Influence of Manure Application on the Soil Bacterial Microbiome in Integrated Crop-Livestock Farms in Maryland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. 16S rRNA Sequencing
2.3. Metagenomic Dataset Processing
2.4. Bacterial Taxonomy and Diversity Analyses
3. Results
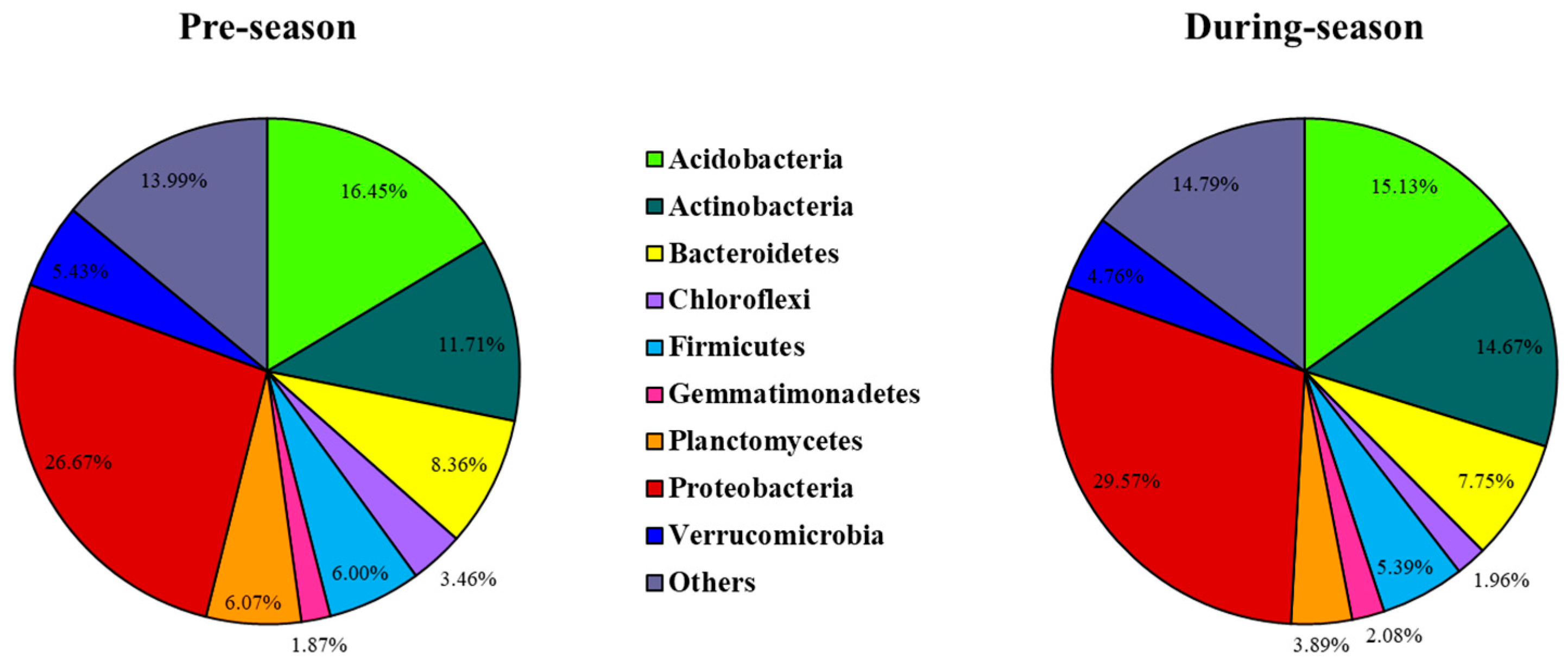
3.1. Relative Abundances of Soil Microbial Phyla
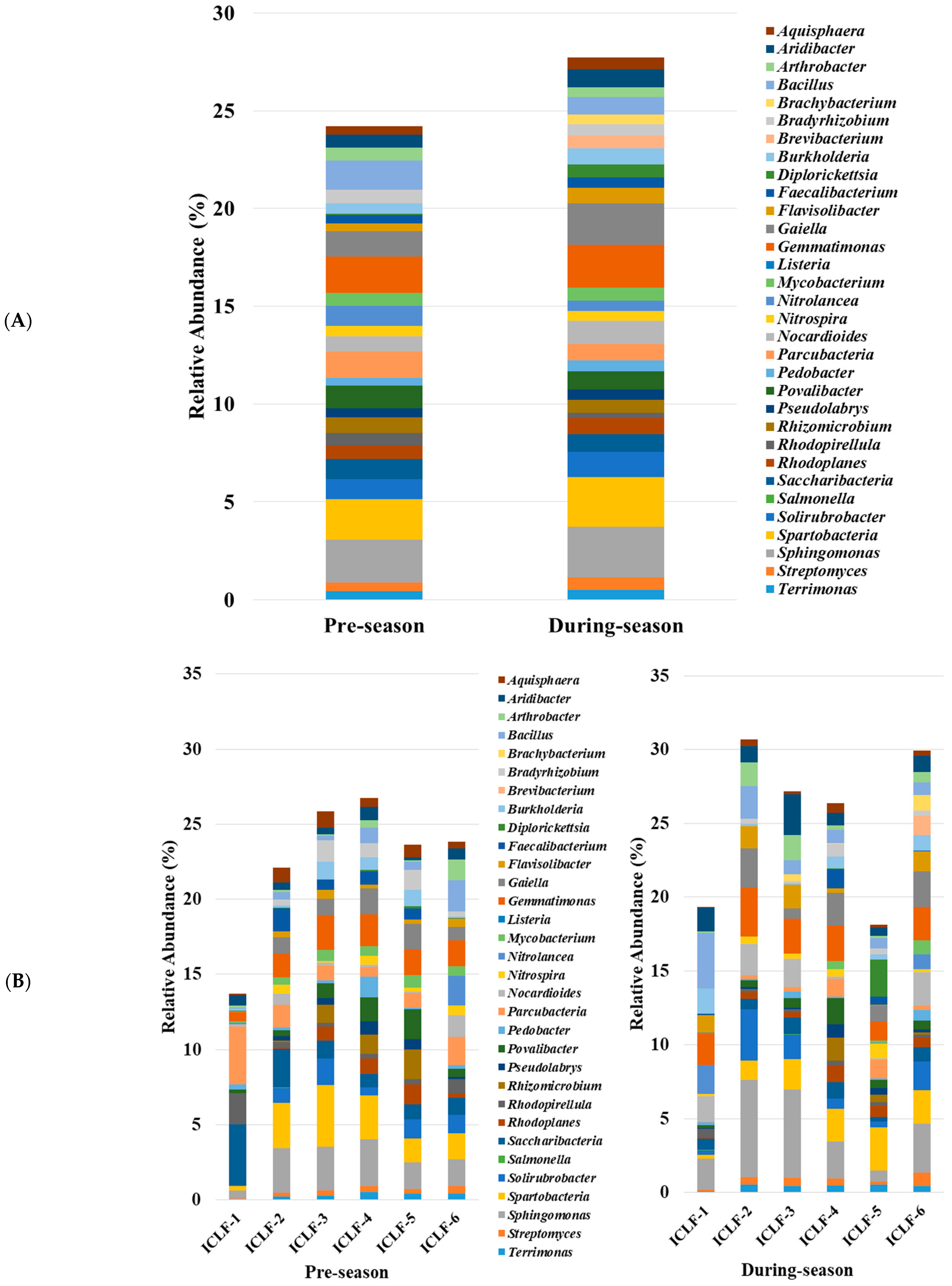
3.2. Genera Compositions of Soil Microbiome
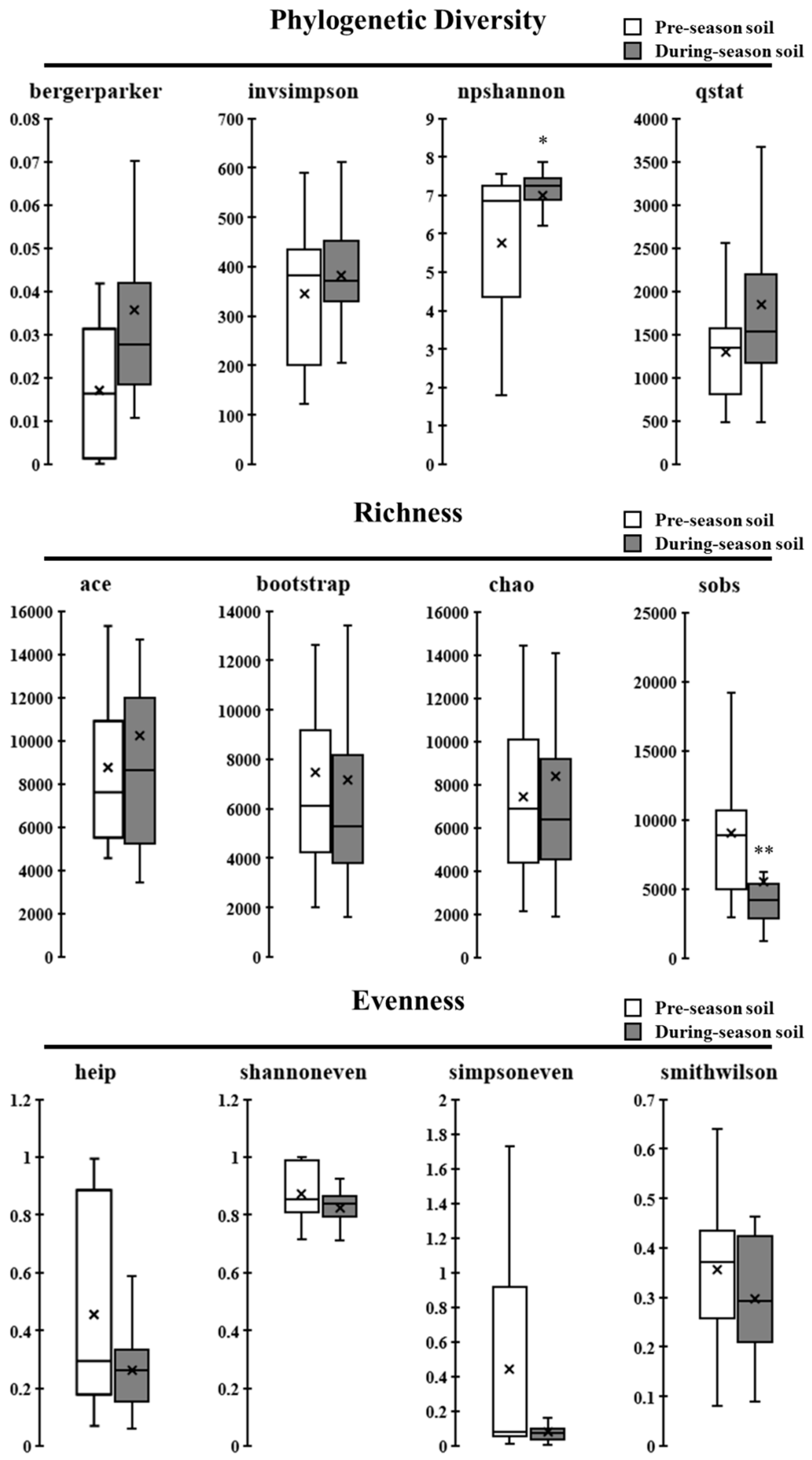
3.3. Species Phylogenetic Diversity, Richness, and Eveness in Soil Bacterial Communities
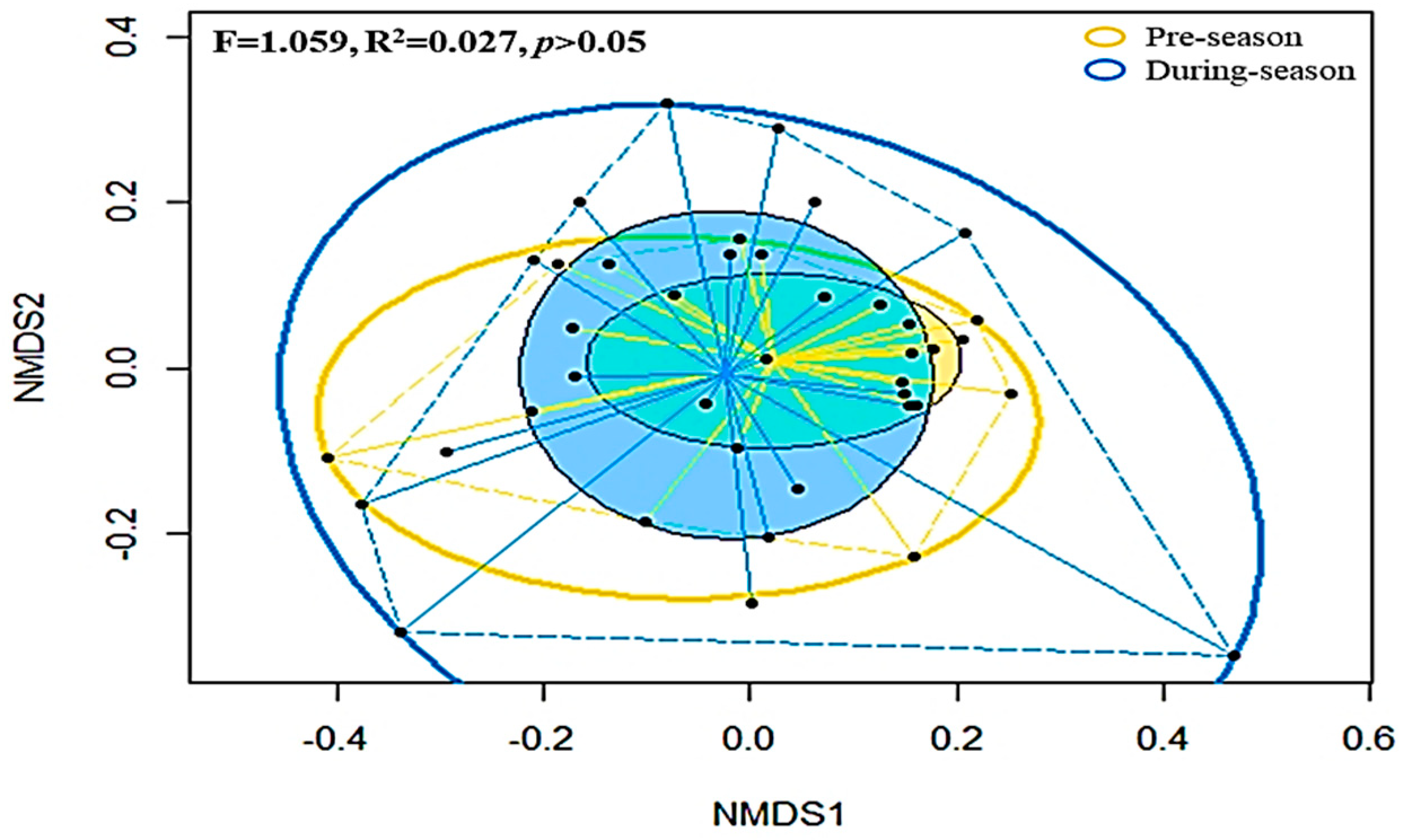
3.4. Dissimilarities in the Clustered Soil Microbial Compositions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peterson, C.A.; Deiss, L.; Gaudin, A.C.M. Commercial integrated crop-livestock systems achieve comparable crop yields to specialized production systems: A meta-analysis. PLoS ONE 2020, 15, e0231840. [Google Scholar] [CrossRef]
- Peng, M. The Growing Market of Organic Foods: Impact on the US and Global Economy. In Safety and Practice for Organic Food; Biswas, D., Micallef, S.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 3–22. [Google Scholar] [CrossRef]
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R Soc. Lond. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mie, A.; Andersen, H.R.; Gunnarsson, S.; Kahl, J.; Kesse-Guyot, E.; Rembialkowska, E.; Quaglio, G.; Grandjean, P. Human health implications of organic food and organic agriculture: A comprehensive review. Environ. Health 2017, 16, 111. [Google Scholar] [CrossRef] [Green Version]
- Lucan, S.C.; Maroko, A.R.; Sanon, O.; Frias, R.; Schechter, C.B. Urban farmers’ markets: Accessibility, offerings, and produce variety, quality, and price compared to nearby stores. Appetite 2015, 90, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebouillat, P.; Bonin, S.; Kestens, Y.; Chaput, S.; Drouin, L.; Mercille, G. Fruit and Vegetable Purchases in Farmer’s Market Stands: Analysing Survey and Sales Data. Int. J. Environ. Res. Public Health 2019, 17, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaheen, S.; Peng, M.; Biswas, D. Ecological Dynamics of Campylobacter in Integrated Mixed Crop-Livestock Farms and Its Prevalence and Survival Ability in Post-Harvest Products. Zoonoses Public Health 2016, 63, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Salaheen, S.; Almario, J.A.; Tesfaye, B.; Buchanan, R.; Biswas, D. Prevalence and antibiotic resistance pattern of Salmonella serovars in integrated crop-livestock farms and their products sold in local markets. Environ. Microbiol. 2016, 18, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Salaheen, S.; Biswas, D. Animal Health: Global Antibiotic Issues. Encycl. Agric. Food Syst. 2014, 346–357. [Google Scholar] [CrossRef]
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.Y.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA 2013, 110, 8399–8404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewey-Mattia, D.; Manikonda, K.; Hall, A.J.; Wise, M.E.; Crowe, S.J. Surveillance for Foodborne Disease Outbreaks—United States, 2009–2015. MMWR Surveill. Summ. 2018, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated With Fresh Produce From 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef]
- Geng, Y.; Cao, G.; Wang, L.; Wang, S. Effects of equal chemical fertilizer substitutions with organic manure on yield, dry matter, and nitrogen uptake of spring maize and soil nitrogen distribution. PLoS ONE 2019, 14, e0219512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, T.; Wang, J.; Chen, Q.; Zhang, F.; Lu, S. The effects of manure and nitrogen fertilizer applications on soil organic carbon and nitrogen in a high-input cropping system. PLoS ONE 2014, 9, e97732. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Salaheen, S.; Buchanan, R.L.; Biswas, D. Alterations of Salmonella enterica Serovar Typhimurium Antibiotic Resistance under Environmental Pressure. Appl. Environ. Microbiol. 2018, 84, e01173-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurtler, J.B.; Doyle, M.P.; Erickson, M.C.; Jiang, X.; Millner, P.; Sharma, M. Composting To Inactivate Foodborne Pathogens for Crop Soil Application: A Review. J. Food Prot. 2018, 81, 1821–1837. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef] [PubMed]
- Delahoy, M.J.; Wodnik, B.; McAliley, L.; Penakalapati, G.; Swarthout, J.; Freeman, M.C.; Levy, K. Pathogens transmitted in animal feces in low- and middle-income countries. Int. J. Hyg. Environ. Health 2018, 221, 661–676. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A Review of the Use of Organic Amendments and the Risk to Human Health. Adv. Agron. 2013, 120, 275–379. [Google Scholar] [CrossRef]
- Peng, M.; Biswas, D. Environmental Influences of High-Density Agricultural Animal Operation on Human Forearm Skin Microflora. Microorganisms 2020, 8, 1481. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Tabashsum, Z.; Patel, P.; Bernhardt, C.; Biswas, C.; Meng, J.; Biswas, D. Prevention of enteric bacterial infections and modulation of gut microbiota with conjugated linoleic acids producing Lactobacillus in mice. Gut. Microbes. 2020, 11, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Mbareche, H.; Dumont-Leblond, N.; Bilodeau, G.J.; Duchaine, C. An Overview of Bioinformatics Tools for DNA Meta-Barcoding Analysis of Microbial Communities of Bioaerosols: Digest for Microbiologists. Life 2020, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Paliy, O.; Shankar, V. Application of multivariate statistical techniques in microbial ecology. Mol. Ecol. 2016, 25, 1032–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Chen, H.; Zhao, J.; Zhang, H.; Chen, W. Potential Functions of the Gastrointestinal Microbiome Inhabiting the Length of the Rat Digest Tract. Int. J. Mol. Sci. 2019, 20, 1232. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Rousk, J.; Baath, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Stres, B.; Danevcic, T.; Pal, L.; Fuka, M.M.; Resman, L.; Leskovec, S.; Hacin, J.; Stopar, D.; Mahne, I.; Mandic-Mulec, I. Influence of temperature and soil water content on bacterial, archaeal and denitrifying microbial communities in drained fen grassland soil microcosms. FEMS Microbiol. Ecol. 2008, 66, 110–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition-Current Knowledge and Future Directions. Front. Plant. Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roesch, L.F.; Fulthorpe, R.R.; Riva, A.; Casella, G.; Hadwin, A.K.; Kent, A.D.; Daroub, S.H.; Camargo, F.A.; Farmerie, W.G.; Triplett, E.W. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007, 1, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Steffan, J.J.; Brevik, E.C.; Burgess, L.C.; Cerda, A. The effect of soil on human health: An overview. Eur. J. Soil. Sci. 2018, 69, 159–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazarika, S.N.; Thakur, D. Actinobacteria. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 443–476. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Mamphweli, S.N.; Meyer, E.L.; Makaka, G.; Simon, M.; Okoh, A.I. An Overview of the Control of Bacterial Pathogens in Cattle Manure. Int. J. Environ. Res. Public Health 2016, 13, 843. [Google Scholar] [CrossRef] [Green Version]
- Buckley, D.H.; Huangyutitham, V.; Nelson, T.A.; Rumberger, A.; Thies, J.E. Diversity of Planctomycetes in soil in relation to soil history and environmental heterogeneity. Appl. Environ. Microbiol. 2006, 72, 4522–4531. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.E.; Sangwan, P.; Janssen, P.H. Acidobacteria, Rubrobacteridae and Chloroflexi are abundant among very slow-growing and mini-colony-forming soil bacteria. Environ. Microbiol. 2011, 13, 798–805. [Google Scholar] [CrossRef]
- Costello, E.K.; Schmidt, S.K. Microbial diversity in alpine tundra wet meadow soil: Novel Chloroflexi from a cold, water-saturated environment. Environ. Microbiol. 2006, 8, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Llado, S.; Lopez-Mondejar, R.; Baldrian, P. Forest Soil Bacteria: Diversity, Involvement in Ecosystem Processes, and Response to Global Change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Jinal, N.H.; Amaresan, N. Evaluation of biocontrol Bacillus species on plant growth promotion and systemic-induced resistant potential against bacterial and fungal wilt-causing pathogens. Arch. Microbiol. 2020, 202, 1785–1794. [Google Scholar] [CrossRef]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial Contamination of Fresh Produce: What, Where, and How? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. [Google Scholar] [CrossRef] [Green Version]
- Todd, E. Food-Borne Disease Prevention and Risk Assessment. Int J. Environ. Res. Public Health 2020, 17, 5129. [Google Scholar] [CrossRef] [PubMed]
- Bastida, F.; Eldridge, D.J.; Garcia, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity-biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, L.; Wang, Y.; Fan, K.; Xu, Q.; Li, Y.; Ma, Q.; Wang, J.; Ren, W.; Ding, Z. Cow manure application effectively regulates the soil bacterial community in tea plantation. BMC Microbiol. 2020, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Rieke, E.L.; Soupir, M.L.; Moorman, T.B.; Yang, F.; Howe, A.C. Temporal Dynamics of Bacterial Communities in Soil and Leachate Water after Swine Manure Application. Front. Microbiol. 2018, 9, 3197. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, M.; Tabashsum, Z.; Millner, P.; Parveen, S.; Biswas, D. Influence of Manure Application on the Soil Bacterial Microbiome in Integrated Crop-Livestock Farms in Maryland. Microorganisms 2021, 9, 2586. https://doi.org/10.3390/microorganisms9122586
Peng M, Tabashsum Z, Millner P, Parveen S, Biswas D. Influence of Manure Application on the Soil Bacterial Microbiome in Integrated Crop-Livestock Farms in Maryland. Microorganisms. 2021; 9(12):2586. https://doi.org/10.3390/microorganisms9122586
Chicago/Turabian StylePeng, Mengfei, Zajeba Tabashsum, Patricia Millner, Salina Parveen, and Debabrata Biswas. 2021. "Influence of Manure Application on the Soil Bacterial Microbiome in Integrated Crop-Livestock Farms in Maryland" Microorganisms 9, no. 12: 2586. https://doi.org/10.3390/microorganisms9122586





