vieira, D.F. Comparative analysis of liver involvement caused by two DENV-2 lineages using an immunocom-petent murine model. Sci. Rep. 2021, 11, 9723. [Google Scholar] [CrossRef] [PubMed]
- Jácome, F.C.; Caldas, G.C.; Rasinhas, A.; de Almeida, A.; de Souza, D.; Paulino, A.C.; Barth, O.M.; Dos Santos, F.B.; Barreto-Vieira, D.F. Brazilian Dengue Virus Type 2-Associated Renal Involvement in a Murine Model: Outcomes after Infection by Two Lineages of the Asian/American Genotype. Pathogens 2021, 10, 1084. [Google Scholar] [CrossRef]
- Kangussu, L.M.; Costa, V.V.; Olivon, V.C.; Queiroz-Junior, C.M.; Gondim, A.; Melo, M.B.; Reis, D.; Nóbrega, N.; Araújo, N.; Rachid, M.A.; et al. Dengue virus infection induces inflammation and oxidative stress on the heart. Heart 2021. Advance online publication. [Google Scholar] [CrossRef]
- Sakinah, S.; Priya, S.P.; Mok, P.L.; Munisvaradass, R.; Teh, S.W.; Sun, Z.; Alzahrani, B.; Abu Bakar, F.; Chee, H.Y.; Awang Hamat, R.; et al. Stem Cell Therapy in Dengue Virus-Infected BALB/C Mice Improves Hepatic Injury. Front. Cell Dev. Biol. 2021, 9, 637270. [Google Scholar] [CrossRef]
- Gubler, D.J.; Kuno, G.; Sather, G.E.; Velez, M.; Oliver, A. Mosquito cell cultures and specific monoclonal antibodies in sur-veillance for dengue viruses. Am. J. Trop. Med. Hyg. 1984, 33, 158–165. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Calisher, C.H.; Gubler, D.J.; Chang, G.J.; Vorndam, A.V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 545–551. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Barreto, D.F.; Barth, M.O.; Schatzmayr, H.G. Modelo Animal Experimental Para o Estudo da Patogênese dos Vírus Dengue Sorotipos 1 e 2; Editora Interciência: Rio de Janeiro, Brazil, 2010; pp. 62–63. [Google Scholar]
- Johnson, B.W.; Russell, B.J.; Lanciotti, R.S. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J. Clin. Microbiol. 2005, 43, 4977–4983. [Google Scholar] [CrossRef]
- Rothman, A.L.; Ennis, F.A. Immunopathogenesis of Dengue hemorrhagic fever. Virology 1999, 257, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.J.; Reis, A.F.; de Almeida, F.C.; Souza, L.A.; Abukater, M.; Gomes, M.A.; Abicair, O.A.; Gonçalves, P.A. Al-teration in the erythrocyte sedimentation rate in dengue patients: Analysis of 1398 cases. Braz. J. Infect. Dis. 2008, 12, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.M.; Schatzmayr, H.G.; de Filippis, A.M.; dos Santos, F.B.; da Cunha, R.V.; Coelho, J.O.; de Souza, L.J.; Guimarães, F.R.; de Araújo, E.S.; De Simone, T.S.; et al. Dengue virus type 3, Brazil, 2002. Emerg. Infect. Dis. 2005, 11, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue Control. Epidemiology. 2018. Available online: http://www.who.int/denguecontrol/epidemiology/en/ (accessed on 10 August 2021).
- Lima, M.; Nogueira, R.M.; Schatzmayr, H.G.; de Filippis, A.M.; Limonta, D.; dos Santos, F.B. A new approach to dengue fatal cases diagnosis: NS1 antigen capture in tissues. PLoS Negl. Trop. Dis. 2011, 5, e1147. [Google Scholar] [CrossRef]
- Cunha, M.; Duarte-Neto, A.N.; Pour, S.Z.; Hajjar, L.A.; Frassetto, F.P.; Dolhnikoff, M.; Saldiva, P.; Zanotto, P. Systemic dengue infection associated with a new dengue virus type 2 introduction in Brazil—A case report. BMC Infect. Dis. 2021, 21, 311. [Google Scholar] [CrossRef]
- Miranda, C.H.; Borges, M.; Schmidt, A.; Pazin-Filho, A.; Rossi, M.A.; Ramos, S.G.; Lopes da Fonseca, B.A. A case presentation of a fatal dengue myocarditis showing evidence for dengue virus-induced lesion. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 127–130. [Google Scholar] [CrossRef]
- Kadam, D.B.; Salvi, S.; Chandanwale, A. Expanded Dengue. JAPI 2016, 64, 59–63. [Google Scholar] [PubMed]
- Nunes, P.; Rioja, L.; Coelho, J.; Salomão, N.G.; Rabelo, K.; José, C.C.; Rodrigues, F.; de Azeredo, E.L.; Basílio-de-Oliveira, C.A.; Basílio-de-Oliveira, R.; et al. Renal Injury in DENV-4 Fatal Cases: Viremia, Immune Response and Cytokine Profile. Pathogens 2019, 8, 223. [Google Scholar] [CrossRef]
- Vabo, K.A.; Torres Neto, G.; dos Santos, A.A.S.M.D.; Vabo, T.P.; Santos, M.L.O.; Machiori, E. Abdominal ultrasound findings in patients with dengue fever. Radiol. Bras. 2004, 37, 159–162. [Google Scholar] [CrossRef]
- De Almeida, R.R.; Paim, B.; de Oliveira, S.A.; Souza, A.S., Jr.; Gomes, A.; Escuissato, D.L.; Zanetti, G.; Marchiori, E. Dengue Hemorrhagic Fever: A State-of-the-Art Review Focused in Pulmonary Involvement. Lung 2017, 195, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Basílio-de-Oliveira, C.A.; Aguiar, G.R.; Baldanza, M.S.; Barth, O.M.; Eyer-Silva, W.A.; Paes, M.V. Pathologic study of a fatal case of dengue-3 virus infection in Rio de Janeiro, Brazil. Braz. J. Infect. Dis. 2005, 9, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Gupta, B.S.; Devpura, G.; Agarwal, A.; Anand, S. Pulmonary haemorrhage syndrome associated with dengue haemorrhagic fever. JAPI 2007, 55, 729–730. [Google Scholar] [PubMed]
- Marchiori, E.; Ferreira, J.L.; Bittencourt, C.N.; de Araújo Neto, C.A.; Zanetti, G.; Mano, C.M.; Santos, A.A.; Vianna, A.D. Pulmonary hemorrhage syndrome associated with dengue fever, high-resolution computed tomography findings: A case report. Orphanet J. Rare Dis. 2009, 4, 8. [Google Scholar] [CrossRef]
- Marchiori, E.; von Ranke, F.; Zanetti, G.; Hochhegger, B. Dengue hemorrhagic fever: Another cause of diffuse alveolar hem-orrhage in immunocompetent patients. Respir. Med. 2012, 106, 1807–1809. [Google Scholar] [CrossRef][Green Version]
- Miranda, C.H.; Borges, M.; Matsuno, A.K.; Vilar, F.C.; Gali, L.G.; Volpe, G.J.; Schmidt, A.; Pazin-Filho, A.; Silva, F.M.; Castro-Jorge, L.A.; et al. Evaluation of cardiac involvement during dengue viral infection. Clin. Infect. Dis. 2013, 57, 812–819. [Google Scholar] [CrossRef]
- Madhavan, S.; Narayanapillai, J. Left ventricular pseudoaneurysm in dengue fever. Heart Asia 2014, 6, 142–143. [Google Scholar] [CrossRef]
- Amin, H.; Siddiqui, W.J. Cardiomegaly. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Oliveira, E.; Póvoa, T.F.; Nuovo, G.J.; Allonso, D.; Salomão, N.G.; Basílio-de-Oliveira, C.A.; Geraldo, L.; Fonseca, C.G.; Lima, F.; Mohana-Borges, R.; et al. Dengue fatal cases present virus-specific HMGB1 response in peripheral organs. Sci. Rep. 2017, 7, 16011. [Google Scholar] [CrossRef]
- Jácome, F.C.; Teixeira, A.L.; Coutinho, D.D.; Costa, A.D.; Caldas, G.C.; Nunes, M.A.; Barth, O.M.; Barreto-Vieira, D.F. Secondary dengue infection in immunocompetent murine model leads to heart tissue damage. Acta Virol. 2019, 63, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, H.A.; Seneviratne, S.L. Liver involvement in dengue viral infections. Rev. Med. Virol. 2018, 28, e1971. [Google Scholar] [CrossRef] [PubMed]







| N = 123 | Histopathology qRT-PCR/IHQ | TEM | CK Level Analysis | Organ Weight | ||||
|---|---|---|---|---|---|---|---|---|
| 72 hpi | 72 hpi | 24 hpi | 48 hpi | 72 hpi | 72 hpi | 7 dpi | 14 dpi | |
| DENV-2/Lineage I | 10/10/5 | 5 | 5 | 5 | 5 | 22 | 15 | 15 |
| DENV-2/Lineage II | 10/10/5 | 5 | 5 | 5 | 5 | 22 | 15 | 15 |
| Negative control | 5/5/5 | 5 | 5 | 19 | ||||
| Total [samples] | 25 | 15 | 35 | 123 | ||||
| Histopathological Alterations | DENV-2 | |||
|---|---|---|---|---|
| Lineage I (%) | Lineage II (%) | Total (%) | ||
| Lung | Alveolar septum thickening | 10/10 (100) | 9/10 (90) | 19/20 (95) |
| Inflammatory infiltrate | 10/10 (100) | 9/10 (90) | 19/20 (95) | |
| Vascular congestion | 8/10 (80) | 6/10 (60) | 14/20 (70) | |
| Alveolar hemorrhage | 6/10 (60) | 6/10 (60) | 12/20 (60) | |
| Edema | 4/10 (40) | 4/10 (40) | 8/20 (40) | |
| Heart | Inflammatory infiltrate | 7/10 (70) | 9/10 (90) | 17/20 (85) |
| Cytoplasmic rarefaction | 7/10 (70) | 4/10 (40) | 11/20 (55) | |
| Increased cellularity | 2/10 (20) | 4/10 (40) | 6/20 (30) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jácome, F.C.; Caldas, G.C.; Rasinhas, A.d.C.; de Almeida, A.L.T.; de Souza, D.D.C.; Paulino, A.C.; da Silva, M.A.N.; Bandeira, D.M.; Barth, O.M.; dos Santos, F.B.; et al. Immunocompetent Mice Infected by Two Lineages of Dengue Virus Type 2: Observations on the Pathology of the Lung, Heart and Skeletal Muscle. Microorganisms 2021, 9, 2536. https://doi.org/10.3390/microorganisms9122536
Jácome FC, Caldas GC, Rasinhas AdC, de Almeida ALT, de Souza DDC, Paulino AC, da Silva MAN, Bandeira DM, Barth OM, dos Santos FB, et al. Immunocompetent Mice Infected by Two Lineages of Dengue Virus Type 2: Observations on the Pathology of the Lung, Heart and Skeletal Muscle. Microorganisms. 2021; 9(12):2536. https://doi.org/10.3390/microorganisms9122536
Chicago/Turabian StyleJácome, Fernanda Cunha, Gabriela Cardoso Caldas, Arthur da Costa Rasinhas, Ana Luisa Teixeira de Almeida, Daniel Dias Coutinho de Souza, Amanda Carlos Paulino, Marcos Alexandre Nunes da Silva, Derick Mendes Bandeira, Ortrud Monika Barth, Flavia Barreto dos Santos, and et al. 2021. "Immunocompetent Mice Infected by Two Lineages of Dengue Virus Type 2: Observations on the Pathology of the Lung, Heart and Skeletal Muscle" Microorganisms 9, no. 12: 2536. https://doi.org/10.3390/microorganisms9122536
APA StyleJácome, F. C., Caldas, G. C., Rasinhas, A. d. C., de Almeida, A. L. T., de Souza, D. D. C., Paulino, A. C., da Silva, M. A. N., Bandeira, D. M., Barth, O. M., dos Santos, F. B., & Barreto-Vieira, D. F. (2021). Immunocompetent Mice Infected by Two Lineages of Dengue Virus Type 2: Observations on the Pathology of the Lung, Heart and Skeletal Muscle. Microorganisms, 9(12), 2536. https://doi.org/10.3390/microorganisms9122536






