Immune Response to BNT162b2 in Solid Organ Transplant Recipients: Negative Impact of Mycophenolate and High Responsiveness of SARS-CoV-2 Recovered Subjects against Delta Variant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrolment
2.2. Humoral Response Elicited by BNT162b2 Vaccine
2.3. SARS-CoV-2 T Cell Response Elicited by BNT162b2 Vaccine
2.4. Statistical Analysis
3. Results
3.1. Humoral and Cell-Mediated Response Elicited by mRNA BNT162b2 Was Suboptimal in SARS-CoV-2 Naïve Transplanted Patients
3.2. Immune Response Elicited by Vaccination in Transplanted Patients Is Associated with Age and Time after Transplant
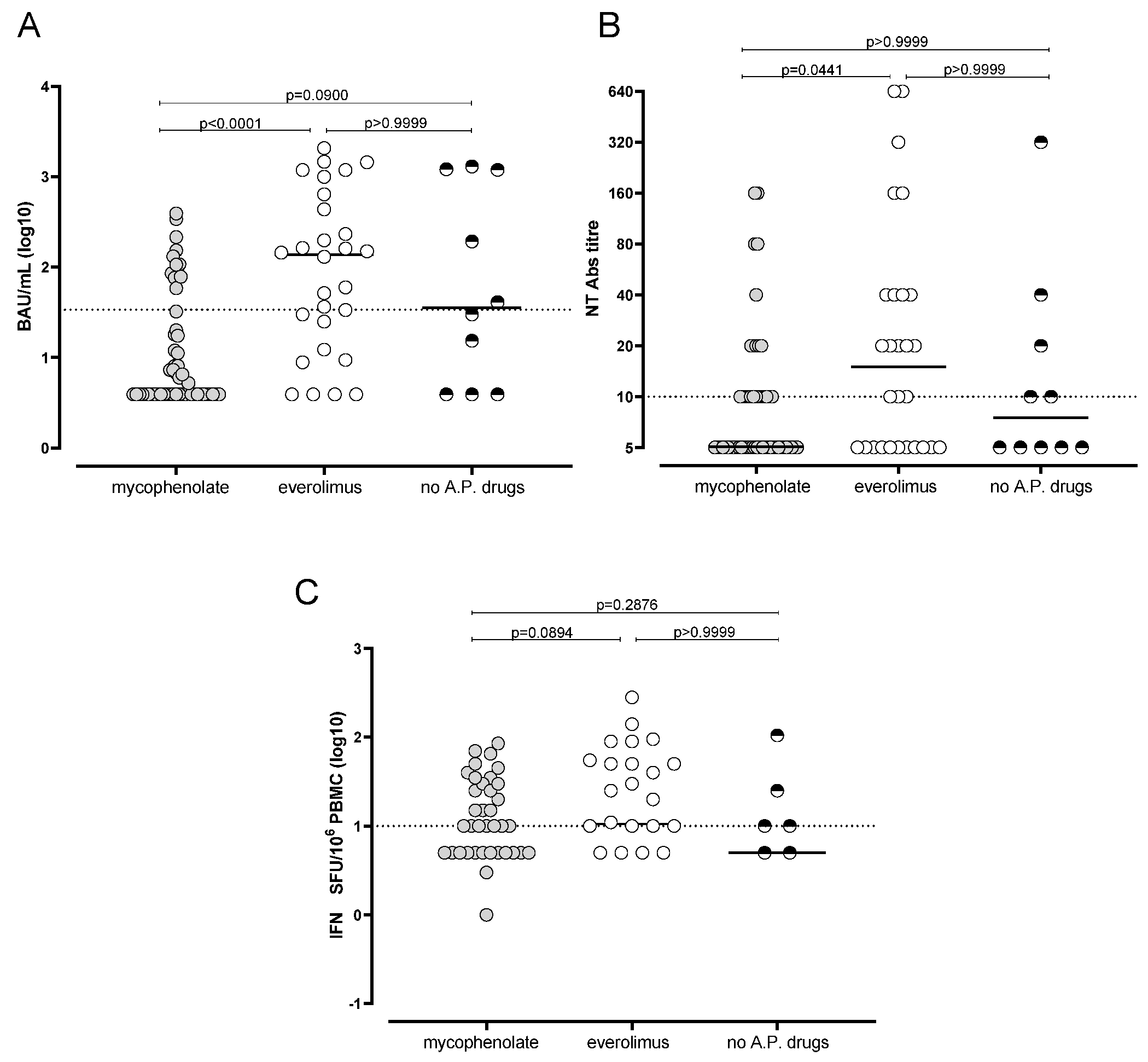
3.3. Reduced Humoral and Cell-Mediated Immune Response to BNT162b2 in Mycophenolate-Treated Patients
3.4. Humoral and T-Cell-Mediated Immune Response against SARS-CoV-2 Variants Were More Efficient in Previously Infected Subjects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azzi, Y.; Bartash, R.; Scalea, J.; Loarte-Campos, P.; Akalin, E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation 2021, 105, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Akalin, E.; Azzi, Y.; Bartash, R.; Seethamraju, H.; Parides, M.; Hemmige, V.; Ross, M.; Forest, S.; Goldstein, Y.D.; Ajaimy, M.; et al. Covid-19 and Kidney Transplantation. N. Engl. J. Med. 2020, 382, 2475–2477. [Google Scholar] [CrossRef]
- Alberici, F.; Delbarba, E.; Manenti, C.; Econimo, L.; Valerio, F.; Pola, A.; Maffei, C.; Possenti, S.; Piva, S.; Latronico, N.; et al. Management of Patients on Dialysis and With Kidney Transplantation During the SARS-CoV-2 (COVID-19) Pandemic in Brescia, Italy. Kidney Int. Rep. 2020, 5, 580–585. [Google Scholar] [CrossRef]
- Bossini, N.; Alberici, F.; Delbarba, E.; Valerio, F.; Manenti, C.; Possenti, S.; Econimo, L.; Maffei, C.; Pola, A.; Terlizzi, V.; et al. Kidney transplant patients with SARS-CoV-2 infection: The Brescia Renal COVID task force experience. Am. J. Transpl. 2020, 20, 3019–3029. [Google Scholar] [CrossRef] [PubMed]
- Caillard, S.; Anglicheau, D.; Matignon, M.; Durrbach, A.; Greze, C.; Frimat, L.; Thaunat, O.; Legris, T.; Moal, V.; Westeel, P.F.; et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020, 98, 1549–1558. [Google Scholar] [CrossRef]
- Crespo, M.; Mazuecos, A.; Rodrigo, E.; Gavela, E.; Villanego, F.; Sánchez-Alvarez, E.; González-Monte, E.; Jiménez-Martín, C.; Melilli, E.; Diekman, F.; et al. Respiratory and Gastrointestinal COVID-19 Phenotypes in Kidney Transplant Recipients. Transplantation 2020, 104, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Tré-Hardy, M.; Cupaiolo, R.; Papleux, E.; Wilmet, A.; Horeanga, A.; Antoine-Moussiaux, T.; Della Vecchia, A.; Beukinga, I.; Vekemans, M.; Blairon, L. Reactogenicity, safety and antibody response, after one and two doses of mRNA-1273 in seronegative and seropositive healthcare workers. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef] [PubMed]
- Keehner, J.; Horton, L.E.; Pfeffer, M.A.; Longhurst, C.A.; Schooley, R.T.; Currier, J.S.; Abeles, S.R.; Torriani, F.J. SARS-CoV-2 Infection after Vaccination in Health Care Workers in California. N. Engl. J. Med. 2021, 384, 1774–1775. [Google Scholar] [CrossRef]
- Callegaro, A.; Borleri, D.; Farina, C.; Napolitano, G.; Valenti, D.; Rizzi, M.; Maggiolo, F. Antibody response to SARS-CoV-2 vaccination is extremely vivacious in subjects with previous SARS-CoV-2 infection. J. Med. Virol. 2021, 93, 4612–4615. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses 2021, 13, 422. [Google Scholar] [CrossRef]
- Korth, J.; Jahn, M.; Dorsch, O.; Anastasiou, O.E.; Sorge-Hädicke, B.; Eisenberger, U.; Gäckler, A.; Dittmer, U.; Witzke, O.; Wilde, B.; et al. Impaired Humoral Response in Renal Transplant Recipients to SARS-CoV-2 Vaccination with BNT162b2 (Pfizer-BioNTech). Viruses 2021, 13, 756. [Google Scholar] [CrossRef]
- Havlin, J.; Svorcova, M.; Dvorackova, E.; Lastovicka, J.; Lischke, R.; Kalina, T.; Hubacek, P. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J. Heart Lung Transpl. 2021, 40, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Bedino, G.; Esposito, P.; Bosio, F.; Corradetti, V.; Valsania, T.; Rocca, C.; Pattonieri, E.F.; Gregorini, M.; Rampino, T.; Dal Canton, A. The role of therapeutic drug monitoring in the treatment of cytomegalovirus disease in kidney transplantation. Int. Urol. Nephrol. 2013, 45, 1809–1813. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef] [PubMed]
- Wadei, H.M.; Gonwa, T.A.; Leoni, J.C.; Shah, S.Z.; Aslam, N.; Speicher, L.L. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am. J. Transpl. 2021, 21, 3496–3499. [Google Scholar] [CrossRef]
- Percivalle, E.; Cambiè, G.; Cassaniti, I.; Nepita, E.V.; Maserati, R.; Ferrari, A.; Di Martino, R.; Isernia, P.; Mojoli, F.; Bruno, R.; et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 6 April 2020. Eurosurveillance 2020, 25, 2001031. [Google Scholar] [CrossRef] [PubMed]
- Rovida, F.; Percivalle, E.; Zavattoni, M.; Torsellini, M.; Sarasini, A.; Campanini, G.; Paolucci, S.; Baldanti, F.; Revello, M.G.; Gerna, G. Monoclonal antibodies versus reverse transcription-PCR for detection of respiratory viruses in a patient population with respiratory tract infections admitted to hospital. J. Med. Virol. 2005, 75, 336–347. [Google Scholar] [CrossRef]
- Cassaniti, I.; Percivalle, E.; Bergami, F.; Piralla, A.; Comolli, G.; Bruno, R.; Vecchia, M.; Sambo, M.; Colaneri, M.; Zuccaro, V.; et al. SARS-CoV-2 specific T-cell immunity in COVID-19 convalescent patients and unexposed controls measured by ex vivo ELISpot assay. Clin. Microbiol. Infect. 2021, 27, 1029–1034. [Google Scholar] [CrossRef]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transpl. 2021, 21, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Marinaki, S.; Adamopoulos, S.; Degiannis, D.; Roussos, S.; Pavlopoulou, I.D.; Hatzakis, A.; Boletis, I.N. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am. J. Transpl. 2021, 21, 2913–2915. [Google Scholar] [CrossRef]
- Benotmane, I.; Gautier-Vargas, G.; Cognard, N.; Olagne, J.; Heibel, F.; Braun-Parvez, L.; Martzloff, J.; Perrin, P.; Moulin, B.; Fafi-Kremer, S.; et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021, 99, 1498–1500. [Google Scholar] [CrossRef]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; El Ouafi, Z.; Ponsard, S.; Berrahal, I.; Achard, J.M.; et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 2153–2158. [Google Scholar] [CrossRef]
- Cucchiari, D.; Egri, N.; Bodro, M.; Herrera, S.; Del Risco-Zevallos, J.; Casals-Urquiza, J.; Cofan, F.; Moreno, A.; Rovira, J.; Banon-Maneus, E.; et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transpl. 2021, 21, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, D.; Hamzaoui, M.; Lemée, V.; Lamulle, J.; Hanoy, M.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; Le Roy, F.; et al. Antibody and T Cell Response to SARS-CoV-2 Messenger RNA BNT162b2 Vaccine in Kidney Transplant Recipients and Hemodialysis Patients. J. Am. Soc. Nephrol. 2021, 32, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
- Miele, M.; Busà, R.; Russelli, G.; Sorrentino, M.C.; Di Bella, M.; Timoneri, F.; Mularoni, A.; Panarello, G.; Vitulo, P.; Conaldi, P.G.; et al. Impaired anti-SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am. J. Transpl. 2021, 21, 2919–2921. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Klemis, V.; Schub, D.; Schneitler, S.; Reichert, M.C.; Wilkens, H.; Sester, U.; Sester, M.; Mihm, J. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transpl. 2021, 21, 3990–4002. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef]
- Stumpf, J.; Tonnus, W.; Paliege, A.; Rettig, R.; Steglich, A.; Gembardt, F.; Kessel, F.; Krooger, H.; Arndt, P.; Sradnick, J.; et al. Cellular And Humoral Immune Responses after Three Doses of BNT162b2 mRNA SARS-CoV-2 Vaccine in Kidney Transplant. Transplantation 2021, 105, e267. [Google Scholar] [CrossRef]
- Struijk, G.H.; Minnee, R.C.; Koch, S.D.; Zwinderman, A.H.; van Donselaar-van der Pant, K.A.; Idu, M.M.; ten Berge, I.J.; Bemelman, F.J. Maintenance immunosuppressive therapy with everolimus preserves humoral immune responses. Kidney Int. 2010, 78, 934–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.G.; Isbel, N.M.; Catton, M.G.; Leydon, J.A.; Becker, G.J.; Walker, R.G. Suppression of the humoral immune response by mycophenolate mofetil. Nephrol. Dial. Transpl. 1998, 13, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Bian, L.; Gao, Q.; Gao, F.; Wang, Q.; He, Q.; Wu, X.; Mao, Q.; Xu, M.; Liang, Z. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev. Vaccines 2021, 20, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Dębska-Ślizień, A.; Ślizień, Z.; Muchlado, M.; Kubanek, A.; Piotrowska, M.; Dąbrowska, M.; Tarasewicz, A.; Chamienia, A.; Biedunkiewicz, B.; Renke, M.; et al. Predictors of Humoral Response to mRNA COVID19 Vaccines in Kidney Transplant Recipients: A Longitudinal Study-The COViNEPH Project. Vaccines 2021, 9, 1165. [Google Scholar] [CrossRef]
- Stumpf, J.; Siepmann, T.; Lindner, T.; Karger, C.; Schwöbel, J.; Anders, L.; Faulhaber-Walter, R.; Schewe, J.; Martin, H.; Schirutschke, H.; et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur 2021, 9, 100178. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Cassaniti, I.; Bergami, F.; Percivalle, E.; Gabanti, E.; Sammartino, J.C.; Ferrari, A.; Guy Adzasehoun, K.M.; Zavaglio, F.; Zelini, P.; Comolli, G.; et al. Humoral and cell-mediated response elicited by SARS-CoV-2 mRNA vaccine BNT162b2 e in healthcare workers: A longitudinal observational study. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Barbur, I.; Chiang, T.P.; Ou, M.T.; Greenberg, R.S.; Teles, A.T.; Krach, M.R.; López, J.I.; Garonzik-Wang, J.M.; Avery, R.K.; et al. SARS-CoV-2 Messenger RNA Vaccine Immunogenicity in Solid Organ Transplant Recipients With Prior COVID-19. Transplantation 2021, 105, e270–e271. [Google Scholar] [CrossRef] [PubMed]


| Number (%) | |
|---|---|
| Male gender | 66 (60%) |
| Type of transplant | |
| Heart | 22 (20%) |
| Lung | 26 (23.6%) |
| Kidney | 62 (56.4%) |
| CNIs | |
| Cyclosporine | 22 (20%) |
| Tacrolimus | 83 (75.5%) |
| No | 5 * (4.5%) |
| Anti-proliferative drug | |
| Mycophenolate | 62 (56.4%) |
| Everolimus | 30 (27.3%) |
| Mycophenolate and everolimus | 6 (5.4%) |
| No | 12 (10.9%) |
| Steroid level | |
| Low doses (<5mg/day) | 68 (61.8%) |
| High doses (>5 mg/day) | 3 (2.7%) |
| No | 39 (35.5%) |
| Time after transplant | |
| Less than 1 year | 12 (10.9%) |
| Between 1 and 5 years | 45 (40.9%) |
| More than 5 years | 53 (48.2%) |
| SARS-CoV-2 positivity at T0 | |
| No | 97 (88.2%) |
| Yes | 13 (11.8%) |
| Dependent Variable | Independent Variable | Estimate β Coefficient | 95% Confidence Interval | p Value |
|---|---|---|---|---|
| S Trimeric (Log10BAU/mL) | Intercept | 2.690 | 1.903 to 3.468 | <0.001 |
| Age | −0.014 | −0.028 to 0.000 | 0.054 | |
| Time after transplant <18 months | −0.561 | −1.030 to −0.091 | 0.020 | |
| Use of mycophenolate | −0.806 | −1.110 to −0.498 | <0.001 | |
| Nt Abs (Log10 titer) | Intercept | 1.480 | 0.930 to 2.030 | <0.001 |
| Age | −0.00437 | −0.014 to 0.005 | 0.380 | |
| Time after transplant <18 months | −0.218 | −0.547 to 0.111 | 0.192 | |
| Use of mycophenolate | −0.264 | −0.480 to −0.048 | 0.017 | |
| Spike-specific T cells (Log10 Spots) | Intercept | 1.280 | 0.530 to 2.020 | 0.001 |
| Age | −0.006 | −0.019 to 0.0078 | 0.407 | |
| Time after transplant <18 months | −0.033 | −0.475 to 0.408 | 0.881 | |
| Use of mycophenolate | −0.200 | −0.489 to 0.090 | 0.174 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassaniti, I.; Bergami, F.; Arena, F.; Sammartino, J.C.; Ferrari, A.; Zavaglio, F.; Curti, I.; Percivalle, E.; Meloni, F.; Pandolfi, L.; et al. Immune Response to BNT162b2 in Solid Organ Transplant Recipients: Negative Impact of Mycophenolate and High Responsiveness of SARS-CoV-2 Recovered Subjects against Delta Variant. Microorganisms 2021, 9, 2622. https://doi.org/10.3390/microorganisms9122622
Cassaniti I, Bergami F, Arena F, Sammartino JC, Ferrari A, Zavaglio F, Curti I, Percivalle E, Meloni F, Pandolfi L, et al. Immune Response to BNT162b2 in Solid Organ Transplant Recipients: Negative Impact of Mycophenolate and High Responsiveness of SARS-CoV-2 Recovered Subjects against Delta Variant. Microorganisms. 2021; 9(12):2622. https://doi.org/10.3390/microorganisms9122622
Chicago/Turabian StyleCassaniti, Irene, Federica Bergami, Francesca Arena, Jose Camilla Sammartino, Alessandro Ferrari, Federica Zavaglio, Irene Curti, Elena Percivalle, Federica Meloni, Laura Pandolfi, and et al. 2021. "Immune Response to BNT162b2 in Solid Organ Transplant Recipients: Negative Impact of Mycophenolate and High Responsiveness of SARS-CoV-2 Recovered Subjects against Delta Variant" Microorganisms 9, no. 12: 2622. https://doi.org/10.3390/microorganisms9122622
APA StyleCassaniti, I., Bergami, F., Arena, F., Sammartino, J. C., Ferrari, A., Zavaglio, F., Curti, I., Percivalle, E., Meloni, F., Pandolfi, L., Pellegrini, C., Turco, A., Seminari, E., Pattonieri, E. F., Gregorini, M., Rampino, T., Sarasini, A., Lilleri, D., & Baldanti, F. (2021). Immune Response to BNT162b2 in Solid Organ Transplant Recipients: Negative Impact of Mycophenolate and High Responsiveness of SARS-CoV-2 Recovered Subjects against Delta Variant. Microorganisms, 9(12), 2622. https://doi.org/10.3390/microorganisms9122622








