Molecular Characterization and Genetic Diversity of Haplogroup E Human Lice in Guinea, West Africa
Abstract
:1. Introduction
2. Materials and Methods
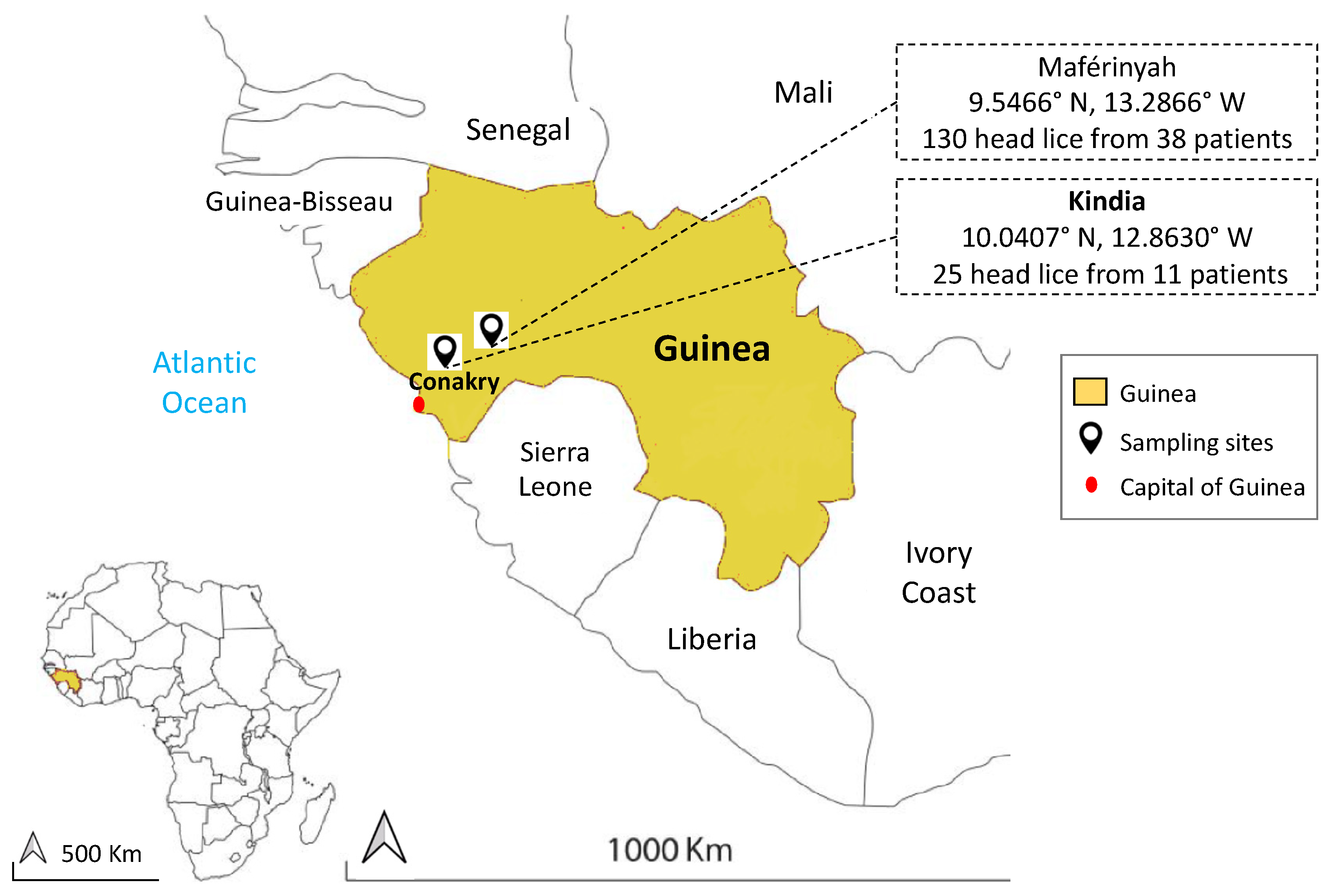
2.1. Lice Collection and DNA Extraction
2.2. Genotypic Status of Lice
2.2.1. Identification of Louse Mitochondrial Haplogroup by qPCR Assays
2.2.2. Identification of Louse Haplotype by Conventional PCR Assays and Sequencing
2.2.3. Sequences and Phylogenetic Diversity Analysis
2.3. Molecular Investigation of Lice Ecotype
2.3.1. Louse Ecotype Investigation by Multiplex qPCR Assays
2.3.2. Louse Ecotype Investigation by Conventional PCR Assays and Sequencing
2.4. Screening for the Presence of Pathogen’s DNA
2.4.1. Identification of Pathogen’s DNA by qPCR Assays
2.4.2. Identification of Pathogen’s DNA by Conventional PCR and Sequencing
2.5. Acinetobacter Resistance to Carbapenem
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Light, J.E.; Smith, V.S.; Allen, J.M.; Durden, L.A.; Reed, D.L. Evolutionary history of mammalian sucking lice (Phthiraptera: Anoplura). BMC Evol. Biol. 2010, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, D.L.; Smith, V.S.; Hammond, S.L.; Rogers, A.R.; Clayton, D.H. Genetic analysis of lice supports direct contact between modern and archaic humans. PLoS Biol. 2004, 2, e340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raoult, D.; Roux, V. The body louse as a vector of re-emerging human diseases. Clin. Infect. Dis. 1999, 29, 888–911. [Google Scholar] [CrossRef] [PubMed]
- Durden, L.A.; Musser, G.G. The sucking lice (Insecta, Anoplura) of the world: A taxonomic checklist with records of mammalian hosts and geographical distributions. Bulletin of the AMNH; no. 218,” in Sucking Lice and Hosts. Available online: http://digitallibrary.amnh.org/handle/2246/825 (accessed on 15 April 2018).
- Light, J.E.; Toups, M.A.; Reed, D.L. What’s in a name: The taxonomic status of human head and body lice. Mol. Phylogenet. Evol. 2008, 47, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D. A personal view of how paleomicrobiology aids our understanding of the role of lice in plague pandemics. Microbiol. Spectr. 2016, 4, 29–37. [Google Scholar]
- Robinson, D.; Leo, N.; Prociv, P.; Barker, S.C. Potential role of head lice, Pediculus humanus capitis, as vectors of Rickettsia prowazekii. Parasitol. Res. 2003, 90, 209–211. [Google Scholar] [CrossRef]
- Kim, J.H.; Min, J.S.; Kang, J.S.; Kwon, D.H.; Yoon, K.S.; Strycharz, J.; Koh, Y.H.; Pittendrigh, B.R.; Clark, J.M.; Lee, S.H. Comparison of the humoral and cellular immune responses between body and head lice following bacterial challenge. Insect Biochem. Mol. Biol. 2011, 41, 332–339. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoon, K.S.; Previte, D.J.; Pittendrigh, B.R.; Clark, J.M.; Lee, S.H. Comparison of the immune response in alimentary tract tissues from body versus head lice following Escherichia coli oral infection. J. Asia Pac. Entomol. 2012, 15, 409–412. [Google Scholar] [CrossRef]
- Kim, J.H.; Previte, D.J.; Yoon, K.S.; Murenzi, E.; Koehler, J.E.; Pittendrigh, B.R.; Lee, S.H.; Clark, J.M. Comparison of the proliferation and excretion of Bartonella quintana between body and head lice following oral challenge. Insect Mol. Biol. 2017, 26, 266–276. [Google Scholar] [CrossRef]
- Amanzougaghene, N.; Fenollar, F.; Raoult, D.; Mediannikov, O. Where are we with human lice? A review of the current state of knowledge. Front. Cell. Infect. Microbiol. 2020, 9, 474. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Poudel SK, S.; Isawa, H.; Hayashi, T.; Seki, N.; Tomita, T.; Kobayashi, M. First molecular evidence of Bartonella quintana in Pediculus humanus capitis (Phthiraptera: Pediculidae), collected from Nepalese children. J. Med. Entomol. 2006, 43, 110–112. [Google Scholar] [CrossRef]
- Piarroux, R.; Abedi, A.A.; Shako, J.-C.; Kebela, B.; Karhemere, S.; Diatta, G.; Davoust, B.; Raoult, D.; Drancourt, M. Plague epidemics and lice, Democratic Republic of the Congo. Emerg. Infect. Dis. 2013, 19, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Drali, R.; Shako, J.-C.; Davoust, B.; Diatta, G.; Raoult, D. A new clade of African body and head lice infected by Bartonella quintana and Yersinia pestis-Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2015, 93, 990–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutellis, A.; Mediannikov, O.; Bilcha, K.D.; Ali, J.; Campelo, D.; Barker, S.C.; Raoult, D. Borrelia recurrentis in head lice, Ethiopia. Emerg. Infect. Dis. 2013, 19, 796–798. [Google Scholar] [CrossRef]
- Angelakis, E.; Rolain, J.-M.; Raoult, D.; Brouqui, P. Altitude-dependent Bartonella quintana genotype C in head lice, Ethiopia. Emerg. Infect. Dis. 2011, 62, 2357–2359. [Google Scholar] [CrossRef]
- Sangaré, A.K.; Rolain, J.-M.; Gaudart, J.; Weber, P.; Raoult, D. Detection of Bartonella quintana in African body and head lice. Am. J. Trop. Med. Hyg. 2014, 47, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Amanzougaghene, N.; Fenollar, F.; Sangaré, A.K.; Sissoko, M.S.; Doumbo, O.K.; Raoult, D. Detection of bacterial pathogens including potential new species in human head lice from Mali. PLoS ONE 2017, 12, e0184621. [Google Scholar] [CrossRef] [Green Version]
- Eremeeva, M.E.; Capps, D.; Winful, E.B.; Warang, S.S.; Braswell, S.E.; Tokarevich, N.K.; Bonilla, D.L.; Durden, L.A. Molecular markers of pesticide resistance and pathogens in human head lice (Phthiraptera: Pediculidae) from rural Georgia, USA. J. Med. Entomol. 2017, 54, 1067–1072. [Google Scholar] [CrossRef]
- Boutellis, A.; Veracx, A.; Angelakis, E.; Diatta, G.; Mediannikov, O.; Trape, J.-F.; Raoult, D. Bartonella quintana in head lice from Sénégal. Vector-Borne Zoonotic Dis. 2012, 12, 564–567. [Google Scholar] [CrossRef]
- Amanzougaghene, N.; Akiana, J.; Mongo Ndombe, G.; Davoust, B.; Nsana, N.S.; Parra, H.-J.; Fenollar, F.; Raoult, D.; Mediannikov, O. Head Lice of Pygmies Reveal the Presence of Relapsing Fever Borreliae in the Republic of Congo. PLoS Negl. Trop. Dis. 2016, 10, e0005142. [Google Scholar] [CrossRef]
- Bouvresse, S.; Socolovschi, C.; Berdjane, Z.; Durand, R.; Izri, A.; Raoult, D.; Chosidow, O.; Brouqui, P. No evidence of Bartonella quintana but detection of Acinetobacter baumannii in head lice from elementary schoolchildren in Paris. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Abdissa, A.; Diatta, G.; Trape, J.-F.; Angelakis, E.; Mediannikov, O.; La Scola, B.; Raoult, D. Detection of Acinetobacter baumannii in human head and body lice from Ethiopia and identification of new genotypes. Int. J. Infect. Dis. 2012, 16, e680–e683. [Google Scholar] [CrossRef] [Green Version]
- Sunantaraporn, S.; Sanprasert, V.; Pengsakul, T.; Phumee, A.; Boonserm, R.; Tawatsin, A.; Thavara, U.; Siriyasatien, P. Molecular survey of the head louse Pediculus humanus capitis in Thailand and its potential role for transmitting Acinetobacter spp. Parasites Vectors 2015, 8, 127. [Google Scholar] [CrossRef] [Green Version]
- Candy, K.; Amanzougaghene, N.; Izri, A.; Brun, S.; Durand, R.; Louni, M.; Raoult, D.; Fenollar, F.; Mediannikov, O. Molecular survey of head and body lice, Pediculus humanus, in France. Vector-Borne Zoonotic Dis. 2018, 18, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Louni, M.; Amanzougaghene, N.; Mana, N.; Fenollar, F.; Raoult, D.; Bitam, I.; Mediannikov, O. Detection of bacterial pathogens in clade E head lice collected from Niger’s refugees in Algeria. Parasites Vectors 2018, 11, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mana, N.; Louni, M.; Parola, P.; Bitam, I. Human head lice and pubic lice reveal the presence of several Acinetobacter species in Algiers, Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2017, 53, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Boumbanda Koyo, C.S.; Amanzougaghene, N.; Davoust, B.; Tshilolo, L.; Lekana-Douki, J.B.; Raoult, D.; Mediannikov, O.; Fenollar, F. Genetic diversity of human head lice and molecular detection of associated bacterial pathogens in Democratic Republic of Congo. Parasites Vectors 2019, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Boumbanda-Koyo, C.S.; Mediannikov, O.; Amanzougaghene, N.; Oyegue-Liabagui, S.L.; Imboumi-Limoukou, R.K.; Raoult, D.; Lekana-Douki, J.B.; Fenollar, F. Molecular identification of head lice collected in Franceville (Gabon) and their associated bacteria. Parasites Vectors 2020, 13, 410. [Google Scholar] [CrossRef]
- Amanzougaghene, N.; Mediannikov, O.; Anh Ly, T.D.; Gautret, P.; Davoust, B.; Fenollar, F.; Izri, A. Molecular investigation and genetic diversity of Pediculus and Pthirus lice in France. Parasites Vectors 2020, 13, 177. [Google Scholar] [CrossRef]
- Ashfaq, M.; Prosser, S.; Nasir, S.; Masood, M.; Ratnasingham, S.; Hebert, P.D.N. High diversity and rapid diversification in the head louse, Pediculus humanus (Pediculidae: Phthiraptera). Sci. Rep. 2015, 5, 14188. [Google Scholar] [CrossRef] [Green Version]
- Amanzougaghene, N.; Mumcuoglu, K.Y.; Fenollar, F.; Alfi, S.; Yesilyurt, G.; Raoult, D.; Mediannikov, O. High Ancient Genetic Diversity of Human Lice, Pediculus humanus, from Israel Reveals New Insights into the Origin of Clade B Lice. PLoS ONE 2016, 11, e0164659. [Google Scholar] [CrossRef] [PubMed]
- Amanzougaghene, N.; Fenollar, F.; Davoust, B.; Djossou, F.; Ashfaq, M.; Bitam, I.; Raoult, D.; Mediannikov, O. Mitochondrial diversity and phylogeographic analysis of Pediculus humanus reveals a new Amazonian clade “F”. Infect. Genet. Evol. 2019, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ascunce, M.S.; Fane, J.; Kassu, G.; Toloza, A.C.; Picollo, M.I.; González-Oliver, A.; Reed, D.L. Mitochondrial diversity in human head louse populations across the Americas. Am. J. Phys. Anthropol. 2013, 152, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Reed, D.L.; Dittmar, K.; Kirchman, J.J.; Rolain, J.; Guillen, S.; Light, J.E. Molecular Identification of Lice from Pre-Columbian Mummies. J. Infect. Dis. 2008, 197, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Boutellis, A.; Bitam, I.; Fekir, K.; Mana, N.; Raoult, D. Evidence that clade A and clade B head lice live in sympatry and recombine in Algeria. Med. Vet. Entomol. 2015, 29, 94–98. [Google Scholar] [CrossRef]
- Al-Shahrani, S.A.; Alajmi, R.A.; Ayaad, T.H.; Al-Shahrani, M.A.; Shaurub, E.-S.H. Genetic diversity of the human head lice, Pediculus humanus capitis, among primary school girls in Saudi Arabia, with reference to their prevalence. Parasitol. Res. 2017, 116, 2637–2643. [Google Scholar] [CrossRef]
- Li, W.; Ortiz, G.; Fournier, P.-E.; Gimenez, G.; Reed, D.L.; Raoult, D. Genotyping of Human Lice Suggests Multiple Emergences of Body Lice from Local Head Louse Populations. PLoS Negl. Trop. Dis. 2010, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Veracx, A.; Boutellis, A.; Merhej, V.; Diatta, G.; Raoult, D. Evidence for an African Cluster of Human Head and Body Lice with Variable Colors and Interbreeding of Lice between Continents. PLoS ONE 2012, 7, e37804. [Google Scholar] [CrossRef]
- Olds, B.P.; Coates, B.S.; Steele, L.D.; Sun, W.; Agunbiade, T.A.; Yoon, K.S.; Strycharz, J.P.; Lee, S.H.; Paige, K.N.; Clark, J.M.; et al. Comparison of the transcriptional profiles of head and body lice. Insect Mol. Biol. 2012, 21, 257–268. [Google Scholar] [CrossRef]
- Drali, R.; Boutellis, A.; Raoult, D.; Rolain, J.M.; Brouqui, P. Distinguishing Body Lice from Head Lice by Multiplex Real-Time PCR Analysis of the Phum_PHUM540560 Gene. PLoS ONE 2013, 8, e58088. [Google Scholar] [CrossRef]
- La Scola, B.; Fournier, P.-E.; Brouqui, P.; Raoult, D. Detection and Culture of Bartonella quintana, Serratia marcescens, and Acinetobacter spp. from Decontaminated Human Body Lice. J. Clin. Microbiol. 2001, 39, 1707–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandelt, H.J.; Forster, P.; Rohl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Louni, M.; Mana, N.; Bitam, I.; Dahmani, M.; Parola, P.; Fenollar, F.; Raoult, D.; Mediannikov, O. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl. Trop. Dis. 2018, 12, e0006397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolain, J.-M.; Stuhl, L.; Maurin, M.; Raoult, D. Evaluation of Antibiotic Susceptibilities of Three Rickettsial Species Including Rickettsia felis by a Quantitative PCR DNA Assay. Antimicrob. Agents Chemother. 2002, 46, 2747–2751. [Google Scholar] [CrossRef] [Green Version]
- Parola, P.; Diatta, G.; Socolovschi, C.; Mediannikov, O.; Tall, A.; Bassene, H.; Trape, J.F.; Raoult, D. Tick-Borne Relapsing Fever Borreliosis, Rural Senegal. Emerg. Infect. Dis. 2011, 17, 883–885. [Google Scholar] [CrossRef] [Green Version]
- Raoult, D.; Angelakis, E.; Socolovschi, C. Bartonella quintana in Cimex hemipterus, Rwanda. Am. J. Trop. Med. Hyg. 2013, 89, 986–987. [Google Scholar] [CrossRef] [Green Version]
- Mediannikov, O.; Fenollar, F.; Socolovschi, C.; Diatta, G.; Bassene, H.; Molez, J.-F.; Sokhna, C.; Trape, J.-F.; Raoult, D. Coxiella burnetii in Humans and Ticks in Rural Senegal. PLoS Negl. Trop. Dis. 2010, 4, e654. [Google Scholar] [CrossRef] [Green Version]
- Dahmani, M.; Davoust, B.; Rousseau, F.; Raoult, D.; Fenollar, F.; Mediannikov, O. Natural Anaplasmataceae infection in Rhipicephalus bursa ticks collected from sheep in the French Basque Country. Ticks Tick-Borne Dis. 2017, 8, 18–24. [Google Scholar] [CrossRef]
- La Scola, B.; Gundi, V.A.K.B.; Khamis, A.; Raoult, D. Sequencing of the rpoB Gene and Flanking Spacers for Molecular Identification of Acinetobacter Species. J. Clin. Microbiol. 2006, 44, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Ly, T.D.A.; Kerbaj, J.; Edouard, S.; Hoang, V.T.; Louni, M.; Dao, T.L.; Benkouiten, S.; Badiaga, S.; Tissot-Dupont, H.; Raoult, D.; et al. The Presence of Acinetobacter baumannii DNA on the skin of homeless people and its relationship with body lice infestation. Preliminary Results. Front. Cell. Infect. Microbiol. 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.-Z.; Cash, D.M.; Chahine, M.A.; Nikolich, M.P.; Craft, D.W. Development and validation of a multiplex TaqMan real-time PCR for rapid detection of genes encoding four types of class D carbapenemase in Acinetobacter baumannii. J. Med. Microbiol. 2012, 61, 1532–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Scola, B.; Raoult, D. Acinetobacter baumannii in Human Body Louse. Emerg. Infect. Dis. 2004, 10, 1671–1673. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, S.; Deng, Y.; Song, C.; Kang, G.; Dong, Y.; Wang, Y.; Gao, F.; Huang, H. Comparative genomics analysis of Acinetobacter haemolyticus isolates from sputum samples of respiratory patients. Genomics 2020, 112, 2784–2793. [Google Scholar]
- Kempf, M.; Rolain, J.-M.; Diatta, G.; Azza, S.; Samb, B.; Mediannikov, O.; Sow, G.A.; Diene, S.M.; Fenollar, F.; Raoult, D. Carbapenem Resistance and Acinetobacter baumannii in Senegal: The Paradigm of a Common Phenomenon in Natural Reservoirs. PLoS ONE 2012, 7, e39495. [Google Scholar] [CrossRef] [Green Version]




| Target | Name | Primers and Probes (5′-3′) | Source |
|---|---|---|---|
| P. humanus | Cytb. Duplex A/D | FAM-CATTCTTGTCTACGTTCATATTTGG-TAMRA | [18] |
| VIC-TATTCTTGTCTACGTTCATGTTTGA-TAMRA | |||
| F_GATGTAAATAGAGGGTGGTT | |||
| R_GAAATTCCTGAAAATCAAAC | |||
| Cytb. Duplex B/C-E | FAM-GAGCTGGATAGTGATAAGGTTTAT-TAMRA | ||
| VIC-CTTGCCGTTTATTTTGTTGGGGTTT-TAMRA | |||
| F_TTAGAGCGMTTRTTTACCC | |||
| R_AYAAACACACAAAAMCTCCT | |||
| Cytb | F_GAGCGACTGTAATTACTAATC | [38] | |
| R_CAACAAAATTATCCGGGTCC | |||
| Phum540560 | FAM-CGATCACTCGAGTGAATTGCCA-TAMRA | [41] | |
| VIC-CTCTTGAATCGACGACCATTCGCT-TAMRA | |||
| GTCACGTTCGACAAATGTT | |||
| TTTCTATAACCACGACACGATAAAT | |||
| Rickettsia spp. citrate synthase (gltA) | RKNDO3 | FAM-CTATTATGCTTGCGGCTGTCGGTTC-TAMRA | [46] |
| F_GTGAATGAAAGATTACACTATTTAT | |||
| R_GTATCTTAGCAATCATTCTAATAGC | |||
| Borrelia spp. 16S ribosomal RNA | Bor16S | FAM-CCGGCCTGAGAGGGTGAACGG-TAMRA | [47] |
| F_AGCCTTTAAAGCTTCGCTTGTAG | |||
| R_GCCTCCCGTAGGAGTCTGG | |||
| Bartonella quintana | yopP-Hypothetical intracellular effector | FAM-GCGCGCGCTTGATAAGCGTG-TAMRA | [48] |
| F_GATGCCGGGGAAGGTTTTC | |||
| R_GCCTGGGAGGACTTGAACCT | |||
| Yersinia pestis | PLA | FAM-TCCCGAAAGGAGTGCGGGTAATAGG-TAMRA | [13] |
| F_ATG GAG CTT ATA CCG GAA AC | |||
| R_GCG ATA CTG GCC TGC AAG | |||
| Coxiella burnetii | IS1111 | FAM- CCGAGTTCGAAACAATGAGGGCTG-TAMRA | [49] |
| F_CGCTGACCTACAGAAATATGTCC | |||
| R_GGGGTAAGTAAATAATACCTTCTGG | |||
| Anaplasma spp. 23S ribosomal RNA | TtAna | FAM-GGATTAGACCCGAAACCAAG-TAMRA | [50] |
| F_TGACAGCGTACCTTTTGCAT | |||
| R_TGGAGGACCGAACCTGTTAC | |||
| Acinetobacter spp. RNA polymerase β subunit gene | rpoB | FAM-CGCGAAGATATCGGTCTSCAAGC-TAMR | [22] |
| F_TACTCATATACCGAAAAGAAACGG | |||
| R_GGYTTACCAAGRCTATACTCAAC | |||
| rpoB (zone1) | F_TAYCGYAAAGAYTTGAAAGAAG | [51] | |
| R_CMACACCYTTGTTMCCRTGA | |||
| rpoB (zone1) | F_TACAARATCTTYGAAGAAGC | This study | |
| R_CCACAACADAGDTTGTARRA | |||
| Acinetobacter baumanii. Type VI secretion system OmpA/MotB | OmpA/MotB | FAM_AAGTCGCCAAGAAACCTTGA_TAMRA | [52] |
| F_TCAACATCACAATCTTTAGTAGCTGA | |||
| R_CGCTCTTGCCAGCATAAAGA | |||
| Carbapenems genes | OXA-23 | 6-FAM-CCAGTCTATCAGGAACTTGCGCGA-BHQ_1 | [53] |
| F_GACACTAGGAGAAGCCATGAAG | |||
| R_CAGCATTACCGAAACCAATACG | |||
| OXA-24 | TET-AGTAACACCCATTCCCCATCCACTTTT-IABkFQ | ||
| F_GATGACCTTGCACATAACCG | |||
| R_CAGTCAACCAACCTACCTGTG | |||
| OXA-58 | Cy5-TGGACCAATACGACGTGCCAATTCT-IAbRQSp | ||
| AAGATTTTACTTTGGGCGAAGC | |||
| CAACTTCCGTGCCTATTTGC |
| Ecotype | N. of HL | Clade and Origin | N. of SNPs | Present SNPs |
|---|---|---|---|---|
| HL | 1 | A-Amazonia | 22 | SNP 1–22 |
| BL | 1 | A-Algeria | 0 | - |
| HL | 1 | E-Guinea | 18 | SNP 1–5, 8–12, 15–22 |
| HL | 2 | E-Guinea | 20 | SNP 1–12, 15–22 |
| HL | 4 | E-Guinea | 3 | SNP 1, 2 and 12 |
| HL | 33 | E-Guinea | 0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammoud, A.; Louni, M.; Baldé, M.C.; Beavogui, A.H.; Gautret, P.; Raoult, D.; Fenollar, F.; Misse, D.; Mediannikov, O. Molecular Characterization and Genetic Diversity of Haplogroup E Human Lice in Guinea, West Africa. Microorganisms 2021, 9, 257. https://doi.org/10.3390/microorganisms9020257
Hammoud A, Louni M, Baldé MC, Beavogui AH, Gautret P, Raoult D, Fenollar F, Misse D, Mediannikov O. Molecular Characterization and Genetic Diversity of Haplogroup E Human Lice in Guinea, West Africa. Microorganisms. 2021; 9(2):257. https://doi.org/10.3390/microorganisms9020257
Chicago/Turabian StyleHammoud, Alissa, Meriem Louni, Mamadou Cellou Baldé, Abdoul Habib Beavogui, Philippe Gautret, Didier Raoult, Florence Fenollar, Dorothée Misse, and Oleg Mediannikov. 2021. "Molecular Characterization and Genetic Diversity of Haplogroup E Human Lice in Guinea, West Africa" Microorganisms 9, no. 2: 257. https://doi.org/10.3390/microorganisms9020257





