Nutritional Modulation of the Immune Response Mediated by Nucleotides in Canine Leishmaniosis
Abstract
:1. Canine Leishmaniosis and Associated Immune Responses
1.1. Canine Leishmaniosis
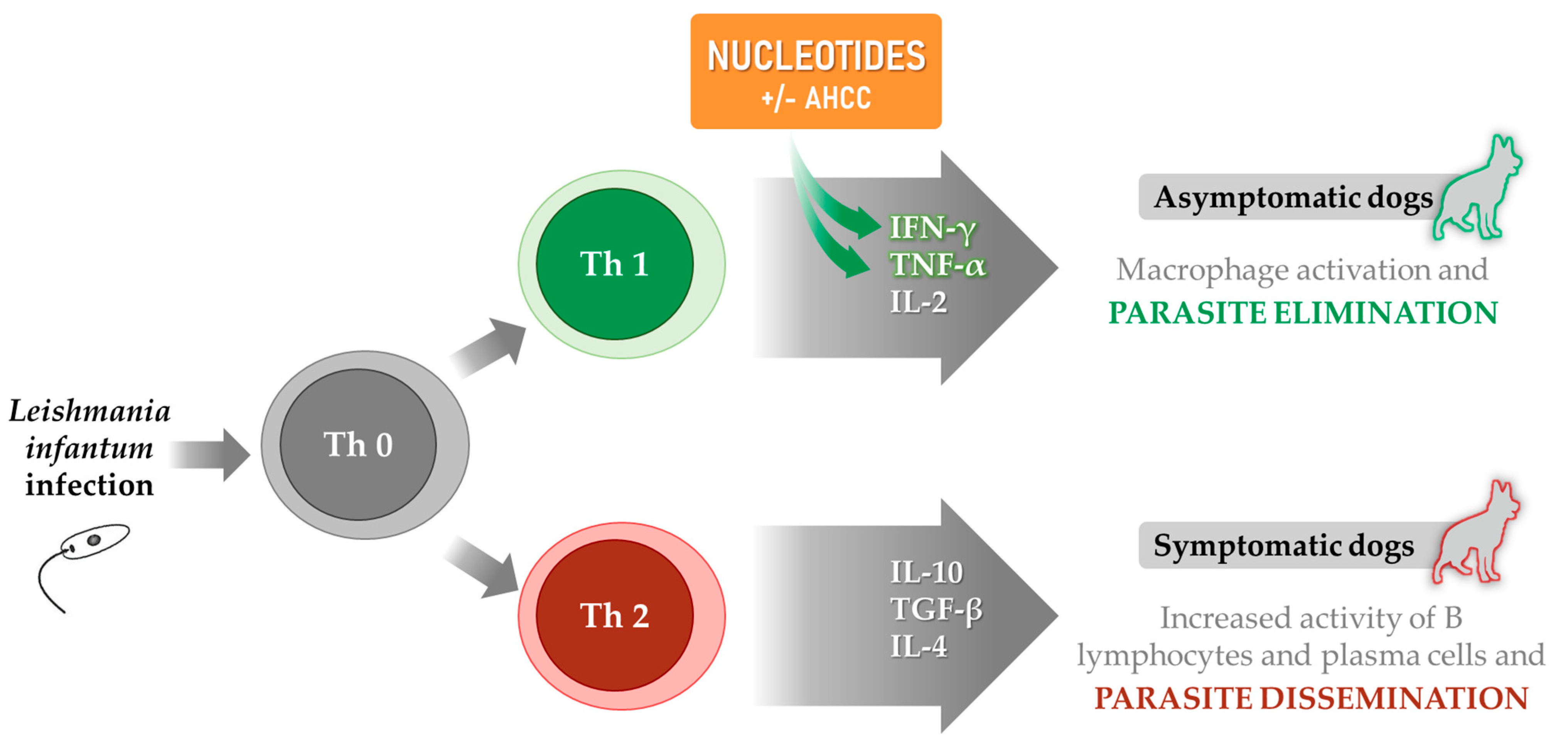
1.2. Immune Response and Disease Prognosis
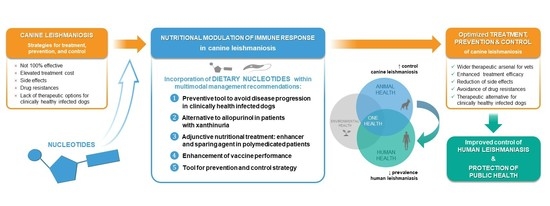
2. Strategies for Treatment, Prevention and Control, and Unresolved Issues
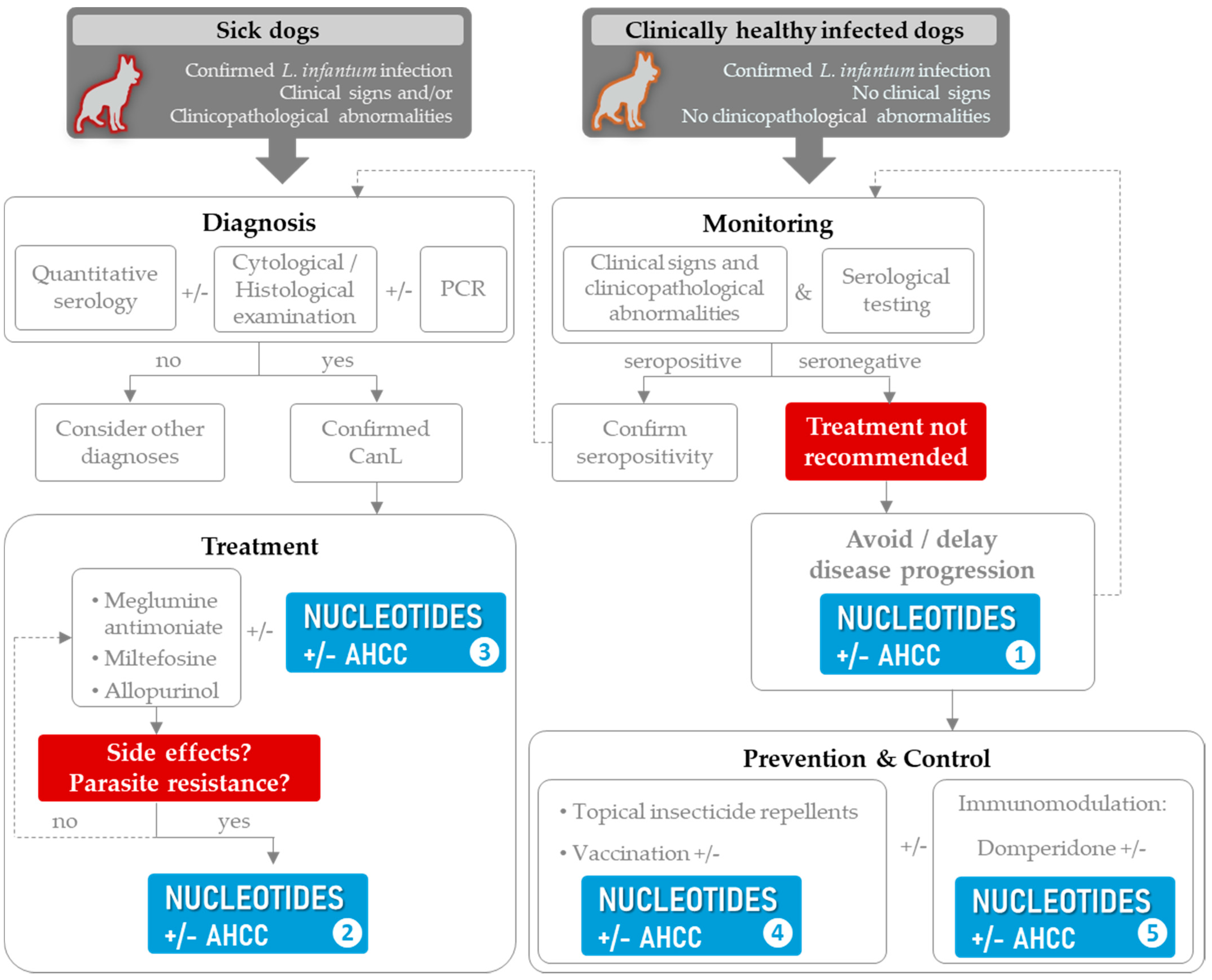
2.1. Recommended Treatment Options
2.2. Prevention, Control, and Public Health Considerations
3. Immunonutrition and Bioactive Compounds
4. Nucleotides in Leishmaniasis
4.1. Reported Effects of Nucleotides and AHCC
4.2. The Role of Nucleotides in CanL Multimodal Management
4.3. Limitations and Unexplored Paths
5. Future Perspectives
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where are we and where are we heading? Parasitol. Res. 2021, 120, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- WHO. Control of the Leishmaniases. In WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2010; Volume 949, pp. 22–26. [Google Scholar]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Leishman, W. On the possibility of the occurrence of trypanosomiasis in India. Br. Med. J. 1903, 1, 1252–1254. [Google Scholar] [CrossRef] [Green Version]
- Donovan, C. On the possibility of the occurrence of trypanosomiasis in India. Natl. Med. J. India 1994, 7, 196, 201–202. [Google Scholar]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Baneth, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasites Vectors 2011, 4, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Podaliri Vulpiani, M.; Iannetti, L.; Paganico, D.; Iannino, F.; Ferri, N. Methods of Control of the Leishmania infantum Dog Reservoir: State of the Art. Vet. Med. Int. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hosein, S.; Blake, D.P.; Solano-Gallego, L. Insights on adaptive and innate immunity in canine leishmaniosis. Parasitology 2017, 144, 95–115. [Google Scholar] [CrossRef] [Green Version]
- Miró, G.; Cardoso, L.; Pennisi, M.G.; Oliva, G.; Baneth, G. Canine leishmaniosis—New concepts and insights on an expanding zoonosis: Part two. Trends Parasitol. 2008, 24, 371–377. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Koutinas, A.; Miró, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009, 165, 1–18. [Google Scholar] [CrossRef]
- Bourdeau, P.; Rowton, E.; Petersen, C. Impact of different Leishmania reservoirs on sand fly transmission: Perspectives from xenodiagnosis and other one health observations. Vet. Parasitol. 2020, 287, 109237. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Gomez, E.A.; Cáceres, A.G.; Uezato, H.; Mimori, T.; Hashiguchi, Y. Molecular epidemiology for vector research on leishmaniasis. Int. J. Environ. Res. Public Health 2010, 7, 814–826. [Google Scholar] [CrossRef]
- Salant, H.; Nachum-Biala, Y.; Feinmesser, B.; Perelmutter, M.; Baneth, G. Early onset of clinical leishmaniosis in a litter of pups with evidence of in utero transmission. Parasites Vectors 2021, 14, 1–9. [Google Scholar] [CrossRef]
- Cardoso, L.; Schallig, H.; Persichetti, M.F.; Pennisi, M.G. New epidemiological aspects of animal leishmaniosis in Europe: The role of vertebrate hosts other than dogs. Pathogens 2021, 10, 307. [Google Scholar] [CrossRef]
- Martín-Sánchez, J.; Torres-Medina, N.; Morillas-Márquez, F.; Corpas-López, V.; Díaz-Sáez, V. Acta Tropica Role of wild rabbits as reservoirs of leishmaniasis in a non-epidemic Mediterranean hot spot in Spain. Acta Trop. 2021, 222, 106036. [Google Scholar] [CrossRef]
- Barbiéri, C.L. Immunology of canine leishmaniasis. Parasite Immunol. 2006, 28, 329–337. [Google Scholar] [CrossRef]
- Azami-Conesa, I.; Sansano-Maestre, J.; Martínez-Díaz, R.A.; Gómez-Muñoz, M.T. Invasive species as hosts of zoonotic infections: The case of american mink (neovison vison) and leishmania infantum. Microorganisms 2021, 9, 1531. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Saz, S.; Giner, J.; Verde, M.; Lacasta, D.; Riera, C.; Fisa, R. Antibodies to Leishmania in naturally exposed domestic ferrets (Mustela putorius furo) in Spain. Vet. Parasitol. 2021, 296, 109492. [Google Scholar] [CrossRef]
- Giner, J.; Villanueva-Saz, S.; Alcover, M.M.; Riera, C.; Fisa, R.; Verde, M.; Fernández, A.; Yzuel, A. Clinical leishmaniosis in a domestic ferret (Mustela putorius furo) treated with miltefosine plus allopurinol: Serological and clinical follow-up. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 143340. [Google Scholar] [CrossRef]
- Fernandez-Gallego, A.; Feo Bernabe, L.; Dalmau, A.; Esteban-Saltiveri, D.; Font, A.; Leiva, M.; Ortuñez-Navarro, A.; Peña, M.T.; Tabar, M.D.; Real-Sampietro, L.; et al. Feline leishmaniosis: Diagnosis, treatment and outcome in 16 cats. J. Feline Med. Surg. 2020, 22, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Alcover, M.M.; Basurco, A.; Fernandez, A.; Riera, C.; Fisa, R.; Gonzalez, A.; Verde, M.; Garrido, A.M.; Ruíz, H.; Yzuel, A.; et al. A cross-sectional study of Leishmania infantum infection in stray cats in the city of Zaragoza (Spain) using serology and PCR. Parasites Vectors 2021, 14, 178. [Google Scholar] [CrossRef]
- Vioti, G.; Dantas, M.; Galvis-Ovallos, F.; Alves, M.L.; Tiago, D.; Augusto, J.; Leonel, F.; Wolfgang, N.; Pereira, B.; Benassi, J.C.; et al. Xenodiagnosis in four domestic cats naturally infected by Leishmania infantum. Transbound. Emerg. Dis. 2021, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Abramo, F.; Albanese, F.; Gattuso, S.; Randone, A.; Fileccia, I.; Dedola, C.; Ibba, F.; Ottaiano, P.; Brianti, E. Skin lesions in feline leishmaniosis: A systematic review. Pathogens 2021, 10, 472. [Google Scholar] [CrossRef]
- Ortuño, M.; Nachum-Biala, Y.; García-Bocanegra, I.; Resa, M.; Berriatua, E.; Baneth, G. An epidemiological study in wild carnivores from Spanish Mediterranean ecosystems reveals association between Leishmania infantum, Babesia spp. and Hepatozoon spp. infection and new hosts for Hepatozoon martis, Hepatozoon canis and Sarcocystis spp. Transbound. Emerg. Dis. 2021, 26, 1–16. [Google Scholar] [CrossRef]
- Azami-Conesa, I.; Alberto, R. A Systematic Review (1990–2021) of Wild Animals Infected with Zoonotic Leishmania. Microorganisms 2021, 9, 1101. [Google Scholar] [CrossRef] [PubMed]
- Maroli, M.; Gradoni, L.; Oliva, G.; Castagnaro, M.; Crotti, A.; Lubas, G.; Paltrinieri, S.; Roura, X.; Zini, E.; Zatelli, A. Guidelines for prevention of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1200–1206. [Google Scholar] [CrossRef]
- Portero, M.; Miró, G.; Checa, R.; Martínez de Merlo, E.; Fragío, C.; Benito, M.; Sainz, Á.; Pérez, C. Role of Leishmania infantum in Meningoencephalitis of Unknown Origin in Dogs from a Canine Leishmaniosis Endemic Area. Microorganisms 2021, 9, 571. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Solano-Gallego, L.; Fondati, A.; Lubas, G.; Gradoni, L.; Castagnaro, M.; Crotti, A.; Maroli, M.; Oliva, G.; Roura, X.; et al. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1184–1191. [Google Scholar] [CrossRef]
- Cabré, M.; Planellas, M.; Ordeix, L.; Solano-gallego, L. Is signalment associated with clinicopathological findings in dogs with leishmaniosis? VetRecord 2021, 189, e451. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Koutinas, A.F.; Solano-Gallego, L.; Bourdeau, P.; Ferrer, L. Canine leishmaniosis—New concepts and insights on an expanding zoonosis: Part one. Trends Parasitol. 2008, 24, 324–330. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Cardoso, L.; Pennisi, M.G.; Petersen, C.; Bourdeau, P.; Oliva, G.; Miró, G.; Ferrer, L.; Baneth, G. Diagnostic Challenges in the Era of Canine Leishmania infantum Vaccines. Trends Parasitol. 2017, 33, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet. Parasitol. 2007, 149, 139–146. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Morell, P.; Arboix, M.; Alberola, J.; Ferrer, L. Prevalence of Leishmania infantum infection in dogs living in an area of canine Leishmaniasis endemicity using PCR on several tissues and serology. J. Clin. Microbiol. 2001, 39, 560–563. [Google Scholar] [CrossRef] [Green Version]
- Miró, G.; Montoya, A.; Roura, X.; Gálvez, R.; Sainz, A. Seropositivity rates for agents of canine vector-borne diseases in Spain: A multicentre study. Parasites Vectors 2013, 6, 117. [Google Scholar] [CrossRef] [Green Version]
- Gizzarelli, M.; Bosco, A.; Foglia Manzillo, V.; Bongiorno, G.; Bianchi, R.; Giaquinto, D.; Ben Fayala, N.E.H.; Varloud, M.; Crippa, A.; Gradoni, L.; et al. Examining the Relationship of Clinical and Laboratory Parameters with Infectiousness to Phlebotomus perniciosus and Its Potential Infectivity in Dogs with Overt Clinical Leishmaniasis. Front. Vet. Sci. 2021, 8, 1–8. [Google Scholar] [CrossRef]
- De Massis, F.; Ippoliti, C.; Simona, I.; Tittarelli, M.; Pelini, S.; Giansante, D.; Ciarrocchi, A. Canine leishmaniasis: Serological results in private and kennel dogs tested over a six-year period (2009–2014) in Abruzzo and Molise regions, Italy. Microorganisms 2020, 8, 1915. [Google Scholar] [CrossRef]
- Castelli, G.; Bruno, F.; Reale, S.; Catanzaro, S.; Valenza, V. Molecular Diagnosis of Leishmaniasis: Quantification of Parasite Load by a Real-Time PCR Assay with High Sensitivity. Pathogens 2021, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Ayene, Y.Y.; Mohebali, M.; Hajjaran, H.; Akhoundi, B.; Shojaee, S. A comparative study of nested—PCR and direct agglutination test (DAT) for the detection of Leishmania infantum infection in symptomatic and asymptomatic domestic dogs. BMC Res. Notes 2021, 14, 270. [Google Scholar] [CrossRef]
- Oliva, G.; Roura, X.; Crotti, A.; Maroli, M.; Castagnaro, M.; Gradoni, L.; Lubas, G.; Paltrinieri, S.; Zatelli, A.; Zini, E. Guidelines for treatment of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Roura, X.; Fondati, A.; Lubas, G.; Gradoni, L.; Maroli, M.; Oliva, G.; Paltrinieri, S.; Zatelli, A.; Zini, E. Prognosis and monitoring of leishmaniasis in dogs: A working group report. Vet. J. 2013, 198, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Cantos-Barreda, A.; Escribano, D.; Siriyasatien, P.; Cerón, J.J.; Thomas, M.C.; Afonso-Lehmann, R.N.; López, M.C.; Bernal, L.J.; Phumee, A.; Lubas, G.; et al. Detection of Leishmania infantum DNA by real-time PCR in saliva of dogs. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101542. [Google Scholar] [CrossRef] [PubMed]
- Cerón, J.J. Acute phase proteins, saliva and education in laboratory science: An update and some reflections. BMC Vet. Res. 2019, 15, 197. [Google Scholar] [CrossRef] [PubMed]
- Peres Rubio, C.; Cerón, J.J. Spectrophotometric assays for evaluation of Reactive Oxygen Species (ROS) in serum: General concepts and applications in dogs and humans. BMC Vet. Res. 2021, 8, 226. [Google Scholar] [CrossRef]
- Ejazi, S.A.; Choudhury, S.T.; Bhattacharyya, A.; Kamran, M.; Pandey, K.; Das, V.N.R.; Das, P.; da Silva, F.O.; Costa, D.L.; Costa, C.H.N.; et al. Development and clinical evaluation of serum and urine-based lateral flow tests for diagnosis of human visceral leishmaniasis. Microorganisms 2021, 9, 1369. [Google Scholar] [CrossRef]
- Cacheiro-Llaguno, C.; Parody, N.; Escutia, M.R.; Carnés, J. Role of circulating immune complexes in the pathogenesis of canine leishmaniasis: New players in vaccine development. Microorganisms 2021, 9, 712. [Google Scholar] [CrossRef]
- Tripathi, P.; Singh, V.; Naik, S. Immune response to leishmania: Paradox rather than paradigm. FEMS Immunol. Med. Microbiol. 2007, 51, 229–242. [Google Scholar] [CrossRef] [Green Version]
- Kushawaha, P.K.; Gupta, R.; Sundar, S.; Sahasrabuddhe, A.A.; Dube, A. Elongation Factor-2, a Th1 Stimulatory Protein of Leishmania donovani, Generates Strong IFN-γ and IL-12 Response in Cured Leishmania-Infected Patients/Hamsters and Protects Hamsters against Leishmania Challenge. J. Immunol. 2011, 187, 6417–6427. [Google Scholar] [CrossRef] [Green Version]
- Dubie, T.; Mohammed, Y. Review on the Role of Host Immune Response in Protection and Immunopathogenesis during Cutaneous Leishmaniasis Infection. J. Immunol. Res. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Esch, K.J.; Petersen, C.A. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin. Microbiol. Rev. 2013, 26, 58–85. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, R.R.; Michalick, M.S.M.; da Silva, M.E.; Dos Santos, C.C.P.; Frézard, F.J.G.; da Silva, S.M. Canine Leishmaniasis: An Overview of the Current Status and Strategies for Control. Biomed Res. Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Gradoni, L. Canine Leishmania vaccines: Still a long way to go. Vet. Parasitol. 2015, 208, 94–100. [Google Scholar] [CrossRef]
- Gonçalves, R.D.S.; De Pinho, F.A.; Dinis-Oliveira, R.J.; Azevedo, R.; Gaifem, J.; Larangeira, D.F.; Ramos-Sanchez, E.M.; Goto, H.; Silvestre, R.; Barrouin-Melo, S.M. Mathematical modelling using predictive biomarkers for the outcome of canine leishmaniasis upon chemotherapy. Microorganisms 2020, 8, 745. [Google Scholar] [CrossRef]
- Reis, A.B.; Giunchetti, R.C.; Carrillo, E.; Martins-Filho, O.A.; Moreno, J. Immunity to Leishmania and the rational search for vaccines against Canine Leishmaniasis. Trends Parasitol. 2010, 26, 341–349. [Google Scholar] [CrossRef]
- Reis, A.B.; Teixeira-Carvalho, A.; Giunchetti, R.C.; Guerra, L.L.; Carvalho, M.G.; Mayrink, W.; Genaro, O.; Corrêa-Oliveira, R.; Martins-Filho, O.A. Phenotypic features of circulating leucocytes as immunological markers for clinical status and bone marrow parasite density in dogs naturally infected by Leishmania chagasi. Clin. Exp. Immunol. 2006, 146, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Manna, L.; Reale, S.; Picillo, E.; Vitale, F.; Gravino, A.E. Interferon-gamma (INF-γ), IL4 expression levels and Leishmania DNA load as prognostic markers for monitoring response to treatment of leishmaniotic dogs with miltefosine and allopurinol. Cytokine 2008, 44, 288–292. [Google Scholar] [CrossRef]
- Almeida, V.; Lima, I.; Fraga, D.; Carrillo, E.; Moreno, J.; Dos-Santos, W.L.C. Hematological Changes in Dogs with Visceral Leishmaniasis Are Associated with Increased IFN-γ and TNF Gene Expression Levels in the Bone Marrow. Microorganisms 2021, 9, 1618. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.; Christensen, S.M.; Mosser, D.M. Humoral immunity in leishmaniasis—Prevention or promotion of parasite growth? Cytokine X 2020, 2, 100046. [Google Scholar] [CrossRef] [PubMed]
- Mendes Roatt, B.; de Oliveira Cardoso, J.M.; de Brito, R.C.F.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Cardoso, L.; Baneth, G.; Bourdeau, P.; Koutinas, A.; Miró, G.; Oliva, G.; Solano-Gallego, L. LeishVet update and recommendations on feline leishmaniosis. Parasites Vectors 2015, 8, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olías-Molero, A.I.; Fontán-Matilla, E.; Cuquerella, M.; Alunda, J.M. Scientometric analysis of chemotherapy of canine leishmaniasis (2000–2020). Parasites Vectors 2021, 14, 36. [Google Scholar] [CrossRef]
- Baneth, G.; Shaw, S.E. Chemotherapy of canine leishmaniosis. Vet. Parasitol. 2002, 106, 315–324. [Google Scholar] [CrossRef]
- Carter, N.S.; Stamper, B.D.; Elbarbry, F.; Nguyen, V.; Lopez, S.; Kawasaki, Y.; Poormohamadian, R.; Roberts, S.C. Natural products that target the arginase in leishmania parasites hold therapeutic promise. Microorganisms 2021, 9, 267. [Google Scholar] [CrossRef]
- Baneth, G.; Africa, N.; Asia, C.; America, C. Treatment of Canine Leishmaniasis and Drug Resistance. In Proceedings of the World Small Animal Veterinary Association Congress, Copenhagen, Denmark, 25–28 September 2017. [Google Scholar]
- Martínez-Salazar, B.; Pereira, V.C.; Hauyon-La-Torre, Y.; Khamesipour, A.; Tacchini-Cottier, F. Evaluation of a new topical treatment for the control of cutaneous leishmaniasis. Microorganisms 2020, 8, 1803. [Google Scholar] [CrossRef]
- Ghaffarifar, F.; Molaei, S.; Mohammad Hassan, Z.; Dayer, M.S.; Dalimi, A.; Nasiri, V.; Foroutan, M.; Hajjaran, H. In Vitro and In Vivo Anti-Parasitic Activity of Artemisinin Combined with Glucantime and Shark Cartilage Extract on Iranian Strain of Leishmania major (MRHO/IR/75/ER). Jundishapur J. Microbiol. 2021, 14, e113313. [Google Scholar] [CrossRef]
- Medkour, H.; Bitam, I.; Laidoudi, Y.; Lafri, I.; Lounas, A.; Hamidat, H.K.; Mekroud, A.; Varloud, M.; Davoust, B.; Mediannikov, O. Potential of Artesunate in the treatment of visceral leishmaniasis in dogs naturally infected by Leishmania infantum: Efficacy evidence from a randomized field trial. PLoS Negl. Trop. Dis. 2020, 14, e0008947. [Google Scholar] [CrossRef] [PubMed]
- Santana, W.; Oliveira, S.S.C.; Ramos, M.H.; Santos, A.L.S.; Dolabella, S.S.; Souto, E.B.; Severino, P.; Jain, S. Exploring Innovative Leishmaniasis Treatment: Drug Targets from Pre-Clinical to Clinical Findings. Chem. Biodivers. 2021, 18, e2100336. [Google Scholar] [CrossRef] [PubMed]
- Manna, L.; Corso, R.; Galiero, G.; Cerrone, A.; Muzj, P.; Gravino, A.E. Long-term follow-up of dogs with leishmaniosis treated with meglumine antimoniate plus allopurinol versus miltefosine plus allopurinol. Parasites Vectors 2015, 8, 289. [Google Scholar] [CrossRef] [Green Version]
- Denerolle, P.; Bourdoiseau, G. Combination allopurinol and antimony treatment versus antimony alone and allopurinol alone in the treatment of canine leishmaniasis (96 cases). J. Vet. Intern. Med. 1999, 13, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Prata, S.; Cardoso, L.; Pereira da Fonseca, I.; Leal, R.O. Diagnosis and clinical management of canine leishmaniosis by general veterinary practitioners: A questionnaire-based survey in Portugal. Parasites Vectors 2021, 14, 306. [Google Scholar] [CrossRef]
- Vismarra, A.; Helen, L.; Moschi, A.; Ciuca, L.; Genchi, M. A survey on canine leishmaniosis: Best practice and guideline awareness among Italian veterinary practitioners. Prev. Vet. Med. 2021, 195, 105450. [Google Scholar] [CrossRef]
- Montoya, A.; Gálvez, R.; Checa, R.; Sarquis, J.; Plaza, A.; Barrera, J.P.; Marino, V.; Miró, G. Latest trends in L. infantum infection in dogs in Spain, Part II: Current clinical management and control according to a national survey of veterinary practitioners. Parasites Vectors 2020, 13, 205. [Google Scholar] [CrossRef] [Green Version]
- Valladares, J.E.; Riera, C.; Alberola, J.; Gállego, M.; Portús, M.; Cristòfol, C.; Franquelo, C.; Arboix, M. Pharmacokinetics of meglumine antimoniate after administration of a multiple dose in dogs experimentally infected with Leishmania infantum. Vet. Parasitol. 1998, 75, 33–40. [Google Scholar] [CrossRef]
- Daza González, M.A.; Miró, G.; Fermín Rodríguez, M.; Rupérez Noguer, C.; Fragío Arnold, C. Short term impacts of meglumine antimoniate treatment on kidney function in dogs with clinical leishmaniosis. Res. Vet. Sci. 2019, 126, 131–138. [Google Scholar] [CrossRef]
- Nelson, D.J.; Bugge, C.J.; Elion, G.B.; Berens, R.L.; Marr, J.J. Metabolism of pyrazolo (3,4-d) pyrimidines in Leishmania braziliensis and Leishmania donovani. Allopurinol, oxipurinol, and 4-aminopyrazolo (3,4-d) pyrimidine. J. Biol. Chem. 1979, 254, 3959–3964. [Google Scholar] [CrossRef]
- Torres, M.; Bardagí, M.; Roura, X.; Zanna, G.; Ravera, I.; Ferrer, L. Long term follow-up of dogs diagnosed with leishmaniosis (clinical stage II) and treated with meglumine antimoniate and allopurinol. Vet. J. 2011, 188, 346–351. [Google Scholar] [CrossRef]
- Torres, M.; Pastor, J.; Roura, X.; Tabar, M.D.; Espada, Y.; Font, A.; Balasch, J.; Planellas, M. Adverse urinary effects of allopurinol in dogs with leishmaniasis. J. Small Anim. Pract. 2016, 57, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Cavaliero, T.; Arnold, P.; Mathis, A.; Glaus, T.; Hofmann-Lehmann, R.; Deplazes, P. Clinical, serologic, and parasitologic follow-up after long-term allopurinol therapy of dogs naturally infected with Leishmania infantum. J. Vet. Intern. Med. 1999, 13, 330–334. [Google Scholar] [CrossRef]
- Gow, A.G.; Fairbanks, L.D.; Simpson, J.W.; Jacinto, A.M.L.; Ridyard, A.E. Xanthine urolithiasis in a Cavalier King Charles spaniel. Vet. Rec. 2011, 169, 2011–2013. [Google Scholar] [CrossRef]
- Osborne, C.A.; Lulich, J.P.; Swanson, L.L.; Albasan, H. Drug-Induced Urolithiasis. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 55–63. [Google Scholar] [CrossRef]
- Yasur-Landau, D.; Jaffe, C.L.; David, L.; Baneth, G. Allopurinol Resistance in Leishmania infantum from Dogs with Disease Relapse. PLoS Negl. Trop. Dis. 2016, 10, e0004341. [Google Scholar] [CrossRef]
- Yasur-landau, D.; Jaffe, C.L.; Doron-Faigenboim, A.; David, L.; Baneth, G. Induction of allopurinol resistance in Leishmania infantum isolated from dogs. PLoS Negl. Trop. Dis. 2017, e0005910. [Google Scholar] [CrossRef] [Green Version]
- Yasur-Landau, D.; Jaffe, C.L.; David, L.; Doron-Faigenboim, A.; Baneth, G. Resistance of Leishmania infantum to allopurinol is associated with chromosome and gene copy number variations including decrease in the S-adenosylmethionine synthetase (METK) gene copy number. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 403–410. [Google Scholar] [CrossRef]
- Miró, G.; Oliva, G.; Cruz, I.; Cañavate, C.; Mortarino, M.; Vischer, C.; Bianciardi, P. Multicentric, controlled clinical study to evaluate effectiveness and safety of miltefosine and allopurinol for canine leishmaniosis. Vet. Dermatol. 2009, 20, 397–404. [Google Scholar] [CrossRef]
- Daza González, M.A.; Fragío Arnold, C.; Fermín Rodríguez, M.; Checa, R.; Montoya, A.; Portero Fuentes, M.; Rupérez Noguer, C.; Martínez Subiela, S.; Cerón, J.J.; Miró, G. Effect of two treatments on changes in serum acute phase protein concentrations in dogs with clinical leishmaniosis. Vet. J. 2019, 245, 22–28. [Google Scholar] [CrossRef]
- Sindermann, H.; Engel, J. Development of miltefosine as an oral treatment for leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 2–5. [Google Scholar] [CrossRef]
- Mateo, M.; Maynard, L.; Vischer, C.; Bianciardi, P.; Miró, G. Comparative study on the short term efficacy and adverse effects of miltefosine and meglumine antimoniate in dogs with natural leishmaniosis. Parasitol. Res. 2009, 105, 155–162. [Google Scholar] [CrossRef]
- Gonçalves, G.; Campos, M.P.; Gonçalves, A.S.; Soares Medeiros, L.C.; Figueiredo, F.B. Treatment of canine visceral leishmaniasis with Milteforan induces Leishmania infantum resistance to miltefosine and amphotericin B. bioRxiv 2021. [Google Scholar] [CrossRef]
- Pineda, C.; Aguilera-Tejero, E.; Morales, M.C.; Belinchon-Lorenzo, S.; Gomez-Nieto, L.C.; Garcia, P.; Martinez-Moreno, J.M.; Rodriguez-Ortiz, M.E.; Lopez, I. Treatment of canine leishmaniasis with marbofloxacin in dogs with renal disease. PLoS ONE 2017, 12, e0185981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Subiela, S.; Strauss-Ayali, D.; Cerón, J.J.; Baneth, G. Acute phase protein response in experimental canine leishmaniosis. Vet. Parasitol. 2011, 180, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Marín, L.; Martínez-Subiela, S.; Pastor, J.; Tvarijonaviciute, A.; Garcia-Martinez, J.D.; Segarra, S.; Cerón, J.J. Evaluation of various biomarkers for kidney monitoring during canine leishmaniosis treatment. BMC Vet. Res. 2017, 13, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roura, X.; Cortadellas, O.; Day, M.J.; Benali, S.L.; D’Anna, N.; Fondati, A.; Gradoni, L.; Lubas, G.; Maroli, M.; Paltrinieri, S.; et al. Canine leishmaniosis and kidney disease: Q & A for an overall management in clinical practice. J. Small Anim. Pract. 2020, 62, E1–E19. [Google Scholar] [CrossRef]
- Gomes de Almeida Leal, G.; Roatt, B.M.; de Oliveira Aguiar-Soares, R.D.; Carneiro, C.M.; Giunchetti, R.C.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Francisco, A.F.; Cardoso, J.M.; Mathias, F.A.S.; et al. Immunological profile of resistance and susceptibility in naturally infected dogs by Leishmania infantum. Vet. Parasitol. 2014, 205, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Miró, G.; Petersen, C.; Cardoso, L.; Bourdeau, P.; Baneth, G.; Solano-Gallego, L.; Pennisi, M.G.; Ferrer, L.; Oliva, G. Novel areas for prevention and control of Canine Leishmaniosis. Trends Parasitol. 2017, 33, 718–730. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Mihalca, A.D.; Traub, R.J.; Lappin, M.; Baneth, G. Zoonotic parasites of Sheltered and Stray Dogs in the Era of the Global Economic and Political Crisis. Trends Parasitol. 2017, 37, 13–40. [Google Scholar] [CrossRef]
- Paradies, P.; Sasanelli, M.; Amato, M.E.; Greco, B.; De Palo, P.; Lubas, G. Monitoring the reverse to normal of clinico-pathological findings and the disease free interval time using four different treatment protocols for canine leishmaniosis in an endemic area. Res. Vet. Sci. 2012, 93, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Panarese, R.; Iatta, R.; Alfonso, J.; Roldan, M.; Zatelli, A.; Beugnet, F.; Otranto, D. Efficacy of afoxolaner (NexGard ®) in preventing the transmission of Leishmania infantum and Dirofilaria immitis to sheltered dogs in a highly endemic area. Parasites Vectors 2021, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.D.; Silva, J.D.; da Fonseca-Martins, A.M.; da Silveira Pratti, J.E.; Firmino-Cruz, L.; Maciel-Oliveira, D.; Dos-Santos, J.S.; Tenorio, J.I.N.; de Araujo, A.F.; Freire-De-Lima, C.G.; et al. Combined therapy with adipose tissue-derived mesenchymal stromal cells and meglumine antimoniate controls lesion development and parasite load in murine cutaneous leishmaniasis caused by Leishmania amazonensis. Stem Cell Res. Ther. 2020, 11, 374. [Google Scholar] [CrossRef]
- Machín, L.; Nápoles, R.; Gille, L.; Monzote, L. Leishmania amazonensis response to artemisinin and derivatives. Parasitol. Int. 2021, 80, 102218. [Google Scholar] [CrossRef]
- Kasabalis, D.; Chatzis, M.K.; Apostolidis, K.; Petanides, T.; Athanasiou, L.V.; Xenoulis, P.G.; Mataragka, A.; Ikonomopoulos, J.; Leontides, L.S.; Saridomichelakis, M.N. A randomized, blinded, controlled clinical trial comparing the efficacy of aminosidine (paromomycin)-allopurinol combination with the efficacy of meglumine antimoniate-allopurinol combination for the treatment of canine leishmaniosis due to Leishmania inf. Exp. Parasitol. 2020, 214, 107903. [Google Scholar] [CrossRef]
- Ready, P.D. Managing the spread of canine leishmaniosis in Europe. Vet. Rec. 2017, 180, 44–46. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Solano-Gallego, L.; Baneth, G.; Ribeiro, V.M.; de Paiva-Cavalcanti, M.; Otranto, D. Canine leishmaniosis in the Old and New Worlds: Unveiled similarities and differences. Trends Parasitol. 2012, 28, 531–538. [Google Scholar] [CrossRef]
- Kamhawi, S.; Coler, R.; Mondal, D.; Kamhawi, S.; Valenzuela, J.; Olivier, M. The yin and yang of leishmaniasis control. PLoS Negl. Trop. Dis. 2017, 11, e0005529. [Google Scholar] [CrossRef] [PubMed]
- Cotton, J.A. The Expanding World of Human Leishmaniasis. Trends Parasitol. 2017, 33, 341–344. [Google Scholar] [CrossRef]
- Berriatua, E.; Maia, C.; Baneth, G.; Jumakanova, Z.; Pereira, A.; Rocha, R.; Gasimov, E.; van der Stede, Y.; Torres, G.; Pereira, A.; et al. Leishmaniases in the European Union and Neighboring Countries. Emerg. Infect. Dis. J. 2021, 27, 1723. [Google Scholar] [CrossRef]
- Semenza, J.C.; Suk, J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018, 365, 244. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Thamsborg, S.M.; Otranto, D.; Guillot, J.; Blaga, R.; Deplazes, P.; Solano-Gallego, L. Major Parasitic Zoonoses Associated with Dogs and Cats in Europe. J. Comp. Pathol. 2015, 155, S54–S74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombolà, P.; Barlozzari, G.; Carvelli, A.; Scarpulla, M.; Iacoponi, F.; Macrì, G. Seroprevalence and risk factors associated with exposure to Leishmania infantum in dogs, in an endemic Mediterranean region. PLoS ONE 2021, 16, e0244923. [Google Scholar] [CrossRef]
- Kaszak, I.; Planellas, M.; Dworecka-Kaszak, B. Canine Leishmaniosis—An emerging disease. Ann. Parasitol. 2015, 61, 69–76. [Google Scholar]
- Osaki, S.C.; Bregonde, R.B.; Dahm, V.; Pereira, P.; Posta, C.; de Campos, M.P.; Figueiredo, F.B. Characterization of a municipality as free of canine visceral leishmaniasis in the context of One Health Caracterização de um município como livre de leishmaniose visceral canina. Braz. J. Vet. Parasitol. 2021, 30, e026720. [Google Scholar] [CrossRef]
- Shaw, S.E.; Langton, D.A.; Hillman, T.J. Canine leishmaniosis in the United Kingdom: A zoonotic disease waiting for a vector? Vet. Parasitol. 2009, 163, 281–285. [Google Scholar] [CrossRef]
- Silvestrini, P.; Batchelor, D.; Allenspach, K.; Maunder, C.; Seth, M.; Mas, A.; Hill, T.; Serrano, G.; Roura, X.; Planellas, M.; et al. Clinical leishmaniasis in dogs living in the UK. J. Small Anim. Pract. 2016, 57, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Alonso, F.H.; Vasilatis, D.M.; Veluvolu, S.M.; Willcox, J.L.; Scorza, B.M.; Petersen, C.A.; Kol, A. Canine leishmaniasis in Northern California—A case report. Vet. Clin. Pathol. 2021, 50, 71–75. [Google Scholar] [CrossRef]
- Curtin, J.M.; Aronson, N.E. Leishmaniasis in the United States: Emerging issues in a region of low endemicity. Microorganisms 2021, 9, 578. [Google Scholar] [CrossRef]
- Daval, N.; Marchal, C.; Guillaumot, L.; Hüe, T.; Ravel, C.; Keck, N.; Kasbari, M. First report of autochthonous non-vectorial canine leishmaniasis in New Caledonia, south-western Pacific: Implications for new control measures and recommendations on importation of dogs. Parasites Vectors 2016, 9, 108. [Google Scholar] [CrossRef] [Green Version]
- Le Rutte, E.A.; van der Wilt, L.S.; Bulstra, C.A.; Nieboer, D.; Kontoroupis, P.; de Vlas, S.J.; Richardus, J.H. Incidence and geographical distribution of canine leishmaniosis in 2016–2017 in Spain and France. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100613. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; Zowalaty, M.E.E.; Rahman, A.M.M.T.; Ashour, H.M. Zoonotic diseases: Etiology, impact, and control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- WHO. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Turner, P.; Laguens, R.; Chiang, J.; Marwick, C.; Mudenda, N.; Tansey, C.; McPherson, T.; Velez Rivas, J.; Blume, H.; Kadian, C.; et al. WVA Position on Leishmaniasis; World Veterinary Association: Bruxelles, Belgium, 2021. [Google Scholar]
- Dantas-Torres, F.; Miró, G.; Baneth, G.; Bourdeau, P.; Breitschwerdt, E.; Capelli, G.; Cardoso, L.; Day, M.J.; Dobler, G.; Ferrer, L.; et al. Canine Leishmaniasis Control in the Context of One Health. Emerg. Infect. Dis. J. 2019, 25, 5–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palatnik-de-Sousa, C.B.; Day, M.J. One Health: The global challenge of epidemic and endemic leishmaniasis. Parasites Vectors 2011, 4, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dye, C. The logic of visceral leishmaniasis control. Am. J. Trop. Med. Hyg. 1996, 55, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Travi, B.L.; Cordeiro-da-Silva, A.; Dantas-Torres, F.; Miró, G. Canine visceral leishmaniasis: Diagnosis and management of the reservoir living among us. PLoS Negl. Trop. Dis. 2018, 12, e0006082. [Google Scholar] [CrossRef] [Green Version]
- Velez, R.; Gállego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, M.C.; Lage, D.P.; Martins, V.T.; Chávez-Fumagalli, M.A.; Roatt, B.M.; Menezes-Souza, D.; Goulart, L.R.; Soto, M.; Tavares, C.A.P.; Coelho, E.A.F. Recent updates and perspectives on approaches for the development of vaccines against visceral leishmaniasis. Rev. Soc. Bras. Med. Trop. 2016, 49, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J. Assessment of vaccine-induced immunity against canine visceral leishmaniasis. Front. Vet. Sci. 2019, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- De Oliviera Aguiar-Soares, R.D.; Roatt, B.M.; Mathias, F.A.S.; Reis, L.E.S.; de Oliviera Cardoso, J.M.; de Brito, R.C.F.; Ker, H.G.; Corrêa-Oliveira, R.; Giunchetti, R.C.; Reis, A.B. Phase I and II clinical trial comparing the LBsap, Leishmune ®, and Leish-Tec ® vaccines against canine visceral leishmaniasis. Vaccines 2020, 8, 690. [Google Scholar] [CrossRef]
- Fernández Cotrina, J.; Iniesta, V.; Monroy, I.; Baz, V.; Hugnet, C.; Marañon, F.; Fabra, M.; Gómez-Nieto, L.C.; Alonso, C. A large-scale field randomized trial demonstrates safety and efficacy of the vaccine LetiFend ® against canine leishmaniosis. Vaccine 2018, 36, 1972–1982. [Google Scholar] [CrossRef]
- Miró Corrales, G.; Acosta de la Corte, C.; Marqués de Brito, N.; Ribas del Río, F.; Tabar Rodríguez, M.; Iniesta Orozco, V.; Montoya Matute, A. Estudio multicéntrico de seguridad post-autorización de la vacuna LetiFend ®: Estudio piloto en España. In Proceedings of the XXXIII Congreso Anual de AMVAC, Madrid, Spain, 3–5 March 2016. [Google Scholar]
- Kumar, R.; Chauhan, S.B.; Ng, S.S.; Sundar, S.; Engwerda, C.R. Immune checkpoint targets for host-directed therapy to prevent and treat leishmaniasis. Front. Immunol. 2017, 8, 1492. [Google Scholar] [CrossRef] [Green Version]
- Silvestrini, P. Leishmaniosis in dogs and cats. Practice 2019, 41, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Ochoa, P.; Castillo, J.A.; Gascón, M.; Zarate, J.J.; Alvarez, F.; Couto, C.G. Use of domperidone in the treatment of canine visceral leishmaniasis: A clinical trial. Vet. J. 2009, 179, 259–263. [Google Scholar] [CrossRef]
- Sabaté, D.; Llinás, J.; Homedes, J.; Sust, M.; Ferrer, L. A single-centre, open-label, controlled, randomized clinical trial to assess the preventive efficacy of a domperidone-based treatment programme against clinical canine leishmaniasis in a high prevalence area. Prev. Vet. Med. 2014, 115, 56–63. [Google Scholar] [CrossRef]
- Cavalera, M.A.; Gernone, F.; Uva, A.; D’Ippolito, P.; Roura, X.; Paltrinieri, S.; Zatelli, A. Effect of domperidone (leisguard ®) on antibody titers, inflammatory markers and creatinine in dogs with leishmaniosis and chronic kidney disease. Parasites Vectors 2021, 14, 525. [Google Scholar] [CrossRef]
- Santiago, M.E.B.; Neto, L.S.; Alexandre, E.C.; Munari, D.P.; Andrade, M.M.C.; Somenzari, M.A.; Ciarlini, P.C.; De Lima, V.M.F. Improvement in clinical signs and cellular immunity of dogs with visceral leishmaniasis using the immunomodulator P-MAPA. Acta Trop. 2013, 127, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Grimble, R.F. Nutritional modulation of immune function. Proc. Nutr. Soc. 2001, 60, 389–397. [Google Scholar] [CrossRef]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional modulation of immune function: Analysis of evidence, mechanisms, and clinical relevance. Front. Immunol. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Gil, A. Modulation of the immune response mediated by dietary nucleotides. Eur. J. Clin. Nutr. 2002, 56, S1–S4. [Google Scholar] [CrossRef]
- Hess, J.R.; Greenberg, N.A. The Role of Nucleotides in the Immune and Gastrointestinal Systems. Nutr. Clin. Pract. 2012, 27, 281–294. [Google Scholar] [CrossRef]
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Fontana, L.; Martínez-Augustin, O.; Gil, Á. Role of Dietary Nucleotides in Immunity. Funct. Food Rev. 2010, 2, 91–100. [Google Scholar] [CrossRef]
- Romano, V.; Martinez-Puig, D.; Torre, C.; Iraculis, N.; Vilaseca, L.; Chetrit, C. Dietary nucleotides improve the immune status of puppies at weaning. J. Anim. Physiol. Anim. Nutr. 2007, 91, 158–162. [Google Scholar] [CrossRef]
- Evangelio, E.; Borda, E.; Tecles, V.F.; Vicente, F.; Martínez-Puig, D.; Ceron, J.J.; Chetrit, C. A dietary nucleotide formula improves the immune status of dogs receiving a chemotherapy treatment. Vet. Clin. Pathol. 2008, 37, 13–40. [Google Scholar] [CrossRef]
- Burkhard, M.J.O.; Sanchez, N.; Torre, C.; Couto, C.G. Dietary nucleotides in dogs undergoing anticancer chemotherapy. Vet. Clin. Pathol. 2011, 40, 595–613. [Google Scholar] [CrossRef]
- Bernal, L.J.; Segarra, S.; Martínez-subiela, S.; Cerón, J.J. Use of Dietary Nucleotides and Active Hexose Correlated Compound in the Treatment of Non-Responsive Canine Demodicosis: A Report of Two Cases. In Proceedings of the Southern European Veterinarian Conference, Barcelona, Spain, 16–18 October 2014; Volume 1, pp. 2–4. [Google Scholar]
- Segarra, S.; Miró, G.; Montoya, A.; Pardo-Marín, L.; Boqué, N.; Ferrer, L.; Cerón, J. Randomized, allopurinol-controlled trial of the effects of dietary nucleotides and active hexose correlated compound in the treatment of canine leishmaniosis. Vet. Parasitol. 2017, 239, 50–56. [Google Scholar] [CrossRef]
- Segarra, S.; Miró, G.; Montoya, A.; Pardo-Marín, L.; Teichenné, J.; Ferrer, L.; Cerón, J.J. Prevention of disease progression in Leishmania infantum-infected dogs with dietary nucleotides and active hexose correlated compound. Parasites Vectors 2018, 11, 103. [Google Scholar] [CrossRef] [Green Version]
- Leal, R.O.; Pereira, H.; Cartaxeiro, C.; Delgado, E.; da Conceição Peleteiro, M.; Pereira da Fonseca, I. Granulomatous rhinitis secondary to feline leishmaniosis: Report of an unusual presentation and therapeutic complications. J. Feline Med. Surg. Open Rep. 2018, 4, 205511691881137. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.; Hernández, J.; Tabar, M. Leishmaniosis en un gato con pancitopenia. In Proceedings of the Southern European Veterinary Conference, Seville, Spain, 7–9 November 2019. [Google Scholar]
- Riera, J.; Pons, V.; Martinez-Puig, D.; Chetrit, C.; Tur, J.A.; Pons, A.; Drobnic, F. Dietary nucleotide improves markers of immune response to strenuous exercise under a cold environment. J. Int. Soc. Sports Nutr. 2013, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Casajús, J.; Martínez-Puig, D.; Sánchez, D.; Aguiló, J.; Anel, A.; Lou, J.; Chetrit, C. The effects of a nucleotide supplement (Inmunactive) on lymphocite proliferation after intensive exercise. In Book of Abstracts, Proceedings of the 14th Annual Congress of the European College of Sport Science, Oslo, Norway, 24–27 June 2009; European College of Sport Science: Malmo, Sweden, 2009; p. 129. [Google Scholar]
- Reda, R.M.; Selim, K.M.; Mahmoud, R.; El-Araby, I.E. Effect of dietary yeast nucleotide on antioxidant activity, non-specific immunity, intestinal cytokines, and disease resistance in Nile Tilapia. Fish Shellfish Immunol. 2018, 80, 281–290. [Google Scholar] [CrossRef]
- Selim, K.M.; Reda, R.M.; Mahmoud, R.; El-Araby, I.E. Effects of nucleotides supplemented diets on growth performance and expressions of ghrelin and insulin-like growth factor genes in Nile tilapia, Oreochromis niloticus. J. Appl. Aquac. 2020, 32, 157–174. [Google Scholar] [CrossRef]
- Romano, N.; Fischer, H.; Rossi, W.; Quintero, H.; Limbaugh, N. Effects of bioprocessed soybean meal and nucleotide supplementation on growth, physiology and histomorphology in largemouth bass, Micropterus salmoides, juveniles. Comp. Biochem. Physiol. 2021, 260, 111038. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.S.; Abdelaziz, M.; Abdel-Moneam, D.A.; Abd-Elhay, E.; Wassif, I.M.; Moustafa, M. Efficacy of dietary nucleotides (Nucleoforce ™) on growth, haemato-immunological response and disease resistance in pangasianodon hypophthalmus fish (sauvage, 1878) in Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 405–424. [Google Scholar] [CrossRef]
- El-Nokrashy, A.M.; El-Banna, R.A.; Edrise, B.M.; Abdel-Rahim, M.M.; Jover-Cerdá, M.; Tomás-Vidal, A.; Prince, A.; Davies, S.J.; El-Haroun, E.R.; Goda, A.M.A.S. Impact of nucleotide enriched diets on the production of gilthead seabream, Sparus aurata fingerlings by modulation of liver mitochondrial enzyme activitity, antioxidant status, immune gene expression, and gut microbial ecology. Aquaculture 2021, 535, 736398. [Google Scholar] [CrossRef]
- Novriadi, R.; Ilham, I.; Roigé, O.; Segarra, S. Effects of dietary nucleotides supplementation on growth, total haemocyte count, lysozyme activity and survival upon challenge with Vibrio harveyi in pacific white shrimp, Litopenaeus vannamei. Aquac. Rep. 2021, 21, 100840. [Google Scholar] [CrossRef]
- Borda, E.; Estévez, A.; Tort, L. Effect of the free nucleotides on the immune response during first stage of fattening in gilthhead bream (Sparus aurata). In Proceedings of the Aquaculture Europe, Trondheim, Norway, 5–9 August 2005; p. 1. [Google Scholar]
- Estruch, G.; Tortosa, I.; Monge, R.; Godoy, S. Inclusión de aditivos en dietas vegetales para doradas. Efectos sobre el crecimiento, parámetros biométricos y nutritivos, digestibilidad e histología. In Actas del XV Congreso Nacional y I Congreso Ibérico de Acuicultura; Instituto de Investigación y Formación Agraria y Pesquera: Palma de Mallorca, Spain, 2015. [Google Scholar]
- Magouz, F.I.; Abdel-Rahim, M.M.; Lotfy, A.M.; Mosbah, A.; Alkafafy, M.; Sewilam, H.; Dawood, M.A.O. Dietary nucleotides enhanced growth performance, carcass composition, blood biochemical, and histology features of European sea bass, Dicentrarchus labrax L. Aquac. Rep. 2021, 20, 100738. [Google Scholar] [CrossRef]
- Borda, E.; de la Fuente, E. Efficacy of dietary nucleotides supplementation on the immune response in atlantic salmon (Salmo salar): Effects on salt water transfer and vaccination. In Proceedings of the Aquaculture Europe, Krakow, Poland, 15–18 September 2008. [Google Scholar]
- Sáenz de Rodrigáñez, M.A.; Barata, M.; Dias, J.; Pousão-Ferreira, P.; Morales, G.; Márquez, L.; Moyano, F.J.; Ribeiro, L. Evaluation of the Effect of Nucleotides on Intestinal Function in juveniles of meagre (Argyrosomus regius) fed on high plant protein diets. In Proceedings of the Aquaculture Europe, Prague, Czech Republic, 1–5 September 2012. [Google Scholar]
- Martinez-Puig, D.; Manzanilla, E.G.; Morales, J.; Borda, E.; Pérez, J.F.; Piñeiro, C.; Chetrit, C. Dietary nucleotide supplementation reduces occurrence of diarrhoea in early weaned pigs. Livest. Sci. 2007, 108, 276–279. [Google Scholar] [CrossRef]
- Superchi, P.; Saleri, R.; Borghetti, P.; De Angelis, E.; Ferrari, L.; Cavalli, V.; Amicucci, P.; Ossiprandi, M.C.; Sabbioni, A. Effects of dietary nucleotide supplementation on growth performance and hormonal and immune responses of piglets. Animal 2012, 6, 902–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Prado, M.; Borda, E.; Sánchez, C.; Calsamiglia, S. Can nucleotides used in the diet of dairy cows act as modulators of the immunity? In Proceedings of the XVII Jornadas sobre Producción Animal, Zaragoza, Spain, 30–31 May 2017; Volume 38, pp. 231–233. [Google Scholar]
- Esteve-Garcia, E.; Martinez-Puig, D.; Borda, E.; Chetrit, C. Efficacy of a nucleotide preparation in broiler chickens. In Proceedings of the 16th European Symposium on Poultry Nutrition, Strasbourg, France, 26–30 August 2007; pp. 511–514. [Google Scholar]
- Khedr, N.; Ahmed, T.; Nagiub, S. Effect of dietary nucleotide supplementation on broiler intestinal histomorphology. Benha Vet. Med. J. 2020, 39, 127–131. [Google Scholar] [CrossRef]
- Mohamed, F.F.; Hady, M.M.; Kamel, N.F.; Ragaa, N.M. The impact of exogenous dietary nucleotides in ameliorating Clostridium perfringens infection and improving intestinal barriers gene expression in broiler chicken. Vet. Anim. Sci. 2020, 10, 100130. [Google Scholar] [CrossRef] [PubMed]
- Kamel, N.F.; Hady, M.M.; Ragaa, N.M.; Mohamed, F.F. Effect of nucleotides on growth performance, gut health, and some immunological parameters in broiler chicken exposed to high stocking density. Livest. Sci. 2021, 253, 104703. [Google Scholar] [CrossRef]
- Borda, E.; Palomo, A.; Fabian, E.; López, A. Effect of nucleotide supplementation in sows diet in the development of weaned piglets. In Proceedings of the Digestive Physiology of Pigs (DDP) Congress, Kliczków, Poland, 19–21 May 2015. [Google Scholar]
- Palomo, A.; Borda, E.; Fabian, E.; López, A. Effect of nucleotide supplementation in sows lactation to improve health and development of small intestine of piglets. In Proceedings of the Digestive Physiology of Pigs (DDP) Congress, Kliczków, Poland, 19–21 May 2015. [Google Scholar]
- Segarra, S.; Pérez, L.; Fabián, E.; Borda, E.; Palomo, A. Dietary nucleotide supplementation in sows during lactation: Effects on piglets per-formance and mortality. In Proceedings of the European Symposium of Porcine Health Management, Prague, Czech Republic, 3–5 May 2017. [Google Scholar]
- Borda, E.; Mejía, A.C.; Herrero, J.; Langeoire, G. Effect of dietary nucleotide supplementation in sows during lactation: Development of piglets at weaning period. In Proceedings of the European Symposium of Porcine Health Management, Barcelona, Spain, 9–11 May 2018. [Google Scholar]
- Roselli, M.; Britti, M.S.; Le Huërou-Luron, I.; Marfaing, H.; Zhu, W.Y.; Mengheri, E. Effect of different plant extracts and natural substances (PENS) against membrane damage induced by enterotoxigenic Escherichia coli K88 in pig intestinal cells. Toxicol. Vitr. 2007, 21, 224–229. [Google Scholar] [CrossRef]
- Andrés-Elias, N.; Pujols, J.; Badiola, I.; Torrallardona, D. Effect of nucleotides and carob pulp on gut health and performance of weanling piglets. Livest. Sci. 2007, 108, 280–283. [Google Scholar] [CrossRef]
- Godlewski, M.M.; Bierła, J.B.; Strzałkowski, A.; Martinez-Puig, D.; Pajak, B.; Kotunia, A.; Chetrit, C.; Zabielski, R. A novel cytometric approach to study intestinal mucosa rebuilding in weaned pigs fed with dietary nucleotides. Livest. Sci. 2009, 123, 215–220. [Google Scholar] [CrossRef]
- Bach, A.; Ferrer, A.; Martínez-Puig, D.; Ahedo, J. Effects of supplementing a mix of nucleotides to dairy calves prior weaning on respiratory afflictions and immune response during the postweaning period. In Proceedings of the ADSA-CSAS-ASAS Joint Meeting, Montreal, QC, Canada, 12–16 July 2009; pp. 2–3. [Google Scholar]
- Smith, J.A.; Mathew, L.; Gaikwad, A.; Rech, B.; Burney, M.N.; Faro, J.P.; Lucci, J.A.; Bai, Y.; Olsen, R.J.; Byrd, T.T. From Bench to Bedside: Evaluation of AHCC Supplementation to Modulate the Host Immunity to Clear High-Risk Human Papillomavirus Infections. Front. Oncol. 2019, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-W.; Lee, N.; Fujii, H.; Kang, I. Active Hexose Correlated Compound promotes T helper (Th) 17 and 1 cell responses via inducing IL-1β production from monocytes in humans. Cell. Immunol. 2012, 275, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Ulbricht, C.; Brigham, A.; Bryan, J.K.; Catapang, M.; Chowdary, D.; Costa, D.; Culwell, S.; D’Auria, D.; Giese, N.; Iovin, R.; et al. An evidence-based systematic review of active hexose correlated compound (AHCC) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2013, 10, 264–308. [Google Scholar] [CrossRef]
- Yin, Z.; Fujii, H.; Walshe, T. Effects of active hexose correlated compound on frequency of CD4+ and CD8+ T cells producing interferon-γ and/or tumor necrosis factor-α in healthy adults. Hum. Immunol. 2010, 71, 1187–1190. [Google Scholar] [CrossRef]
- Daddaoua, A.; Martínez-Plata, E.; López-Posadas, R.; Vieites, J.M.; González, M.; Requena, P.; Zarzuelo, A.; Suárez, M.D.; Sánchez de Medina, F.; Martínez-Augustin, O. Active hexose correlated compound acts as a prebiotic and is antiinflammatory in rats with hapten-induced colitis. J. Nutr. 2007, 137, 1222–1228. [Google Scholar] [CrossRef]
- Shin, M.S.; Park, H.J.; Maeda, T.; Nishioka, H.; Fujii, H.; Kang, I. The Effects of AHCC®, a Standardized Extract of Cultured Lentinura edodes Mycelia, on Natural Killer and T Cells in Health and Disease: Reviews on Human and Animal Studies. J. Immunol. Res. 2019, 2019, 3758576. [Google Scholar] [CrossRef] [Green Version]
- Mathew, L.; Gaikwad, A.; Gonzalez, A.; Nugent, E.K.; Smith, J.A. Evaluation of Active Hexose Correlated Compound (AHCC) in Combination with Anticancer Hormones in Orthotopic Breast Cancer Models. Integr. Cancer Ther. 2017, 16, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Mallet, J.F.; Graham, É.; Ritz, B.W.; Homma, K.; Matar, C. Active Hexose Correlated Compound (AHCC) promotes an intestinal immune response in BALB/c mice and in primary intestinal epithelial cell culture involving toll-like receptors TLR-2 and TLR-4. Eur. J. Nutr. 2016, 55, 139–146. [Google Scholar] [CrossRef]
- Corradetti, B.; Vaiasicca, S.; Mantovani, M.; Virgili, E.; Bonucci, M.; Hammarberg Ferri, I. Bioactive Immunomodulatory Compounds: A Novel Combinatorial Strategy for Integrated Medicine in Oncology? BAIC Exposure in Cancer Cells. Integr. Cancer Ther. 2019, 18, 1534735419866908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dea-Ayuela, M.A.; Segarra, S.; Serrano, D.R.; Bolás-Fernández, F. Nucleotides and AHCC Enhance Th1 Responses in Vitro in Leishmania-Stimulated/Infected Murine Cells. Molecules 2020, 25, 3918. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Maia, C. Leishmania infection in cats and feline leishmaniosis: An updated review with a proposal of a diagnosis algorithm and prevention guidelines. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, 100035. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Villanueva Saz, S.; Cardoso, L.; Ordeix i Esteve, L.; Miró Corrales, G.; Fondati, A.; Peña Giménez, M.T.; Leiva Repiso, M.; Naranjo Freixa, C.; Dantas-Torres, F.; et al. Tratamiento y Pronóstico. In Leishmaniosis Una Revisión Actualizada; Servet: Zaragoza, Spain, 2013; pp. 151–164. ISBN 978-84-941389-1-1. [Google Scholar]
- Baxarias, M.; Martínez-Orellanaa, P.; Banethb, G.; Solano-Gallego, L. Immunotherapy in clinical canine leishmaniosis: A comparative update. Res. Vet. Sci. 2019, 125, 218–226. [Google Scholar] [CrossRef]
- Lombardi, P.; Palatucci, A.T.; Giovazzino, A.; Mastellone, V.; Ruggiero, G.; Rubino, V.; Musco, N.; Crupi, R.; Cutrignelli, M.I.; Britti, D.; et al. Naturally Infected by L. infantum Treated with a Nutritional Supplement. Animals 2019, 9, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sousa, R.; Alves, F.; Pinho, D.; Dinis-Oliveira, R.J.; Oliveira, M.; Sena, T.; Andrade, D.; Solc, S.; Farias, D.; Silvestre, R.; et al. Nutritional adjuvants with antioxidant properties in the treatment of canine leishmaniasis. Vet. Parasitol. 2021, 298, 109526. [Google Scholar] [CrossRef]
- Cortese, L.; Annunziatella, M.; Palatucci, A.T.; Lanzilli, S.; Rubino, V.; Di Cerbo, A.; Centenaro, S.; Guidetti, G.; Canello, S.; Terrazzano, G. An immune-modulating diet increases the regulatory T cells and reduces T helper 1 inflammatory response in Leishmaniosis affected dogs treated with standard therapy. BMC Vet. Res. 2015, 11, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastellone, V.; Musco, N.; Vassalotti, G.; Piantedosi, D.; Vastolo, A.; Cutrignelli, M.I.; Britti, D.; Cortese, L.; Lombardi, P. A nutritional supplement (Dìlsh ™) improves the inflammatory cytokines response, oxidative stress markers and clinical signs in dogs naturally infected by leishmania infantum. Animals 2020, 10, 938. [Google Scholar] [CrossRef]
- Plevraki, K.; Koutinas, A.F.; Kaldrymidou, H.; Roumpies, N.; Papazoglou, L.G.; Saridomichelakis, M.N.; Savvas, I.; Leondides, L. Effects of Allopurinol Treatment on the Progression of Chronic Nephritis in Canine Leishmaniosis (Leishmania infantum). J. Vet. Intern. Med. 2006, 20, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Feo, L.; Planellas, M.; Monné, J.M.; Dalmau, A.; Pastor, J.; Espada, I. Alteraciones urinarias secundarias al tratamiento prolongado con alopurinol en perros con leishmaniosis. In Proceedings of the Southern European Veterinary Conference, Barcelona, Spain, 18–20 October 2012; pp. 6–7. [Google Scholar]
- Borja, L.S.; de Sousa, O.M.F.; da Solcà, M.S.; Bastos, L.A.; Bordoni, M.; Magalhaes, J.T.; Larangeira, D.F.; Barrouin-Melo, S.M.; Fraga, D.B.M.; Veras, P.S.T. Parasite load in the blood and skin of dogs naturally infected by Leishmania infantum is correlated with their capacity to infect sand fly vectors. Vet. Parasitol. 2016, 229, 110–117. [Google Scholar] [CrossRef] [Green Version]
- De Jesus, L.C.F.; Leal, R.A.O. Xanthinuria Secondary to Allopurinolt Therapy in Dogs with Canine Leishmaniosis: Current Perspectives of the Iberian Veterinary Community. Master’s Thesis, Universidade de Lisboa, Lisbon, Portugal, 2021. [Google Scholar]
- Warburg, A.; Gelman, S.; Deutsch, J. Xanthine in urine stimulates growth of Leishmania promastigotes in vitro. J. Med. Microbiol. 2008, 57, 136–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Gálvez, G.; López-Alonso, M.; Pechova, A.; Mayo, B.; Dierick, N.; Gropp, J. Alternatives to antibiotics and trace elements (copper and zinc) to improve gut health and zootechnical parameters in piglets: A review. Anim. Feed Sci. Technol. 2021, 271, 114727. [Google Scholar] [CrossRef]
- Miró, G. Canine leishmaniosis. In Proceedings of the 9th World Congress of Veterinary Dermatology, Sydney, Australia, 20–24 October 2020. [Google Scholar]
- Travi, B.L.; Miró, G. Use of domperidone in canine visceral leishmaniasis: Gaps in veterinary knowledge and epidemiological implications. Mem. Inst. Oswaldo Cruz 2018, 113, e180301. [Google Scholar] [CrossRef] [PubMed]
- Zerbe, C.A.; Clark, T.P.; Sartia, J.L.; Kemppainen, R.J. Domperidone treatment enhances corticotropin-releasing hormone stimulated adrenocorticotropic hormone release from the dog pituitary. Neuroendocrinology 1993, 57, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Nasello, A.G.; Vanzeler, M.L.A.; Madureira, E.H.; Felicio, L.F. Effects of acute and long-term domperidone treatment on prolactin and gonadal hormone levels and sexual behavior of male and female rats. Pharmacol. Biochem. Behav. 1997, 58, 1089–1094. [Google Scholar] [CrossRef]
- Mazzi, C.; Riva, L.P.; Morandi, G.; Mainini, E.; Martinelli, I.; Bertaccini, G. Drug-induced alterations of the hypothalamus-hypophysis axis. Acta Psychiatr. Belg. 1980, 80, 619–637. [Google Scholar] [PubMed]
- Pennisi, M.G.; Hartmann, K.; Lloret, A.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hosie, M.J.; et al. Leishmaniosis in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2013, 15, 638–642. [Google Scholar] [CrossRef]
- Alexandre-Pires, G.; Santos, M.; Rodrigues, M.A.; Pereira, M.A.; Gomes, J.; Diaz, S.A.; Gomes, L.; Basso, A.; Reisinho, A.; Gomes, J.; et al. Leishmaniosis: New Insights in a Changing World. In Advances in Animal Health, Medicine and Production; Springer: Cham, Switzerland, 2020; pp. 301–320. ISBN 9783030619800. [Google Scholar]
- Charoensakulchai, S.; Bualert, L.; Manomat, J.; Mungthin, M.; Leelayoova, S.; Tan-Ariya, P.; Siripattanapipong, S.; Naaglor, T.; Piyaraj, P. Risk factors of leishmania infection among hiv-infected patients in trang province, southern Thailand: A study on three prevalent species. Am. J. Trop. Med. Hyg. 2020, 103, 1502–1509. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- WHO. Impact of the COVID-19 Pandemic on Seven Neglected Tropical Diseases: A Model-Based Analysis; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Guimarães Carvalho, S.F.; Vieira, T.M.; Moura, A.P.V.; Andrade, M.C. Should an intersection between visceral leishmaniasis endemicity and the COVID-19 pandemic be considered? Med. Hypotheses 2020, 144, 19–22. [Google Scholar] [CrossRef]
- Francis, M.J. How has COVID-19 changed our views of the One Health agenda? Vet. Rec. 2021, 188, 361. [Google Scholar] [CrossRef]
- Trilla, A. One world, one health: The novel coronavirus COVID-19 epidemic. Med. Clin. 2020, 154, 175–177. [Google Scholar] [CrossRef]
- Decaro, N.; Martella, V.; Saif, L.J.; Buonavoglia, C. COVID-19 from veterinary medicine and one health perspectives: What animal coronaviruses have taught us. Res. Vet. Sci. 2020, 131, 21–23. [Google Scholar] [CrossRef]
| Animal Species | Main Effects | Reference |
|---|---|---|
| Domestic dog, Canis familiaris | Increased antibody titers against parvovirus 14 days post-vaccination, higher unspecific immunoglobulin levels, and improved peripheral blood mononuclear cells test in puppies at weaning. | Romano et al., 2007 [143] |
| Domestic dog, Canis familiaris | Increased lymphocyte proliferation and higher levels of IgA, IgG, and IgM in dogs receiving chemotherapy treatment (in combination with AHCC). | Evangelio et al., 2008 [144] |
| Domestic dog, Canis familiaris | Improved leukopenia and neutropenia associated with chemotherapy, increased IgA and IgM levels, and expansion of CD3 and CD4 lymphocytes. | Burkhart et al., 2011 [145] |
| Domestic dog, Canis familiaris | Clinical and parasitological improvements in two cases of canine demodicosis unresponsive to ivermectin (in combination with AHCC). | Bernal et al., 2014 [146] |
| Domestic dog, Canis familiaris | In dogs with clinical leishmaniosis receiving an initial course of MGA, clinical superiority vs. allopurinol after 6 months, without producing xanthinuria (in combination with AHCC). | Segarra et al., 2017 [147] |
| style="border-bottom:solid thin">Domestic dog, Canis familiaris | style="border-bottom:solid thin">In clinically healthy L. infantum-infected dogs, significant reduction in serology and disease progression rate after 1 year (in combination with AHCC). | style="border-bottom:solid thin">Segarra et al., 2018 [148] |
| Domestic cat, Felis silvestris catus | Clinical efficacy of nucleotides and AHCC combined with miltefosine in a cat with leishmaniosis, which had developed side effects following treatment with allopurinol, as well as side effects to MGA treatment. | Leal et al., 2018 [149] |
| style="border-bottom:solid thin">Domestic cat, Felis silvestris catus | style="border-bottom:solid thin">Use of nucleotides with AHCC in a cat with leishmaniosis, which had developed xanthinuria secondary to allopurinol treatment. | style="border-bottom:solid thin">Domínguez et al., 2019 [150] |
| Human, Homo sapiens | Protective effect on biomarkers of immune response in athletes after four weeks of strenuous exercise. | Casajús et al., 2009 [152] |
| style="border-bottom:solid thin">Human, Homo sapiens | style="border-bottom:solid thin">Beneficial effect on biomarkers of immune response in athletes after four weeks of strenuous exercise under a cold environment. | style="border-bottom:solid thin">Riera et al., 2013 [151] |
| Nile tilapia, Oreochromis niloticus | Increased survival upon exposure to Aeromonas sobria, and improved levels of blood proteins, leukocytes, antioxidant activity, non-specific immunity, cytokines, and gene expression. | Reda et al., 2018 [153] |
| Nile tilapia, Oreochromis niloticus | Improved body protein and fat content, and increased expression of ghrelin and insulin-like growth factor genes. | Selim et al., 2020 [154] |
| Striped catfish, Pangasianodon hypophthalmus | Increased lymphocytic proliferation activity, nitric oxide concentration and lysozyme activity, and improved resistance to Pseudomonas aeruginosa challenge. | Yaseen et al., 2020 [156] |
| Gilthead seabream, Sparus aurata | Improved performance parameters, positive impact on liver enzymes, improvements in gene expression, and modulation of gut microbiome. | El-Nokrashy et al., 2020 [157] |
| Pacific white shrimp, Litopenaeus vannamei | Positive impact of nucleotides on the immune system and disease resistance against Vibrio harveyi in Pacific white shrimp. | Novriadi et al., 2021 [158] |
| Gilthead seabream, Sparus aurata | Increased gut associated lymphoid tissue (GALT) and enhanced leucocyte phagocytic capacity. | Borda et al., 2005 [159] |
| Gilthead seabream, Sparus aurata | Improved performance parameters, including final weight, feed conversion rate, and growth efficiency. | Estruch et al., 2015 [160] |
| European sea bass, Dicentrarchus labrax | Improved performance and biochemical parameters as well as improved gastrointestinal histological evaluation. | Magouz et al., 2021 [161] |
| Atlantic salmon, Salmo salar | Reduced mortality and improved immune response upon challenge with Piscirickettsia salmonis | Borda et al., 2008 [162] |
| Meagre, Argyrosomus regius | Increased relative growth rate in meagre fed diets with high levels of vegetable proteins. | Sáenz de Rodrigáñez et al., 2012 [163] |
| style="border-bottom:solid thin">Largemouth bass, Micropterus salmoides | style="border-bottom:solid thin">Improved histomorphology and enhanced expression of genes associated with immune response in juveniles fed with soybean-based diets. | style="border-bottom:solid thin">Romano et al., 2021 [155] |
| Piglets, Sus scrofa | Prevention of post-weaning diarrhea and attenuated reduction of villous height in weaned piglets. | Martínez-Puig et al., 2007 [164] |
| Piglets, Sus scrofa | Protective effect on intestinal cells against increased membrane permeability caused by enterotoxigenic Escherichia coli. | Roselli et al., 2007 [175] |
| Piglets, Sus scrofa | Modulation of gut microbiota composition in piglets after weaning, acting especially in the ileum. | Andrés-Elías et al., 2007 [176] |
| Piglets, Sus scrofa | Supplementation before weaning can improve the adaptive capabilities of weaned piglets to stressors, enhancing their growth performance. | Superchi et al., 2012 [165] |
| Piglets, Sus scrofa | Nucleotide supplementation in sows one week before farrowing until weaning significantly improves the performance of the weaned piglets. | Borda et al., 2015 [171] |
| Piglets, Sus scrofa | Nucleotide supplementation in sows one week before farrowing until weaning significantly improves the health and development of the small intestine of piglets at weaning. | Palomo et al., 2015 [172] |
| Piglets, Sus scrofa | Dietary nucleotide supplementation in sows during lactation results in the transmission of nucleotides to their piglets, leading to improvements in performance parameters and reduced mortality rates. | Segarra et al., 2017 [173] |
| style="border-bottom:solid thin">Piglets, Sus scrofa | style="border-bottom:solid thin">Nucleotide transmission from sows to piglets, allowing significantlyimproved growth and consumption by weaned piglets. | style="border-bottom:solid thin">Borda et al., 2018 [174] |
| style="border-bottom:solid thin">Calves, Bos taurus | style="border-bottom:solid thin">Reduced incidence of respiratory upset during transition from liquid to solid feeds. | style="border-bottom:solid thin">Bach et al., 2009 [178] |
| style="border-bottom:solid thin">Calves, Bos taurus | style="border-bottom:solid thin">Improved parameters related to immunity and health of the reproductive system. | style="border-bottom:solid thin">Rodríguez-Prado et al., 2017 [166] |
| Broiler chicken, Gallus gallus domesticus | Improved performance parameters during the first 21 days of life, including increased body weight and enhanced feed–to-gain ratio. | Esteve-Garcia et al., 2007 [167] |
| Broiler chicken, Gallus gallus domesticus | Increase in length of intestinal villi. | Khedr et al., 2020 [168] |
| Broiler chicken, Gallus gallus domesticus | Nucleotide supplementation counteracted the negative effects of C. perfringens challenge and led to the improved intestinal barrier function and intestinal histomorphology, with positive impact on performance. | Mohamed et al., 2020 [169] |
| style="border-bottom:solid thin">Broiler chicken, Gallus gallus domesticus | style="border-bottom:solid thin">Improve gut health and immunity during stressconditions. | style="border-bottom:solid thin">Kamel et al., 2021 [170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segarra, S. Nutritional Modulation of the Immune Response Mediated by Nucleotides in Canine Leishmaniosis. Microorganisms 2021, 9, 2601. https://doi.org/10.3390/microorganisms9122601
Segarra S. Nutritional Modulation of the Immune Response Mediated by Nucleotides in Canine Leishmaniosis. Microorganisms. 2021; 9(12):2601. https://doi.org/10.3390/microorganisms9122601
Chicago/Turabian StyleSegarra, Sergi. 2021. "Nutritional Modulation of the Immune Response Mediated by Nucleotides in Canine Leishmaniosis" Microorganisms 9, no. 12: 2601. https://doi.org/10.3390/microorganisms9122601