Adaptation of Cupriavidus metallidurans CH34 to Toxic Zinc Concentrations Involves an Uncharacterized ABC-Type Transporter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Media, and Culture Conditions
2.2. Isolation of Zinc-Resistant Mutants
2.3. Assessment of Zinc-Resistant Phenotype
2.4. Construction of Plasmids
2.5. Construction of Deletion Mutant Strains
2.6. Whole-Genome Gene Expression Analysis and qRT-PCR
2.7. Genome Sequencing
3. Results
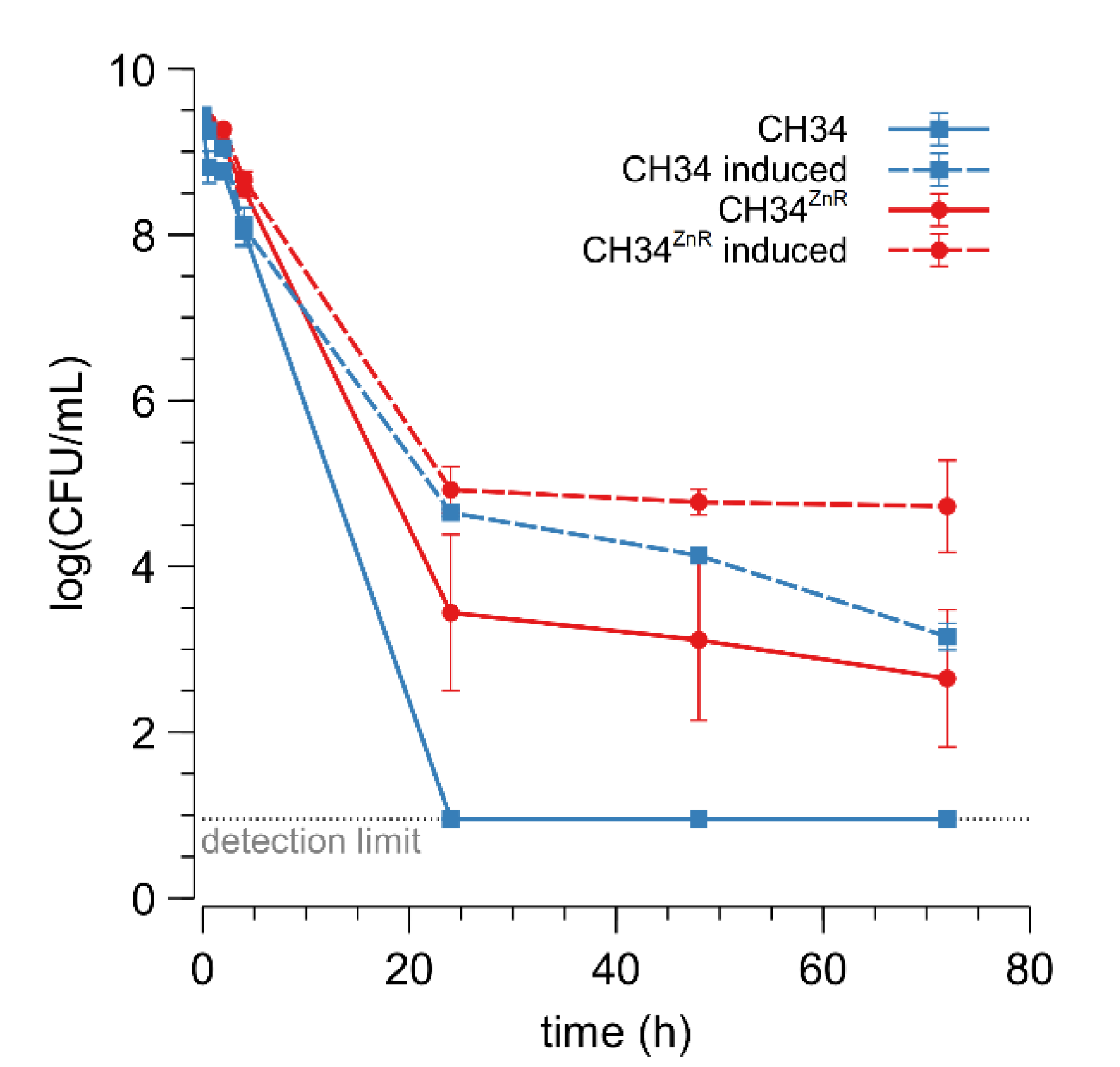
3.1. Isolation and Characterization of Zinc-Resistant CH34 Derivatives
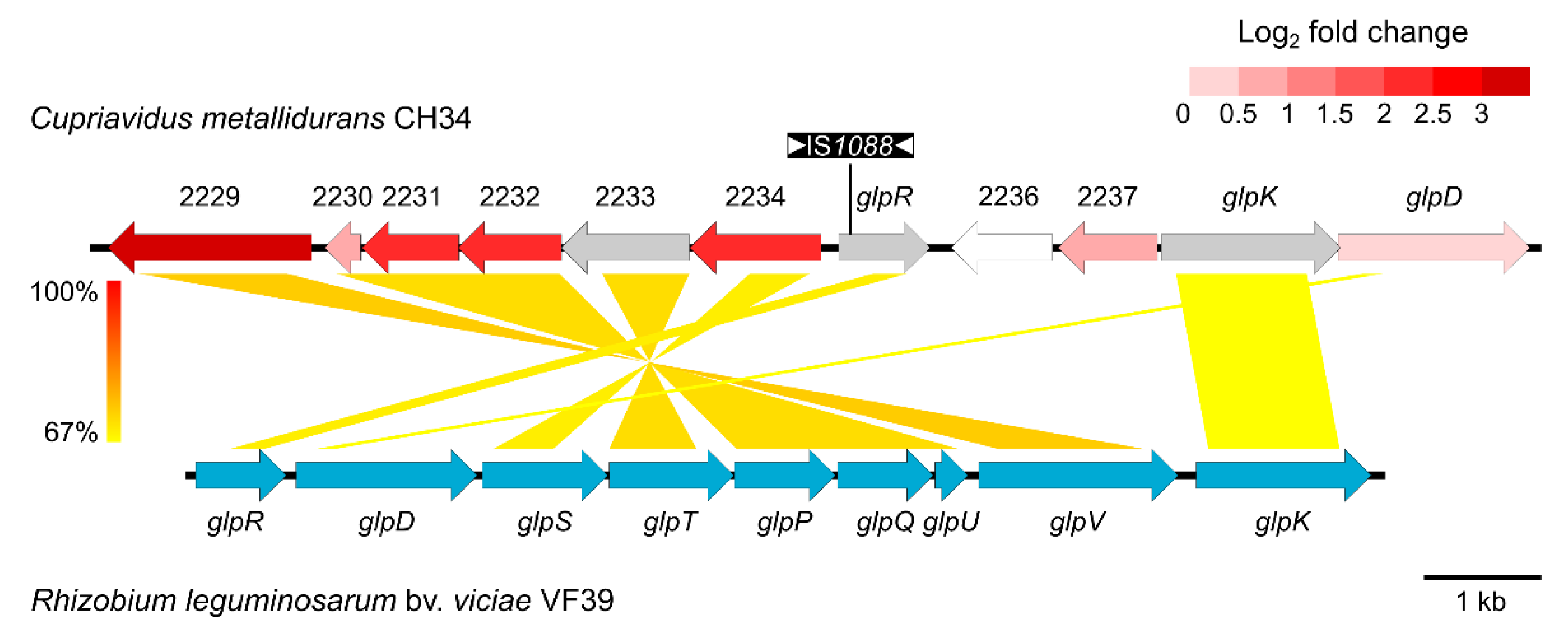
3.2. Whole-Genome Expression Profile and Genome Sequence Analysis of CH34ZnR
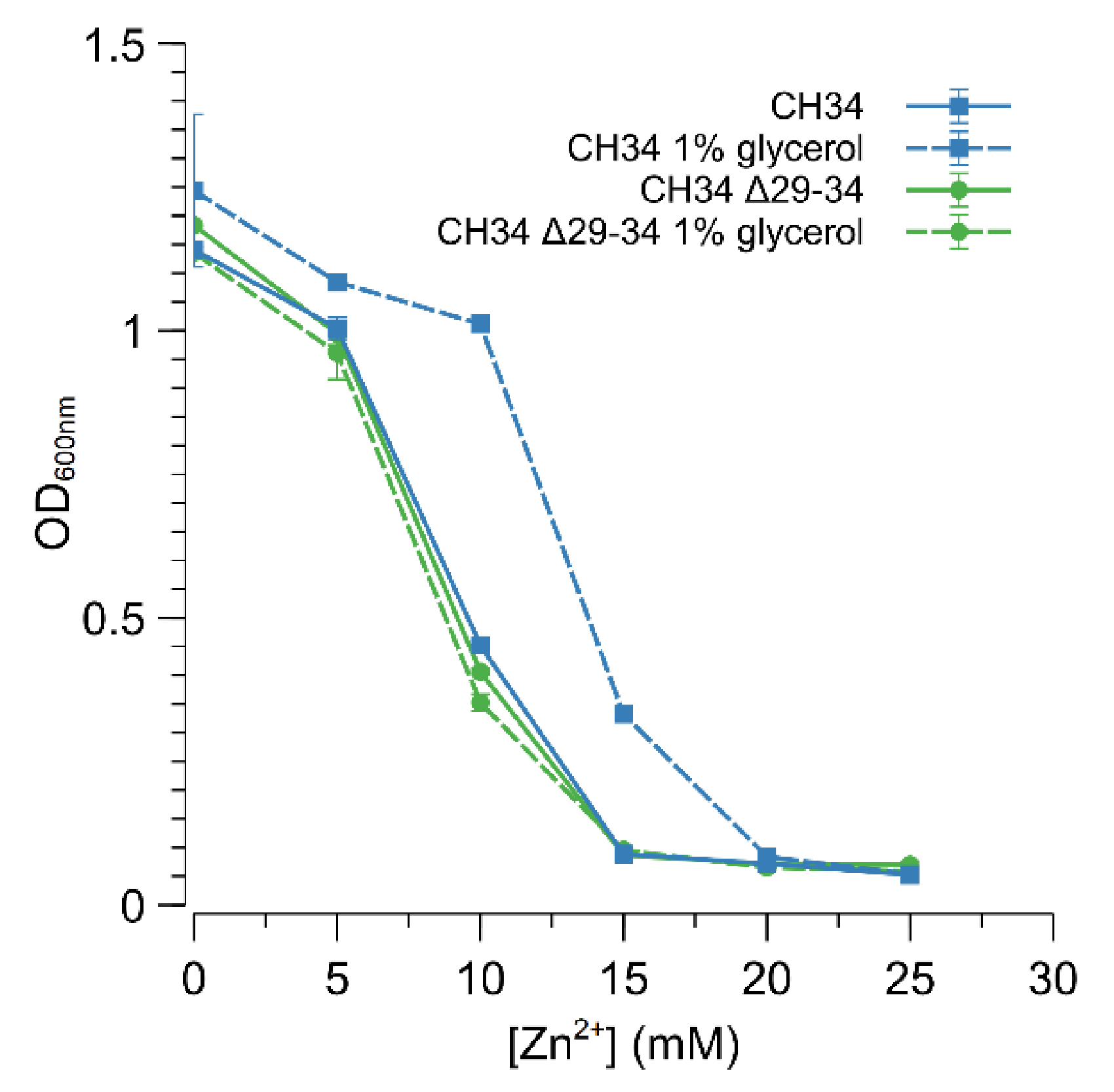
3.3. ABC-Type Transporter (Rmet_2229-2234) Is Responsible for Increased Zinc Resistance
3.4. Glycerol Induces Increased Zinc Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, C.M.; Helmann, J.D. Metal ion homeostasis in Bacillus subtilis. Curr. Opin. Microbiol. 2005, 8, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Hobman, J.; Yamamoto, K.; Oshima, T. Transcriptomic Responses of Bacterial Cells to Sublethal Metal Ion Stress. In Molecular Microbiology of Heavy Metals; Nies, D., Silver, S., Eds.; Springer: Berlin/Heidelberg, Gemany, 2007; Volume 6, pp. 73–115. [Google Scholar]
- Nies, D.H. The biological chemistry of the transition metal “transportome” of Cupriavidus metallidurans. Metallomics 2016, 8, 481–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hantke, K. Bacterial zinc transporters and regulators. BioMetals 2001, 14, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Staicu, L.C.; Wojtowicz, P.J.; Pósfai, M.; Pekker, P.; Gorecki, A.; Jordan, F.L.; Barton, L.L. PbS biomineralization using cysteine: Bacillus cereus and the sulfur rush. FEMS Microbiol. Ecol. 2020, 96, fiaa151. [Google Scholar] [CrossRef] [PubMed]
- Dopson, M.; Baker-Austin, C.; Koppineedi, P.R.; Bond, P.L. Growth in sulfidic mineral environments: Metal resistance mechanisms in acidophilic micro-organisms. Microbiology 2003, 149, 1959–1970. [Google Scholar] [CrossRef] [Green Version]
- Monsieurs, P.; Hobman, J.; Vandenbussche, G.; Mergeay, M.; Van Houdt, R. Response of Cupriavidus metallidurans to Metals. In Metal Response in Cupriavidus Metallidurans, Volume I: From Habitats to Genes and Proteins; Mergeay, M., Van Houdt, R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 45–89. [Google Scholar]
- Grosse, C.; Friedrich, S.; Nies, D.H. Contribution of extracytoplasmic function sigma factors to transition metal homeostasis in Cupriavidus metallidurans strain CH34. J. Mol. Microbiol. Biotechnol. 2007, 12, 227–240. [Google Scholar] [CrossRef]
- Malle, K.G. Zink in der Umwelt. Acta Hydrochim. Hydrobiol. 1992, 20, 196–204. [Google Scholar] [CrossRef]
- Tan, S.Y.; Praveena, S.M.; Abidin, E.Z.; Cheema, M.S. A review of heavy metals in indoor dust and its human health-risk implications. Rev. Environ. Health 2016, 31, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Gillan, D.C.; Van Camp, C.; Mergeay, M.; Provoost, A.; Thomas, N.; Vermard, L.; Billon, G.; Wattiez, R. Paleomicrobiology to investigate copper resistance in bacteria: Isolation and description of Cupriavidus necator B9 in the soil of a medieval foundry. Environ. Microbiol. 2017, 19, 770–787. [Google Scholar] [CrossRef]
- John, M.; Heuss-Aßbichler, S.; Ullrich, A. Recovery of Zn from wastewater of zinc plating industry by precipitation of doped ZnO nanoparticles. Int. J. Environ. Sci. Technol. 2016, 13, 2127–2134. [Google Scholar] [CrossRef] [Green Version]
- Gillan, D.C.; Van Houdt, R. The Impact of Metal Contamination on Soil Microbial Community Dynamics. In Modern Soil Microbiology, 3rd ed.; Van Elsas, J.D., Trevors, J.T., Rosado, A.S., Nannipieri, A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 403–419. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 548. [Google Scholar]
- Ellis, R.J.; Morgan, P.; Weightman, A.J.; Fry, J.C. Cultivation-Dependent and -Independent Approaches for Determining Bacterial Diversity in Heavy-Metal-Contaminated Soil. Appl. Environ. Microbiol. 2003, 69, 3223–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogiers, T.; Claesen, J.; Van Gompel, A.; Vanhoudt, N.; Mysara, M.; Williamson, A.; Leys, N.; Van Houdt, R.; Boon, N.; Mijnendonckx, K. Soil microbial community structure and functionality changes in response to long-term metal and radionuclide pollution. Environ. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Frassinetti, S.; Bronzetti, G.; Caltavuturo, L.; Cini, M.; Croce, C.D. The role of zinc in life: A review. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, D.K.; Morby, A.P. Zn(II) metabolism in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 291–311. [Google Scholar] [CrossRef]
- Mergeay, M.; Nies, D.; Schlegel, H.G.; Gerits, J.; Charles, P.; Vangijsegem, F. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy-metals. J. Bacteriol. 1985, 162, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Patzer, S.I.; Hantke, K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 1998, 28, 1199–1210. [Google Scholar] [CrossRef]
- Saier, M.H., Jr.; Tran, C.V.; Barabote, R.D. TCDB: The Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 2006, 34, D181–D186. [Google Scholar] [CrossRef] [Green Version]
- Herzberg, M.; Bauer, L.; Nies, D.H. Deletion of the zupT gene for a zinc importer influences zinc pools in Cupriavidus metallidurans CH34. Metallomics 2014, 6, 421–436. [Google Scholar] [CrossRef]
- Kirsten, A.; Herzberg, M.; Voigt, A.; Seravalli, J.; Grass, G.; Scherer, J.; Nies, D.H. Contributions of five secondary metal uptake systems to metal homeostasis of Cupriavidus metallidurans CH34. J. Bacteriol. 2011, 193, 4652–4663. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.; Schwarzenberger, C.; Grosse, C.; Nies, D.H. FurC regulates expression of zupT for the central zinc importer ZupT of Cupriavidus metallidurans. J. Bacteriol. 2014, 196, 3461–3471. [Google Scholar] [CrossRef] [Green Version]
- Janssen, P.J.; Van Houdt, R.; Moors, H.; Monsieurs, P.; Morin, N.; Michaux, A.; Benotmane, M.A.; Leys, N.; Vallaeys, T.; Lapidus, A.; et al. The complete genome sequence of Cupriavidus metallidurans strain CH34, a master survivalist in harsh and anthropogenic environments. PLoS ONE 2010, 5, e10433. [Google Scholar] [CrossRef] [PubMed]
- Mergeay, M.; Monchy, S.; Vallaeys, T.; Auquier, V.; Benotmane, A.; Bertin, P.; Taghavi, S.; Dunn, J.; van der Lelie, D.; Wattiez, R. Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: Towards a catalogue of metal-responsive genes. FEMS Microbiol. Rev. 2003, 27, 385–410. [Google Scholar] [CrossRef]
- Nies, D.H. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 313–339. [Google Scholar] [CrossRef]
- Diels, L.; Van Roy, S.; Taghavi, S.; Van Houdt, R. From industrial sites to environmental applications with Cupriavidus metallidurans. Antonie van Leeuwenhoek 2009, 96, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Hynninen, A.; Touze, T.; Pitkanen, L.; Mengin-Lecreulx, D.; Virta, M. An efflux transporter PbrA and a phosphatase PbrB cooperate in a lead-resistance mechanism in bacteria. Mol. Microbiol. 2009, 74, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Grosse, C.; Grass, G.; Anton, A.; Franke, S.; Santos, A.N.; Lawley, B.; Brown, N.L.; Nies, D.H. Transcriptional organization of the czc heavy-metal homeostasis determinant from Alcaligenes eutrophus. J. Bacteriol. 1999, 181, 2385–2393. [Google Scholar] [CrossRef] [Green Version]
- Nies, D.; Mergeay, M.; Friedrich, B.; Schlegel, H.G. Cloning of plasmid genes encoding resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus CH34. J. Bacteriol. 1987, 169, 4865–4868. [Google Scholar] [CrossRef] [Green Version]
- Tseng, T.T.; Gratwick, K.S.; Kollman, J.; Park, D.; Nies, D.H.; Goffeau, A.; Saier, M.H., Jr. The RND permease superfamily: An ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol. 1999, 1, 107–125. [Google Scholar]
- Vandenbussche, G.; Mergeay, M.; Van Houdt, R. Metal Response in Cupriavidus Metallidurans, Volume II: Insights into the Structure-Function Relationship of Proteins; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Rensing, C.; Pribyl, T.; Nies, D.H. New functions for the three subunits of the CzcCBA cation-proton antiporter. J. Bacteriol. 1997, 179, 6871–6879. [Google Scholar] [CrossRef] [Green Version]
- Seeger, M.A.; Schiefner, A.; Eicher, T.; Verrey, F.; Diederichs, K.; Pos, K.M. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science 2006, 313, 1295–1298. [Google Scholar] [CrossRef] [Green Version]
- Murakami, S.; Nakashima, R.; Yamashita, E.; Matsumoto, T.; Yamaguchi, A. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 2006, 443, 173–179. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Totrov, M. Vacuuming the periplasm. J Bacteriol 2005, 187, 1879–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherer, J.; Nies, D.H. CzcP is a novel efflux system contributing to transition metal resistance in Cupriavidus metallidurans CH34. Mol. Microbiol. 2009, 73, 601–621. [Google Scholar] [CrossRef]
- van der Lelie, D.; Schwuchow, T.; Schwidetzky, U.; Wuertz, S.; Baeyens, W.; Mergeay, M.; Nies, D.H. Two-component regulatory system involved in transcriptional control of heavy-metal homoeostasis in Alcaligenes eutrophus. Mol. Microbiol. 1997, 23, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Legatzki, A.; Grass, G.; Anton, A.; Rensing, C.; Nies, D.H. Interplay of the Czc system and two P-type ATPases in conferring metal resistance to Ralstonia metallidurans. J. Bacteriol. 2003, 185, 4354–4361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munkelt, D.; Grass, G.; Nies, D.H. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. J. Bacteriol. 2004, 186, 8036–8043. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, F.; Lee, J.K.; O’Connell, J.D., 3rd; Miercke, L.J.; Verschueren, K.H.; Srinivasan, V.; Bauvois, C.; Govaerts, C.; Robbins, R.A.; Ruysschaert, J.M.; et al. Metal-induced conformational changes in ZneB suggest an active role of membrane fusion proteins in efflux resistance systems. Proc. Natl. Acad. Sci. USA 2010, 107, 11038–11043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pak, J.E.; Ekende, E.N.; Kifle, E.G.; O’Connell, J.D., 3rd; De Angelis, F.; Tessema, M.B.; Derfoufi, K.M.; Robles-Colmenares, Y.; Robbins, R.A.; Goormaghtigh, E.; et al. Structures of intermediate transport states of ZneA, a Zn(II)/proton antiporter. Proc. Natl. Acad. Sci. USA 2013, 110, 18484–18489. [Google Scholar] [CrossRef] [Green Version]
- Mergeay, M.; Van Houdt, R. Metal Response in Cupriavidus Metallidurans: Volume I: From Habitats to Genes and Proteins; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Kovach, M.E.; Elzer, P.H.; Hill, D.S.; Robertson, G.T.; Farris, M.A.; Roop, R.M., 2nd; Peterson, K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 1995, 166, 175–176. [Google Scholar] [CrossRef]
- Chang, A.C.; Cohen, S.N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J. Bacteriol. 1978, 134, 1141–1156. [Google Scholar] [CrossRef] [Green Version]
- Katzen, F.; Becker, A.; Ielmini, M.V.; Oddo, C.G.; Ielpi, L. New mobilizable vectors suitable for gene replacement in gram-negative bacteria and their use in mapping of the 3′ end of the Xanthomonas campestris pv. campestris gum operon. Appl. Environ. Microb. 1999, 65, 278–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monsieurs, P.; Moors, H.; Van Houdt, R.; Janssen, P.J.; Janssen, A.; Coninx, I.; Mergeay, M.; Leys, N. Heavy metal resistance in Cupriavidus metallidurans CH34 is governed by an intricate transcriptional network. BioMetals 2011, 24, 1133–1151. [Google Scholar] [CrossRef] [PubMed]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nies, D.H. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc, and cadmium (czc system) in Alcaligenes eutrophus. J. Bacteriol. 1992, 174, 8102–8110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monchy, S.; Benotmane, M.A.; Janssen, P.; Vallaeys, T.; Taghavi, S.; van der Lelie, D.; Mergeay, M. Plasmids pMOL28 and pMOL30 of Cupriavidus metallidurans are specialized in the maximal viable response to heavy metals. J. Bacteriol. 2007, 189, 7417–7425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenski, R.E. What is adaptation by natural selection? Perspectives of an experimental microbiologist. PLoS Genet. 2017, 13, e1006668. [Google Scholar] [CrossRef]
- LaCroix, R.A.; Sandberg, T.E.; O’Brien, E.J.; Utrilla, J.; Ebrahim, A.; Guzman, G.I.; Szubin, R.; Palsson, B.O.; Feist, A.M. Use of adaptive laboratory evolution to discover key mutations enabling rapid growth of Escherichia coli K-12 MG1655 on glucose minimal medium. Appl. Environ. Microbiol. 2015, 81, 17–30. [Google Scholar] [CrossRef] [Green Version]
- McCloskey, D.; Xu, S.; Sandberg, T.E.; Brunk, E.; Hefner, Y.; Szubin, R.; Feist, A.M.; Palsson, B.O. Growth Adaptation of gnd and sdhCB Escherichia coli Deletion Strains Diverges From a Similar Initial Perturbation of the Transcriptome. Front. Microbiol. 2018, 9, 1793. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, T.E.; Pedersen, M.; LaCroix, R.A.; Ebrahim, A.; Bonde, M.; Herrgard, M.J.; Palsson, B.O.; Sommer, M.; Feist, A.M. Evolution of Escherichia coli to 42 degrees C and subsequent genetic engineering reveals adaptive mechanisms and novel mutations. Mol. Biol. Evol. 2014, 31, 2647–2662. [Google Scholar] [CrossRef] [Green Version]
- Maertens, L.; Leys, N.; Matroule, J.Y.; Van Houdt, R. The Transcriptomic Landscape of Cupriavidus metallidurans CH34 Acutely Exposed to Copper. Genes 2020, 11, 1049. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yip, C.B.; Geddes, B.A.; Oresnik, I.J.; Hynes, M.F. Glycerol utilization by Rhizobium leguminosarum requires an ABC transporter and affects competition for nodulation. Microbiology 2012, 158, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Ye, S.; Larson, T.J. Repressor for the sn-glycerol 3-phosphate regulon of Escherichia coli K-12: Primary structure and identification of the DNA-binding domain. J. Bacteriol. 1996, 178, 7080–7089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollig, J.; Kloubert, V.; Weßels, I.; Haase, H.; Rink, L. Parameters Influencing Zinc in Experimental Systems in Vivo and in Vitro. Metals 2016, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.H.; Sherwood Rawls, K.; Chan, J.C.; Hwang, S.; Martinez-Pastor, M.; McMillan, L.J.; Prunetti, L.; Schmid, A.K.; Maupin-Furlow, J.A. GlpR Is a Direct Transcriptional Repressor of Fructose Metabolic Genes in Haloferax volcanii. J. Bacteriol. 2018, 200, e00218–e00244. [Google Scholar] [CrossRef] [Green Version]
- Lin, E.C. Glycerol dissimilation and its regulation in bacteria. Annu. Rev. Microbiol. 1976, 30, 535–578. [Google Scholar] [CrossRef]
- Saier, M.H., Jr. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 2000, 64, 354–411. [Google Scholar] [CrossRef] [Green Version]
- Ammendola, S.; Pasquali, P.; Pistoia, C.; Petrucci, P.; Petrarca, P.; Rotilio, G.; Battistoni, A. High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect. Immun. 2007, 75, 5867–5876. [Google Scholar] [CrossRef] [Green Version]
- Mikolay, A.; Nies, D.H. The ABC-transporter AtmA is involved in nickel and cobalt resistance of Cupriavidus metallidurans strain CH34. Antonie Van Leeuwenhoek 2009, 96, 183–191. [Google Scholar] [CrossRef]
- Edgar, R.J.; van Hensbergen, V.P.; Ruda, A.; Turner, A.G.; Deng, P.; Le Breton, Y.; El-Sayed, N.M.; Belew, A.T.; McIver, K.S.; McEwan, A.G.; et al. Discovery of glycerol phosphate modification on streptococcal rhamnose polysaccharides. Nat. Chem. Biol. 2019, 15, 463–471. [Google Scholar] [CrossRef]
- Newsome, L.; Morris, K.; Trivedi, D.; Bewsher, A.; Lloyd, J.R. Biostimulation by Glycerol Phosphate to Precipitate Recalcitrant Uranium(IV) Phosphate. Environ. Sci. Technol. 2015, 49, 11070–11078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarret, G.; Saumitou-Laprade, P.; Bert, V.; Proux, O.; Hazemann, J.L.; Traverse, A.; Marcus, M.A.; Manceau, A. Forms of zinc accumulated in the hyperaccumulator Arabidopsis halleri. Plant Physiol. 2002, 130, 1815–1826. [Google Scholar] [CrossRef] [Green Version]
- Vandecraen, J.; Monsieurs, P.; Mergeay, M.; Leys, N.; Aertsen, A.; Van Houdt, R. Zinc-induced transposition of insertion sequence elements contributes to increased adaptability of Cupriavidus metallidurans. Front. Microbiol. 2016, 7, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandecraen, J.; Chandler, M.; Aertsen, A.; Van Houdt, R. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit. Rev. Microbiol. 2017, 43, 709–730. [Google Scholar] [CrossRef] [PubMed]


| Strain | Genotype/Relevant Characteristic 1 | References 2 |
|---|---|---|
| Cupriavidus metallidurans | ||
| CH34 | pMOL28 pMOL30 | [19] |
| CH34ZnR | pMOL28 pMOL30; increased resistance to Zn2+ | This study |
| CH34 ∆glpR | glpR replaced by tet, TcR | This study |
| CH34 ∆29-34 | Rmet_2229 to Rmet_2234 replaced by tet, TcR | This study |
| CH34ZnR ∆29-34 | Rmet_2229 to Rmet_2234 replaced by tet, TcR | This study |
| Escherichia coli | ||
| DG1 | mcrA ∆(mrr-hsdRMS-mcrBC, modification-, restriction-) ϕ80lacZ∆M15 ∆lacX74 recA1 araD139 ∆(ara-leu)7697 galU galK rpsL endA1 nupG | Eurogentec |
| HB101 | F- mcrB mrr hsdS20(rB- mB-) recA13 leuB6 ara-14 proA2 lacY1 galK2 xyl-5 mtl-1 rpsL20 glnV44 λ- | Lab |
| Strain | Genotype/Relevant Characteristic 1 | References 2 |
|---|---|---|
| pRK600 | Helper plasmid; CmR tra | Lab |
| pBBR1MCS2 | lacZα KmR ori pBBR1 oriT | [45] |
| pBBR-glpR | glpR in pBBR1MCS2 | This study |
| pBBR-glpRR | glpR::IS1088 in pBBR1MCS2 | This study |
| pACYC184 | CmR TcR p15A ori | [46] |
| pK18mob | lacZα KmR oriT oriV | [47] |
| pglpR | glpR in pK18mob, KmR | This study |
| pRmet_2229-34 | Rmet_2229-2234 in pK18mob, KmR | This study |
| pglpR::tet | glpR::tet in pK18mob, TcR KmR | This study |
| pRmet_2229-34::tet | Rmet_2229-2234::tet in pK18mob, TcR KmR | This study |
| C. metallidurans | Zn2+ | Ni2+ | Co2+ | Cd2+ |
|---|---|---|---|---|
| CH34 | 12 | 2.5 | 5 | 1.5 |
| CH34ZnR | 24 | 2.5 | 5 | 3 |
| CH34 ∆glpR | 24 | 2.5 | 5 | 3 |
| CH34 ∆29-34 | 12 | 2.5 | 5 | 1.5 |
| Gene | Protein | Inserted Element |
|---|---|---|
| Rmet_2146 | ABC superfamily transporter subunit | IS1088 |
| Rmet_2171 | Conserved hypothetical protein | ISRme15 |
| Rmet_2235 | DNA-binding transcriptional repressor | IS1088 |
| Rmet_4452 | Response regulator receiver domain protein (CheY-like) | IS1088 |
| Rmet_4521 | Transcriptional regulator | ISRme5 |
| Rmet_4574 | Acyl-CoA dehydrogenase protein | IS1088 |
| Rmet_5200 | Putative glyoxalase/bleomycin resistance protein/dihydroxy-biphenyl dioxygenase | IS1088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Houdt, R.; Vandecraen, J.; Leys, N.; Monsieurs, P.; Aertsen, A. Adaptation of Cupriavidus metallidurans CH34 to Toxic Zinc Concentrations Involves an Uncharacterized ABC-Type Transporter. Microorganisms 2021, 9, 309. https://doi.org/10.3390/microorganisms9020309
Van Houdt R, Vandecraen J, Leys N, Monsieurs P, Aertsen A. Adaptation of Cupriavidus metallidurans CH34 to Toxic Zinc Concentrations Involves an Uncharacterized ABC-Type Transporter. Microorganisms. 2021; 9(2):309. https://doi.org/10.3390/microorganisms9020309
Chicago/Turabian StyleVan Houdt, Rob, Joachim Vandecraen, Natalie Leys, Pieter Monsieurs, and Abram Aertsen. 2021. "Adaptation of Cupriavidus metallidurans CH34 to Toxic Zinc Concentrations Involves an Uncharacterized ABC-Type Transporter" Microorganisms 9, no. 2: 309. https://doi.org/10.3390/microorganisms9020309
APA StyleVan Houdt, R., Vandecraen, J., Leys, N., Monsieurs, P., & Aertsen, A. (2021). Adaptation of Cupriavidus metallidurans CH34 to Toxic Zinc Concentrations Involves an Uncharacterized ABC-Type Transporter. Microorganisms, 9(2), 309. https://doi.org/10.3390/microorganisms9020309







