The Manganese Peroxidase Gene Family of Trametes trogii: Gene Identification and Expression Patterns Using Various Metal Ions under Different Culture Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. DNA and RNA Isolation and Cloning of Manganese Peroxidase Genes
2.3. Manganese Peroxidase Activity Assay
2.4. Real-Time PCR
2.5. Sequence and Phylogenetic Analysis
2.6. Data Analysis
3. Results
3.1. Identification of the MnP Gene Family of T. trogii S0301
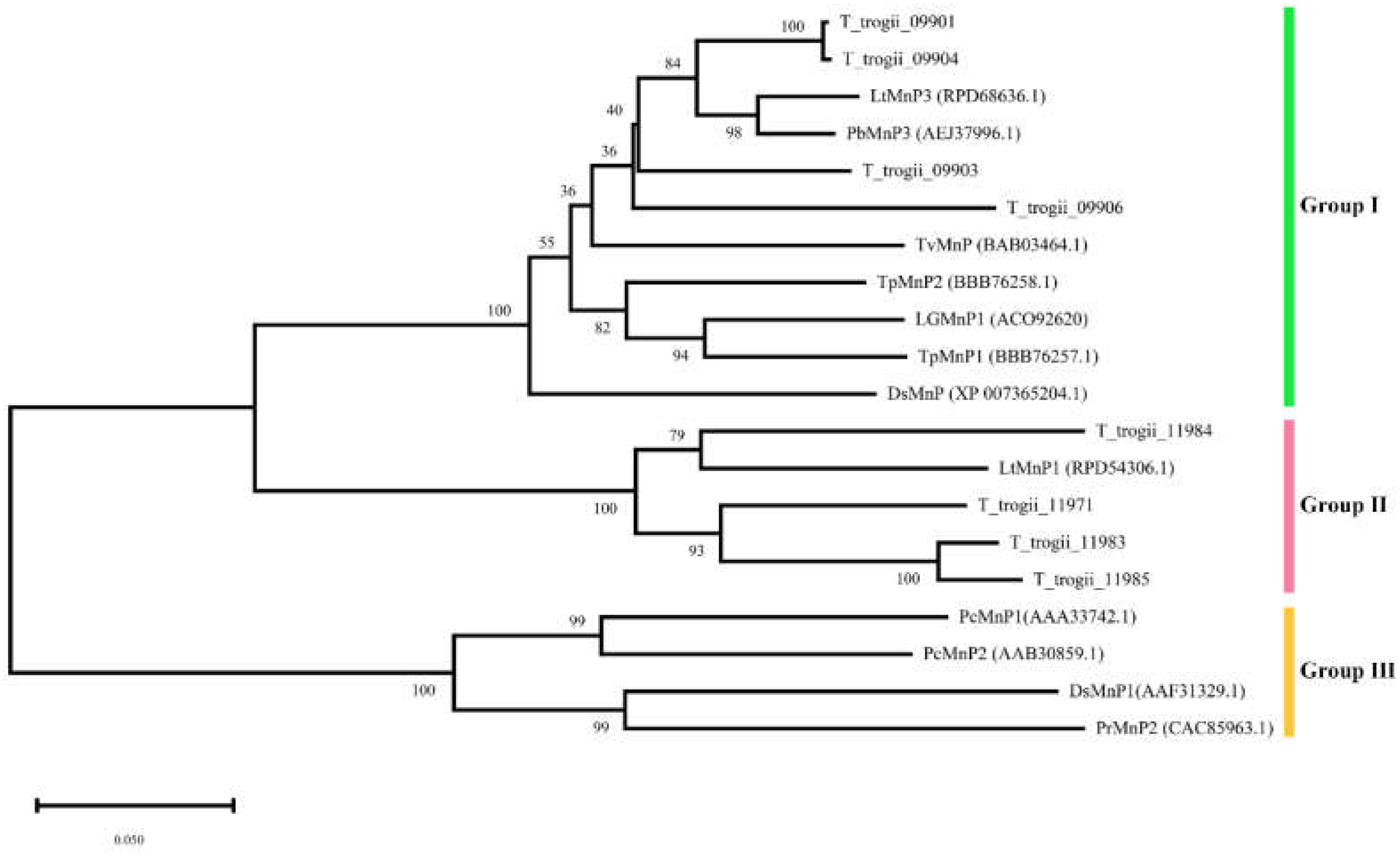
3.2. Phylogenetic Analyses of the MnP Gene Family of T. trogii S0301
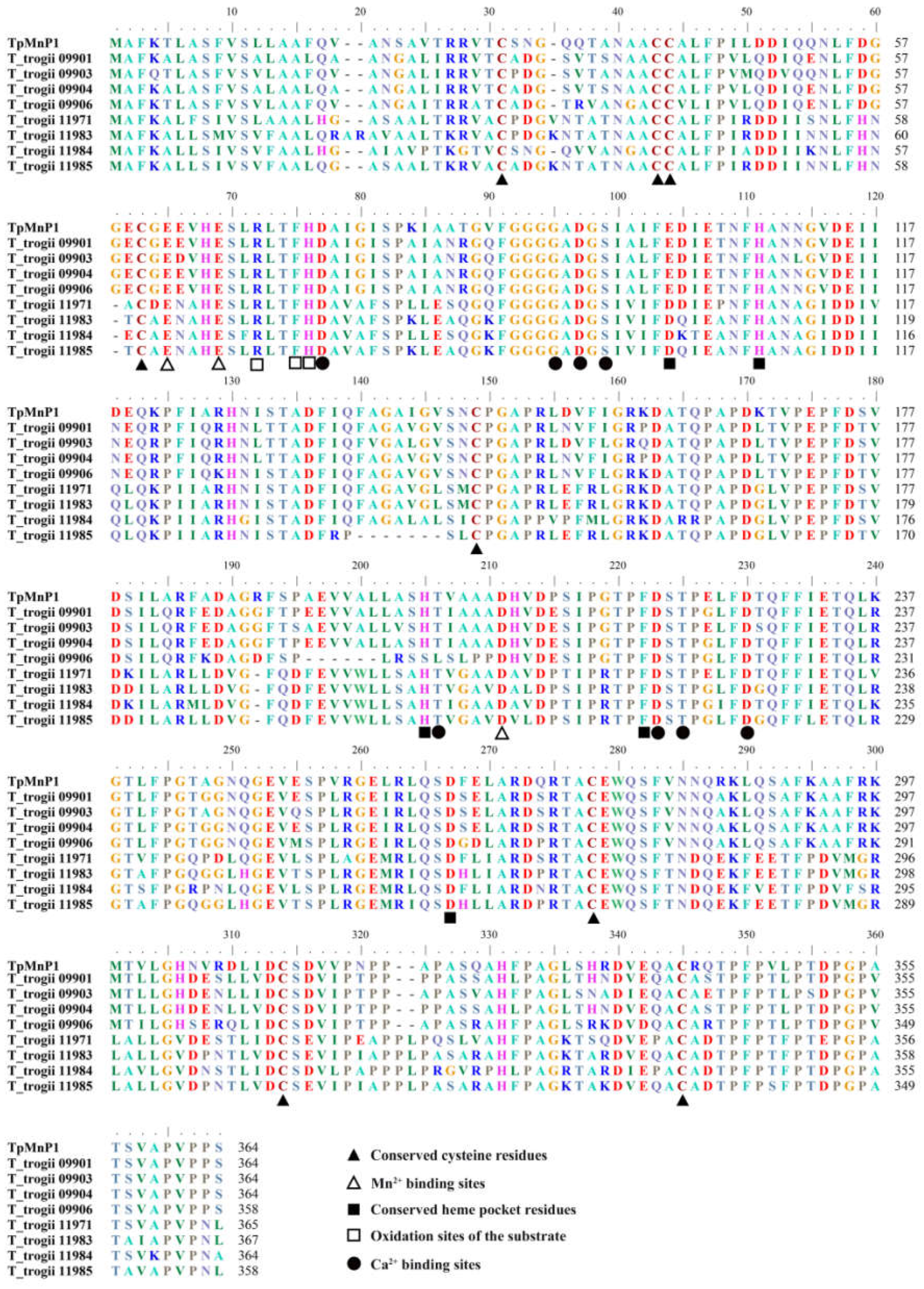
3.3. Gene Structure and Conserved Motifs of TtMnP Gene Family Members
3.4. Promoter of TtMnPs
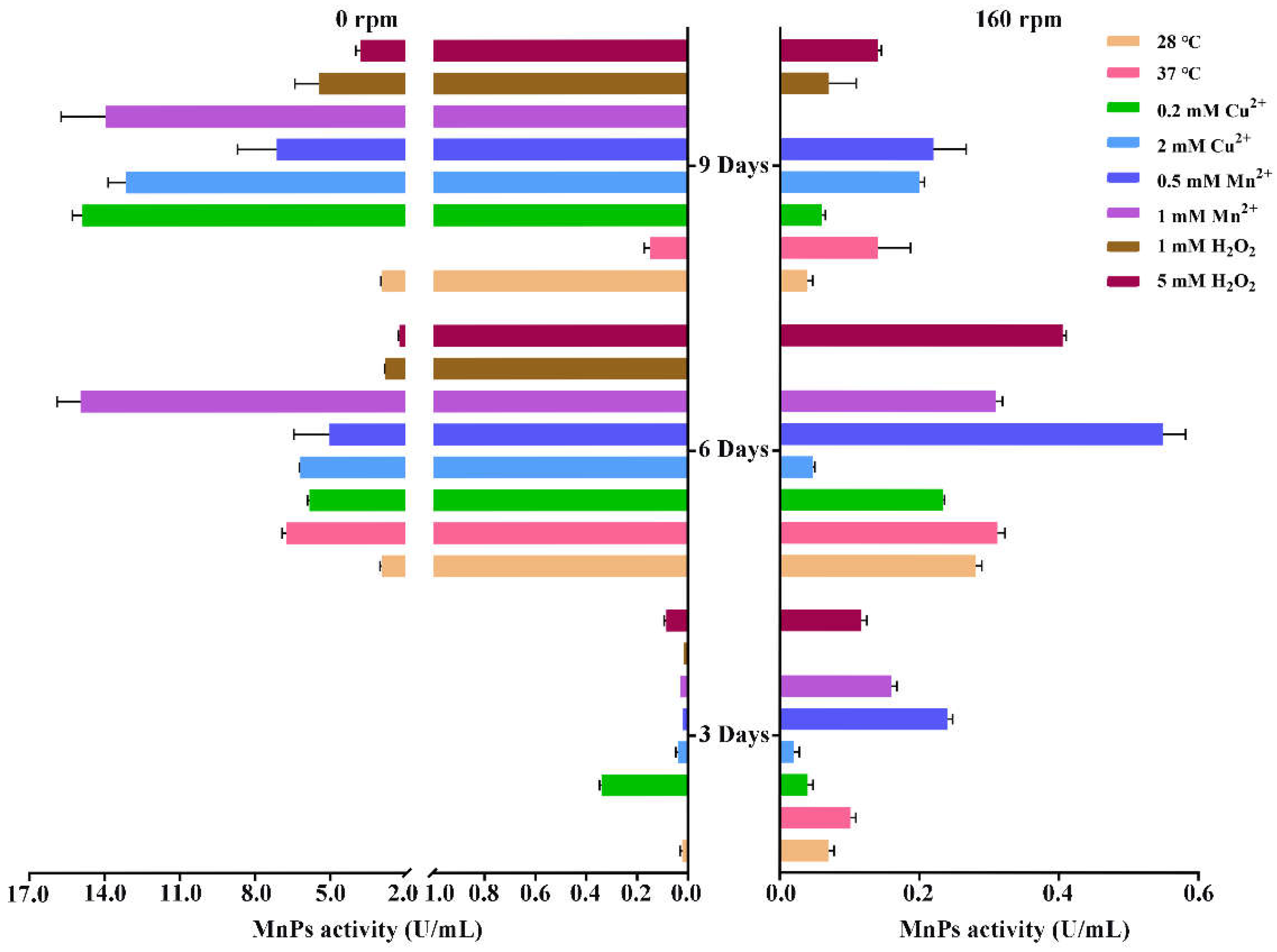
3.5. Factors Affecting MnP Activity of T. trogii S0301
3.6. Expression Patterns of TtMnPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sutzl, L.; Laurent, C.; Abrera, A.T.; Schutz, G.; Ludwig, R.; Haltrich, D. Multiplicity of enzymatic functions in the CAZy AA3 family. Appl. Microbiol. Biotechnol. 2018, 102, 2477–2492. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Zainith, S.; Purchase, D.; Saratale, G.D.; Ferreira, L.F.R.; Bilal, M.; Bharagava, R.N. Isolation and characterization of lignin-degrading bacterium Bacillus aryabhattai from pulp and paper mill wastewater and evaluation of its lignin-degrading potential. 3 Biotech 2019, 9, 92. [Google Scholar] [CrossRef]
- Rekik, H.; Zarai Jaouadi, N.; Bouacem, K.; Zenati, B.; Kourdali, S.; Badis, A.; Annane, R.; Bouanane-Darenfed, A.; Bejar, S.; Jaouadi, B. Physical and enzymatic properties of a new manganese peroxidase from the white-rot fungus Trametes pubescens strain i8 for lignin biodegradation and textile-dyes biodecolorization. Int. J. Biol. Macromol. 2019, 125, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, L.; Zeng, G.; Chen, J.; Wang, J.; Fan, C.; Yang, G.; Zhang, Y.; Xie, X. Amplified and selective detection of manganese peroxidase genes based on enzyme-scaffolded-gold nanoclusters and mesoporous carbon nitride. Biosens. Bioelectron. 2015, 65, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, T.; Palvannan, T.; Kim, D.H.; Park, S.M. Manganese peroxidase h4 isozyme mediated degradation and detoxification of triarylmethane dye malachite green: Optimization of decolorization by response surface methodology. Appl. Biochem. Biotechnol. 2013, 171, 1178–1193. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zheng, J.; Lu, Y.; Jia, R. Degradation and detoxification of the triphenylmethane dye malachite green catalyzed by crude manganese peroxidase from Irpex lacteus F17. Environ. Sci. Pollut. Res. Int. 2016, 23, 9585–9597. [Google Scholar] [CrossRef]
- Coconi-Linares, N.; Magana-Ortiz, D.; Guzman-Ortiz, D.A.; Fernandez, F.; Loske, A.M.; Gomez-Lim, M.A. High-yield production of manganese peroxidase, lignin peroxidase, and versatile peroxidase in Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2014, 98, 9283–9294. [Google Scholar] [CrossRef]
- Janusz, G.; Kucharzyk, K.H.; Pawlik, A.; Staszczak, M.; Paszczynski, A.J. Fungal laccase, manganese peroxidase and lignin peroxidase: Gene expression and regulation. Enzyme Microb. Technol. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Knop, D.; Ben-Ari, J.; Salame, T.M.; Levinson, D.; Yarden, O.; Hadar, Y. Mn2+-deficiency reveals a key role for the Pleurotus ostreatus versatile peroxidase (VP4) in oxidation of aromatic compounds. Appl. Microbiol. Biotechnol. 2014, 98, 6795–6804. [Google Scholar] [CrossRef]
- Pech-Canul, A.C.; Carrillo-Campos, J.; Ballinas-Casarrubias, M.L.; Solis-Oviedo, R.L.; Hernandez-Rascon, S.K.; Hernandez-Ochoa, L.R.; Gutierrez-Mendez, N.; Garcia-Triana, A. Functional Expression and One-Step Protein Purification of Manganese Peroxidase 1 (rMnP1) from Phanerochaete chrysosporium Using the E. coli-Expression System. Int. J. Mol. Sci. 2020, 21, 416. [Google Scholar] [CrossRef] [Green Version]
- Salame, T.M.; Knop, D.; Levinson, D.; Yarden, O.; Hadar, Y. Redundancy among manganese peroxidases in Pleurotus ostreatus. Appl. Environ. Microbiol. 2013, 79, 2405–2415. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Fueyo, E.; Ruiz-Duenas, F.J.; Martinez, M.J.; Romero, A.; Hammel, K.E.; Medrano, F.J.; Martinez, A.T. Ligninolytic peroxidase genes in the oyster mushroom genome: Heterologous expression, molecular structure, catalytic and stability properties, and lignin-degrading ability. Biotechnol. Biofuels 2014, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elisashvili, V.; Kachlishvili, E. Physiological regulation of laccase and manganese peroxidase production by white-rot Basidiomycetes. J. Biotechnol. 2009, 144, 37–42. [Google Scholar] [CrossRef]
- Kachlishvili, E.; Metreveli, E.; Elisashvili, V. Modulation of Cerrena unicolor laccase and manganese peroxidase production. Springerplus 2014, 3, 463. [Google Scholar] [CrossRef] [Green Version]
- Scheel, T.; Hofer, M.; Ludwig, S.; Holker, U. Differential expression of manganese peroxidase and laccase in white-rot fungi in the presence of manganese or aromatic compounds. Appl. Microbiol. Biotechnol. 2000, 54, 686–691. [Google Scholar] [CrossRef]
- Pawlik, A.; Jaszek, M.; Sulej, J.; Janusz, G. Light-regulated synthesis of extra- and intracellular enzymes related to wood degradation by the white rot fungus Cerrena unicolor during solid-state fermentation on ash sawdust-based medium. Acta Biochim. Polonica 2019, 66, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Vrsanska, M.; Voberkova, S.; Langer, V.; Palovcikova, D.; Moulick, A.; Adam, V.; Kopel, P. Induction of Laccase, Lignin Peroxidase and Manganese Peroxidase Activities in White-Rot Fungi Using Copper Complexes. Molecules 2016, 21, 1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachlishvili, E.; Asatiani, M.; Kobakhidze, A.; Elisashvili, V. Trinitrotoluene and mandarin peels selectively affect lignin-modifying enzyme production in white-rot basidiomycetes. Springerplus 2016, 5, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lueangjaroenkit, P.; Teerapatsakul, C.; Sakka, K.; Sakka, M.; Kimura, T.; Kunitake, E.; Chitradon, L. Two Manganese Peroxidases and a Laccase of Trametes polyzona KU-RNW027 with Novel Properties for Dye and Pharmaceutical Product Degradation in Redox Mediator-Free System. Mycobiology 2019, 47, 217–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachlishvili, E.; Asatiani, M.D.; Kobakhidze, A.; Elisashvili, V. Evaluation of Lignin-Modifying Enzyme Activity of Trametes spp. (Agaricomycetes) Isolated from Georgian Forests with an Emphasis on T. multicolor Biosynthetic Potential. Int. J. Med. Mushrooms 2018, 20, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.; Zhang, Y.; Yang, X.; Yang, E.; Xu, H.; Yang, Q.; Chagan, I.; Cui, X.; Chen, W.; et al. Lignin degradation potential and draft genome sequence of Trametes trogii S0301. Biotechnol. Biofuels 2019, 12, 256. [Google Scholar] [CrossRef]
- Ferreira, D.S.S.; de Santana, C.S.; Santana, I.B.; Araujo, J.S.C.; Souza, B.C.; Leite, F.H.A.; Kato, R.B.; Benevides, R.G.; Goes-Neto, A. Functional annotation and comparative modeling of ligninolytic enzymes from Trametes villosa (SW.) Kreisel for biotechnological applications. J. Biomol. Struct. Dyn. 2021, 1–10. [Google Scholar] [CrossRef]
- Yan, J.; Niu, J.; Chen, D.; Chen, Y.; Irbis, C. Screening of Trametes strains for efficient decolorization of malachite green at high temperatures and ionic concentrations. Int. Biodeterior. Biodegrad. 2014, 87, 109–115. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Yang, X.; Yang, E.; Xu, H.; Chen, Y.; Chagan, I.; Yan, J. Alternative Splicing of Heat Shock Transcription Factor 2 Regulates the Expression of Laccase Gene Family in Response to Copper in Trametes trogii. Appl. Environ. Microbiol. 2021, 87, e00055-21. [Google Scholar] [CrossRef]
- Kondo, R.; Harazono, K.; Sakai, K. Bleaching of Hardwood Kraft Pulp with Manganese Peroxidase Secreted from Phanerochaete sordida YK-624. Appl. Environ. Microbiol. 1994, 60, 4359–4363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Morgenstern, I.; Robertson, D.L.; Hibbett, D.S. Characterization of three mnp genes of Fomitiporia mediterranea and report of additional class II peroxidases in the order hymenochaetales. Appl. Environ. Microbiol. 2010, 76, 6431–6440. [Google Scholar] [CrossRef] [Green Version]
- Clough, R.C.; Pappu, K.; Thompson, K.; Beifuss, K.; Lane, J.; Delaney, D.E.; Harkey, R.; Drees, C.; Howard, J.A.; Hood, E.E. Manganese peroxidase from the white-rot fungus Phanerochaete chrysosporium is enzymatically active and accumulates to high levels in transgenic maize seed. Plant Biotechnol. J. 2006, 4, 53–62. [Google Scholar] [CrossRef]
- Ho, P.Y.; Namasivayam, P.; Sundram, S.; Ho, C.L. Expression of Genes Encoding Manganese Peroxidase and Laccase of Ganoderma boninense in Response to Nitrogen Sources, Hydrogen Peroxide and Phytohormones. Genes 2020, 11, 1263. [Google Scholar] [CrossRef]
- Lueangjaroenkit, P.; Kunitake, E.; Sakka, M.; Kimura, T.; Teerapatsakul, C.; Sakka, K.; Chitradon, L. Light Regulation of Two New Manganese Peroxidase-Encoding Genes in Trametes polyzona KU-RNW027. Microorganisms 2020, 8, 852. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.M.; Canessa, P.; Mancilla, R.A.; Polanco, R.; Santibanez, P.A.; Vicuna, R. Expression of genes encoding laccase and manganese-dependent peroxidase in the fungus Ceriporiopsis subvermispora is mediated by an ACE1-like copper-fist transcription factor. Fungal Genet. Biol. 2009, 46, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Catal, T.; Liu, H.; Bermek, H. Selenium induces manganese-dependent peroxidase production by the white-rot fungus Bjerkandera adusta (Willdenow) P. Karsten. Biol. Trace Elem. Res. 2008, 123, 211–217. [Google Scholar] [CrossRef]
- Galgani, F.; Cadiou, Y.; Bocquene, G. Routine determination of enzyme kinetics using plate reader. Biotechnol. Bioeng. 1991, 38, 434–437. [Google Scholar] [CrossRef]
- Johansson, T.; Nyman, P.O.; Cullen, D. Differential regulation of mnp2, a new manganese peroxidase-encoding gene from the ligninolytic fungus Trametes versicolor PRL 572. Appl Environ Microbiol 2002, 68, 2077–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Strain | MnP Isoenzyme | Factor Up (↑) or Down (↓) Regulated | Culturecondition | MnP Activity (Substrate) | References |
|---|---|---|---|---|---|
| G. boninense | Gb_U6011 | Nitrogen source ↑ H2O2 ↑ Methyl jasmonic acid ↓ | Static liquid at 30 °C | 18–110 U/mL (MnSO4) | [30] |
| Gb_U87 | JA ↑ | ||||
| Gb_35959 | ammonium nitrate ↑ sodium nitrate↓ H2O2 ↓ | ||||
| T. pubescens | MnP TP55 | Mn2+ ↑ | shake liquid at 30 °C | 3.56 U/mg (2,6-DMP) | [4] |
| P. ostreatus | MnP4 | Mn2+ ↑ | shake liquid at 30 °C | 1.2 U/mL (2,6-DMP) | [10] |
| T. polyzona | MnP1, MnP2 | Light ↑ | shake liquid at 30 °C | 0.8–3.5 U/mL (2,6-DMP) | [31] |
| Ceriporiopsis subvermispora | MnP1, MnP2 | Mn2+ and Cu2+ ↑ | shake liquid at 30 °C | - | [32] |
| Bjerkandera adusta | - | Se2+ ↑ | shake liquid at 28 °C | 0.39–0.81 U/mL (MnSO4) | [33] |
| Dichomitus squalens | - | Mn2+ ↑ | shake or static at 28 °C | - | [34] |
| T. trogii | T_trogii_09901, 09904, 09903 and 09906 | Mn2+ ↑ | Static liquid at 28 °C | 2.9–15 U/mL (2,6-DMP) | This study |
| T_trogii_11984, 11971, 11985 and 11983 | Cu2+, H2O2 and temperature ↑ | Static liquid at 28 °C | |||
| T_trogii_09901, 09904, 09903 and 11984 | Cu2+ ↑ | shake liquid at 28 °C | <0.55 U/mL (2,6-DMP) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Dong, Z.; Luo, Y.; Yang, E.; Xu, H.; Chagan, I.; Yan, J. The Manganese Peroxidase Gene Family of Trametes trogii: Gene Identification and Expression Patterns Using Various Metal Ions under Different Culture Conditions. Microorganisms 2021, 9, 2595. https://doi.org/10.3390/microorganisms9122595
Zhang Y, Dong Z, Luo Y, Yang E, Xu H, Chagan I, Yan J. The Manganese Peroxidase Gene Family of Trametes trogii: Gene Identification and Expression Patterns Using Various Metal Ions under Different Culture Conditions. Microorganisms. 2021; 9(12):2595. https://doi.org/10.3390/microorganisms9122595
Chicago/Turabian StyleZhang, Yu, Zhongqi Dong, Yuan Luo, En Yang, Huini Xu, Irbis Chagan, and Jinping Yan. 2021. "The Manganese Peroxidase Gene Family of Trametes trogii: Gene Identification and Expression Patterns Using Various Metal Ions under Different Culture Conditions" Microorganisms 9, no. 12: 2595. https://doi.org/10.3390/microorganisms9122595
APA StyleZhang, Y., Dong, Z., Luo, Y., Yang, E., Xu, H., Chagan, I., & Yan, J. (2021). The Manganese Peroxidase Gene Family of Trametes trogii: Gene Identification and Expression Patterns Using Various Metal Ions under Different Culture Conditions. Microorganisms, 9(12), 2595. https://doi.org/10.3390/microorganisms9122595





