Impact of Ceftiofur Administration in Steers on the Prevalence and Antimicrobial Resistance of Campylobacter spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Steers and Treatment
2.2. Gastrointestinal (GI) Fluid Collection, Active Drug Concentration Determination, and Pharmacokinetic Analysis
2.3. Sample Collection and Processing for Campylobacter
2.4. Campylobacter Species Determination, Multilocus Sequence Typing (MLST), and Minimum Spanning Tree (MST)
2.5. Campylobacter Antimicrobial Susceptibility and MIC Determinations
2.6. Statistical Analysis
3. Results
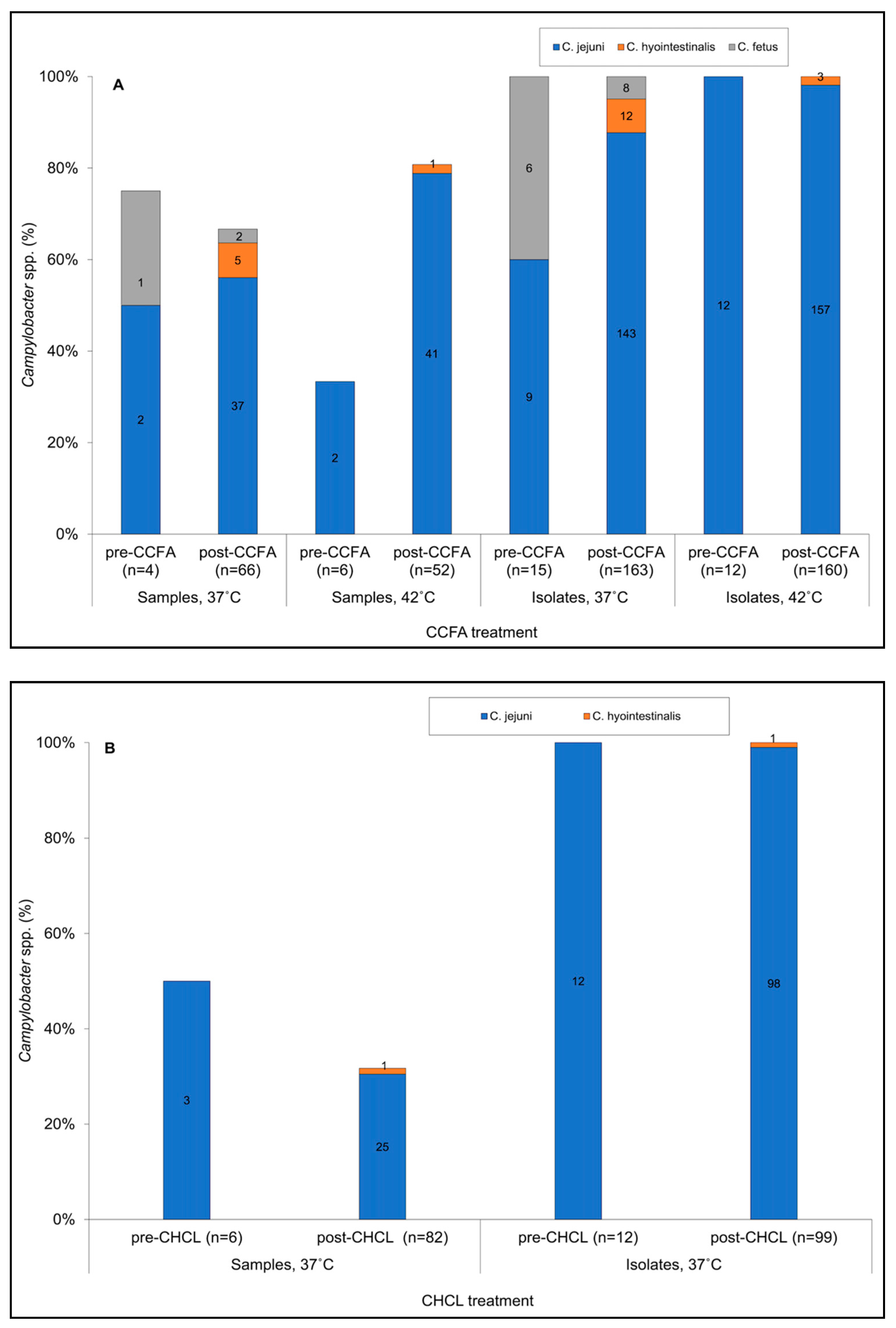
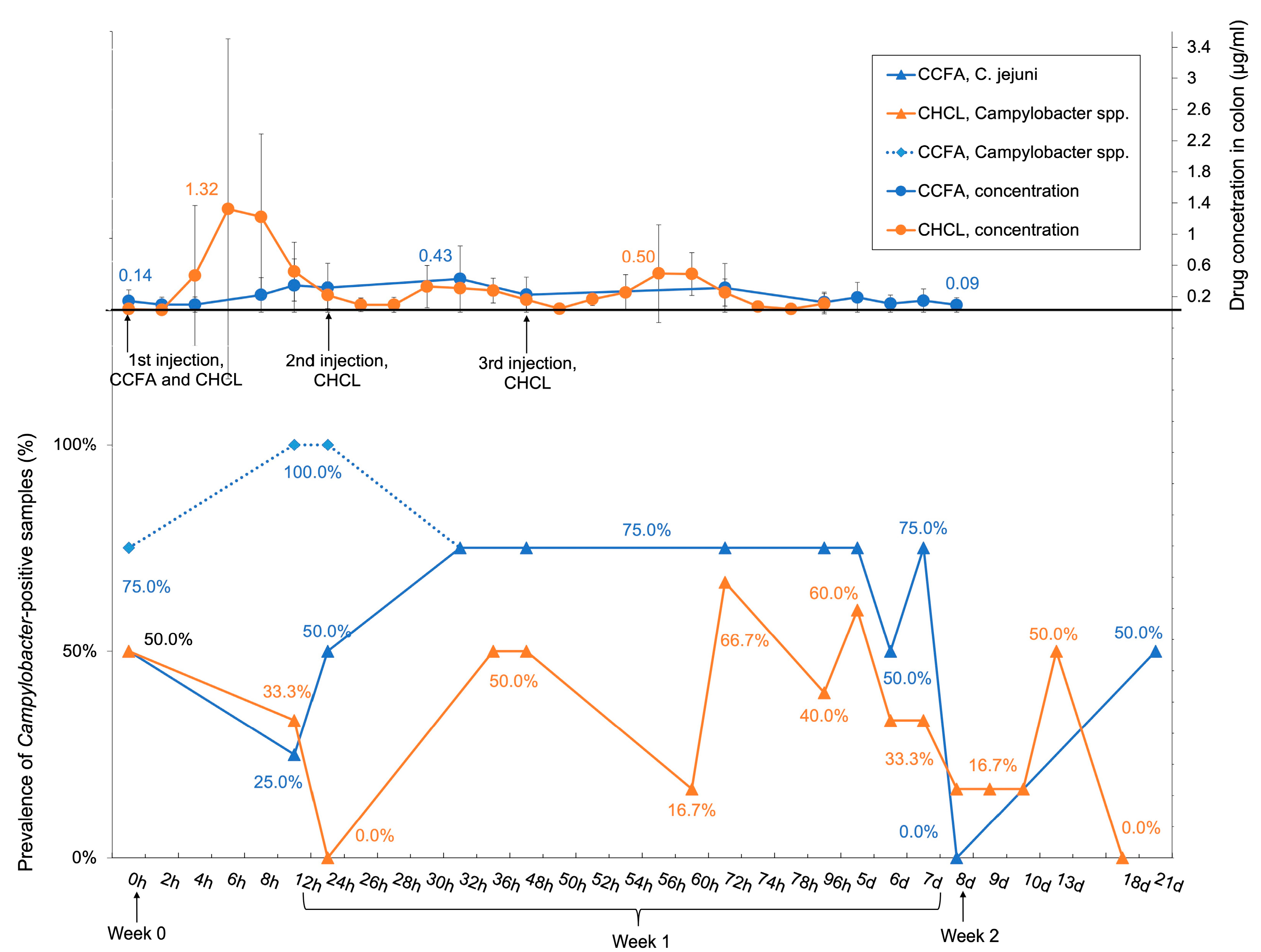
3.1. Impacts of CCFA and CHCL on Prevalence of Campylobacter spp.
3.2. Incubation Temperature Impacted the Estimated Prevalence of C. jejuni and Other Campylobacter spp.
3.3. The Prevalence of Campylobacter-Positive Samples Reflected the Drug Concentration in the Colon, But C. jejuni Ceftiofur MIC Was Not Impacted by Either Treatment
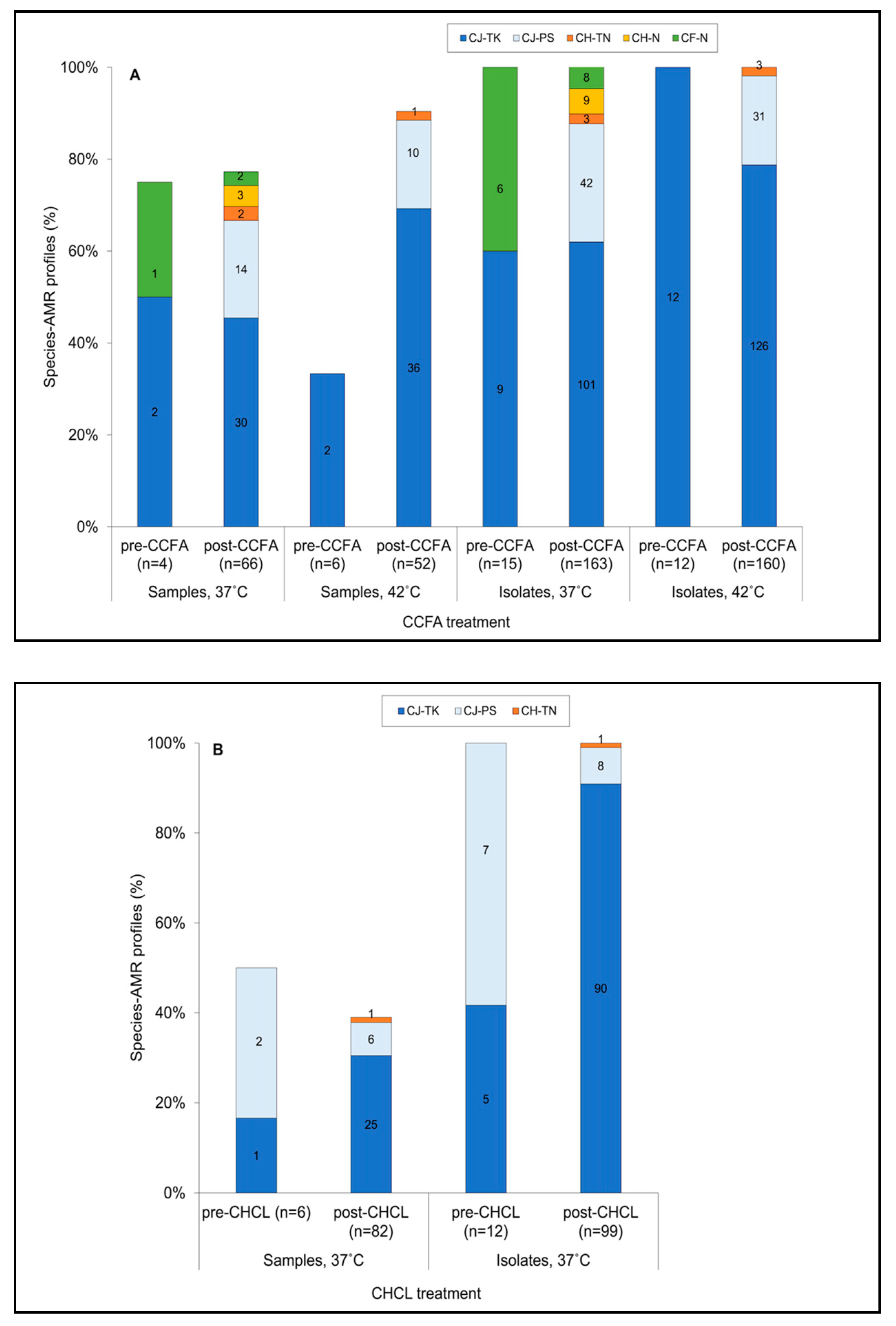
3.4. Isolates Resistant to Both Tetracycline and Kanamycin Predominated Pre- and Post-Treatment in BOTH Treatment Groups
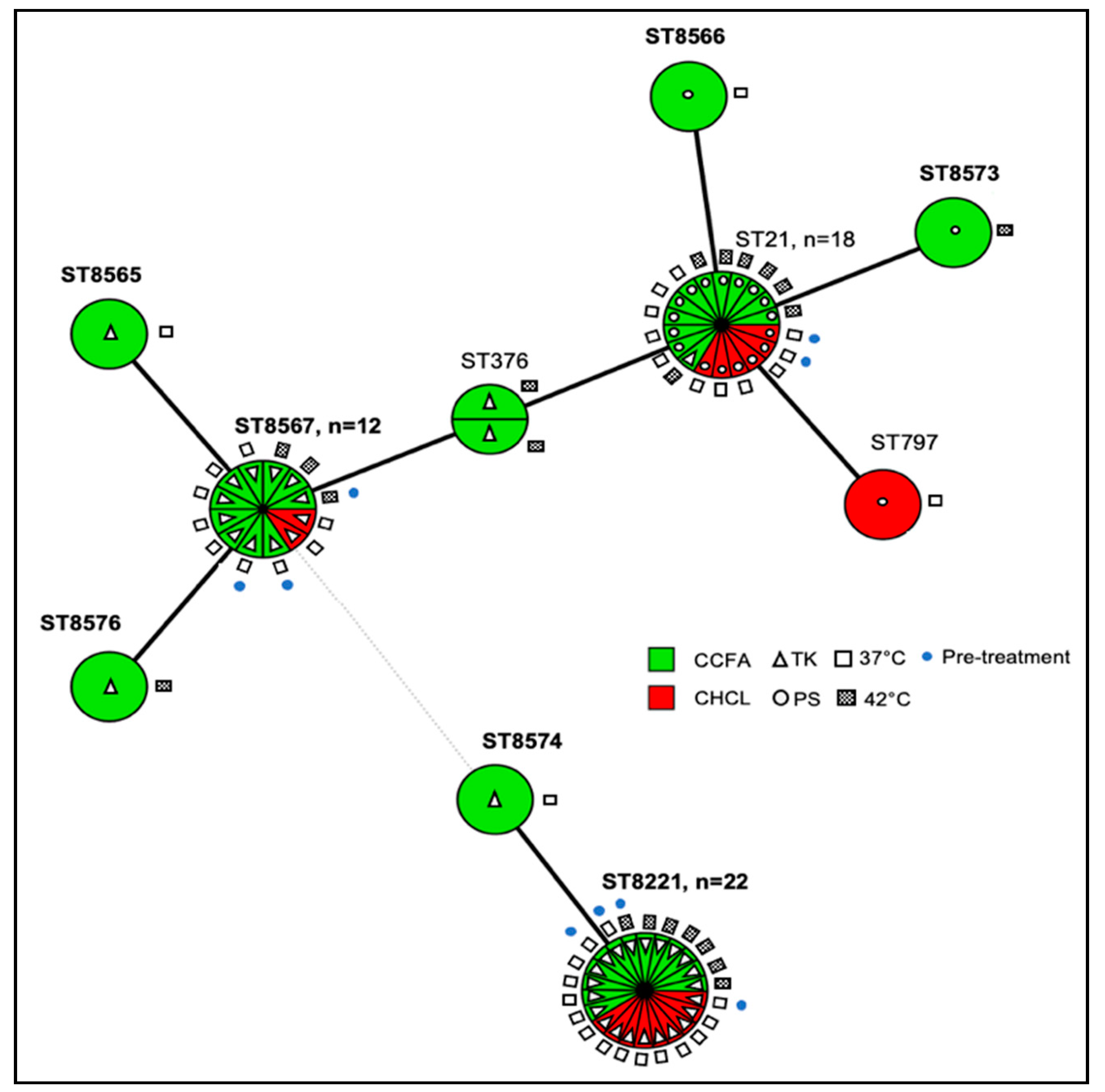
3.5. Multilocus Sequence Typing (MLST) Analysis Suggests that CCFA Treatment May Favor the Diversity of C. jejuni
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rushton, J.; Ferreira, J.P.; Stärk, K.D.C. Antimicrobial Resistance: The Use of Antimicrobials in the Livestock Sector; OECD Publishing: Paris, France, 2014; Volume 68. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, S.P.; Murinda, S.E.; Jayarao, B.M. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: A comprehensive review. Foodborne Pathog. Dis. 2011, 8, 337–355. [Google Scholar] [CrossRef]
- FDA. 2018 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. Available online: https://www.fda.gov/media/133411/download (accessed on 11 December 2020).
- USDA APHIS VS CEAH NAHMS. Dairy 2014: Health and Management Practices on U.S. Dairy Operations. 2014. Available online: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy14/Dairy14_dr_PartIII.pdf (accessed on 11 December 2020).
- WHO. WHO List of Critically Important Antimicrobials for Human Medicine (WHO CIA List). Available online: https://www.who.int/foodsafety/publications/cia2017.pdf?ua=1 (accessed on 11 December 2020).
- USDA APHIS VS CEAH NAHMS. Feedlot 2011: Part IV: Health and Health Management on U.S. Feedlots with a Capacity of 1000 or More Head. Available online: https://www.aphis.usda.gov/animal_health/nahms/feedlot/downloads/feedlot2011/Feed11_dr_PartIV_1.pdf (accessed on 6 January 2021).
- USDA: APHIS VS:CEAH. Dairy 2007: Part II: Changes in the U.S. Dairy Cattle Industry, 1991–2007. Available online: https://naldc.nal.usda.gov/download/38398/PDF (accessed on 6 January 2021).
- USDA APHIS VS CEAH NAHMS. Dairy 2014: Milk Quality, Milking Procedures, and Mastitis on U.S. Dairies. 2014. Available online: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy14/Dairy14_dr_Mastitis.pdf (accessed on 6 January 2021).
- Pereira, R.V.; Siler, J.D.; Ng, J.C.; Davis, M.A.; Grohn, Y.T.; Warnick, L.D. Effect of on-farm use of antimicrobial drugs on resistance in fecal Escherichia coli of preweaned dairy calves. J. Dairy Sci. 2014, 97, 7644–7654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA; HHS. New Animal Drug; Cephalosporins Drugs; Extralabel Animal Drug Use; Order of Prohibition. Fed. Regist. 2012, 77, 735–745. [Google Scholar]
- Salmon, S.A.; Watts, J.L.; Yancey, R.J., Jr. In vitro activity of ceftiofur and its primary metabolite, desfuroylceftiofur, against organisms of veterinary importance. J. Vet. Diagn. Investig. 1996, 8, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Haggett, E.F.; Wilson, W.D. Overview of the use of antimicrobials for the treatment of bacterial infections in horses. Equine Vet. Educ. 2008, 20, 433–448. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. EXCEDE—Ceftiofur Injection, Suspension. Available online: https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=187510 (accessed on 11 December 2020).
- U.S. National Library of Medicine. EXCENEL RTU STERILE—Ceftiofur Hydrochloride Injection, Suspension. Available online: https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=188500 (accessed on 11 December 2020).
- Chambers, L.; Yang, Y.; Littier, H.; Ray, P.; Zhang, T.; Pruden, A.; Strickland, M.; Knowlton, K. Metagenomic analysis of antibiotic resistance genes in dairy cow feces following therapeutic administration of third generation cephalosporin. PLoS ONE 2015, 10, e0133764. [Google Scholar] [CrossRef] [Green Version]
- Foster, D.M.; Jacob, M.E.; Farmer, K.A.; Callahan, B.J.; Theriot, C.M.; Kathariou, S.; Cernicchiaro, N.; Prange, T.; Papich, M.G. Ceftiofur formulation differentially affects the intestinal drug concentration, resistance of fecal Escherichia coli, and the microbiome of steers. PLoS ONE 2019, 14, e0223378. [Google Scholar] [CrossRef] [Green Version]
- Gorden, P.J.; Kleinhenz, M.D.; Wulf, L.W.; Rajewski, S.J.; Wang, C.; Gehring, R.; Coetzee, J.F. Comparative plasma and interstitial fluid pharmacokinetics of flunixin meglumine and ceftiofur hydrochloride following individual and co-administration in dairy cows. J. Vet. Pharmacol. Ther. 2018, 41, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Hornish, R.E.; Kotarski, S.F. Cephalosporins in veterinary medicine—Ceftiofur use in food animals. Curr. Top. Med. Chem. 2002, 2, 717–731. [Google Scholar] [CrossRef]
- Agga, G.E.; Schmidt, J.W.; Arthur, T.M. Antimicrobial-resistant fecal bacteria from ceftiofur-treated and nonantimicrobial-treated comingled beef cows at a cow-calf operation. Microb. Drug Resist. 2016, 22, 598–608. [Google Scholar] [CrossRef]
- Dutil, L.; Irwin, R.; Finley, R.; Ng, L.K.; Avery, B.; Boerlin, P.; Bourgault, A.M.; Cole, L.; Daignault, D.; Desruisseau, A.; et al. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerg. Infect. Dis. 2010, 16, 48–54. [Google Scholar] [CrossRef]
- Dunne, E.F.; Fey, P.D.; Kludt, P.; Reporter, R.; Mostashari, F.; Shillam, P.; Wicklund, J.; Miller, C.; Holland, B.; Stamey, K.; et al. Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with AmpC beta-lactamase. JAMA 2000, 284, 3151–3156. [Google Scholar] [CrossRef] [Green Version]
- Apley, M.D.; Coetzee, J.F. Antimicrobial Drug Use in Cattle. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Giguère, S., Prescott, J.F., Dowling, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 495–518. [Google Scholar] [CrossRef]
- Zoetis. AIF Product Comparison. Available online: https://www.zoetisus.com/dairy/avoidresidues/PDF/EXD13065_AIF_ComparisonChart.pdf (accessed on 11 December 2020).
- Foster, D.M.; Jacob, M.E.; Warren, C.D.; Papich, M.G. Pharmacokinetics of enrofloxacin and ceftiofur in plasma, interstitial fluid, and gastrointestinal tract of calves after subcutaneous injection, and bactericidal impacts on representative enteric bacteria. J. Vet. Pharmacol. Ther. 2016, 39, 62–71. [Google Scholar] [CrossRef]
- Taylor, E.A.; Jordan, E.R.; Garcia, J.A.; Hagevoort, G.R.; Norman, K.N.; Lawhon, S.D.; Piñeiro, J.M.; Scott, H.M. Effects of two-dose ceftiofur treatment for metritis on the temporal dynamics of antimicrobial resistance among fecal Escherichia coli in Holstein-Friesian dairy cows. PLoS ONE 2019, 14, e0220068. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. as a foodborne pathogen: A review. Front. Microbiol. 2011, 2, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D.J.; Gabriel, E.; Leatherbarrow, A.J.H.; Cheesbrough, J.; Gee, S.; Bolton, E.; Fox, A.; Fearnhead, P.; Hart, C.A.; Diggle, P.J. Tracing the source of campylobacteriosis. PLoS Genet. 2008, 6, e1000203. [Google Scholar] [CrossRef] [Green Version]
- Tack, D.M.; Ray, L.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Rissman, T.; Jervis, R.; Lathrop, S.; Muse, A.; Duwell, M.; et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food—Foodborne diseases active surveillance network, 10 U.S. sites, 2016–2019. Morb. Mortal. Wkly. Rep. 2020, 69, 509–514. [Google Scholar] [CrossRef]
- Young, K.T.; Davis, L.M.; Dirita, V.J. Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 5, 665–679. [Google Scholar] [CrossRef]
- Bolinger, H.; Kathariou, S. The current state of macrolide resistance in Campylobacter spp.: Trends and impacts of resistance mechanisms. Appl. Environ. Microbiol. 2017, 83, e00416-17. [Google Scholar] [CrossRef] [Green Version]
- CDC. Antibiotic Resistance Threats in the United States, 2019. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 11 January 2021).
- Ellis-Iversen, J.; Cook, A.J.; Smith, R.P.; Pritchard, G.C.; Nielen, M. Temporal patterns and risk factors for Escherichia coli O157 and Campylobacter spp. in young cattle. J. Food Prot. 2009, 72, 490–496. [Google Scholar] [CrossRef]
- Epps, S.V.; Harvey, R.B.; Hume, M.E.; Phillips, T.D.; Anderson, R.C.; Nisbet, D.J. Foodborne Campylobacter: Infections, metabolism, pathogenesis and reservoirs. Int. J. Environ. Res. Public Health 2013, 10, 6292–6304. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.A.; Moore, D.L.; Baker, K.N.; French, N.P.; Patnode, M.; Hensley, J.; Macdonald, K.; Besser, T.E. Risk factors for campylobacteriosis in two Washington State counties with high numbers of dairy farms. J. Clin. Microbiol. 2013, 51, 3921–3927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, C.R.; Hoekstra, R.M.; Samuel, M.; Marcus, R.; Bender, J.; Shiferaw, B.; Reddy, S.; Ahuja, S.D.; Helfrick, D.L.; Hardnett, F.; et al. Emerging Infections Program FoodNet Working Group. Risk factors for sporadic Campylobacter infection in the United States: A case-control study in FoodNet sites. Clin. Infect. Dis. 2004, 38, S285–S296. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.R.; Dunn, A.C.; Burnett, C.; McCullough, L.; Dimond, M.; Wagner, J.; Smith, L.; Carter, A.; Willardson, S.; Nakashima, A.K. Campylobacter jejuni infections associated with raw milk consumption—Utah, 2014. Morb. Mortal. Wkly. Rep. 2016, 65, 301–305. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Balasegaram, S.; Willis, C.; Wimalarathna, H.M.; Maiden, M.C.; McCarthy, N.D. Partial failure of milk pasteurization as a risk for the transmission of Campylobacter from cattle to humans. Clin. Infect. Dis. 2015, 61, 903–909. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.L.; Foster, D.M.; Papich, M.G. Pharmacokinetics and tissue distribution of enrofloxacin and its active metabolite ciprofloxacin in calves. J. Vet. Pharmacol. Ther. 2007, 30, 564–571. [Google Scholar] [CrossRef]
- Ferguson, K.M.; Jacob, M.E.; Theriot, C.M.; Callahan, B.J.; Prange, T.; Papich, M.G.; Foster, D.M. Dosing regimen of enrofloxacin impacts intestinal pharmacokinetics and the fecal microbiota in steers. Front. Microbiol. 2018, 9, 2190. [Google Scholar] [CrossRef]
- Jaglan, P.S.; Cox, B.L.; Arnold, T.S.; Kubicek, M.F.; Stuart, D.J.; Gilbertson, T.J. Liquid chromatographic determination of desfuroylceftiofur metabolite of ceftiofur as residue in cattle plasma. J. Assoc. Off. Anal. Chem. 1990, 73, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Gharst, G.; Hanson, D.; Kathariou, S. Effect of direct culture versus selective enrichment on the isolation of thermophilic Campylobacter from feces of mature cattle at harvest. J. Food Prot. 2006, 69, 1024–1027. [Google Scholar] [CrossRef]
- Smith, K.; Reimers, N.; Barnes, H.J.; Lee, B.C.; Siletzky, R.; Kathariou, S. Campylobacter colonization of sibling turkey flocks reared under different management conditions. J. Food Prot. 2004, 67, 1463–1468. [Google Scholar] [CrossRef]
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef] [Green Version]
- Miller, W.G.; Chapman, M.H.; Yee, E.; On, S.L.; McNulty, D.K.; Lastovica, A.J.; Carroll, A.M.; McNamara, E.B.; Duffy, G.; Mandrell, R.E. Multilocus sequence typing methods for the emerging Campylobacter species C. hyointestinalis, C. lanienae, C. sputorum, C. concisus, and C. curvus. Front. Cell. Infect. Microbiol. 2012, 2, 45. [Google Scholar] [CrossRef] [Green Version]
- Miller, W.G.; Englen, M.D.; Kathariou, S.; Wesley, I.V.; Wang, G.; Pittenger-Alley, L.; Siletz, R.M.; Muraoka, W.; Fedorka-Cray, P.J.; Mandrell, R.E. Identification of host-associated alleles by multilocus sequence typing of Campylobacter coli strains from food animals. Microbiology 2006, 152, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, W.; Siletzky, R.M.; Wright, S.; Islam, M.; Kathariou, S. Antimicrobial susceptibility profiles and strain type diversity of Campylobacter jejuni isolates from turkeys in eastern North Carolina. Appl. Environ. Microbiol. 2009, 75, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Niedermeyer, J.A.; Ring, L.; Miller, W.G.; Genger, S.; Lindsey, C.P.; Osborne, J.; Kathariou, S. Proximity to other commercial turkey farms affects colonization onset, genotypes, and antimicrobial resistance profiles of Campylobacter spp. in turkeys: Suggestive evidence from a paired-farm model. Appl. Environ. Microbiol. 2018, 84, e01212–e01218. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, P.G.; Bhavnani, S.M.; Rubino, C.M.; Louie, A.; Gumbo, T.; Forrest, A.; Drusano, G.L. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin. Infect. Dis. 2007, 44, 79–86. [Google Scholar] [CrossRef]
- Zhang, Q.; Plummer, P.J. Mechanisms of antibiotic resistance in Campylobacter. In Campylobacter, 3rd ed.; Nachamkin, I., Szymanski, C.M., Blaser, M.J., Eds.; American Society for Microbiology Press: Washington, DC, USA, 2008; pp. 263–276. [Google Scholar]
- Volkova, V.V.; Lanzas, C.; Lu, Z.; Gröhn, Y.T. Mathematical model of plasmid-mediated resistance to ceftiofur in commensal enteric Escherichia coli of cattle. PLoS ONE 2012, 7, e36738. [Google Scholar] [CrossRef] [Green Version]
- Wagenaar, J.A.; Van Bergen, M.A.; Blaser, M.J.; Tauxe, R.V.; Newell, D.G.; Van Putten, J.P. Campylobacter fetus infections in humans: Exposure and disease. Clin. Infect. Dis. 2014, 58, 1579–1586. [Google Scholar] [CrossRef] [Green Version]
- On, S.; Miller, W.G.; Houf, K.; Fox, J.G.; Vandamme, P. Minimal standards for describing new species belonging to the families Campylobacteraceae and Helicobacteraceae: Campylobacter, Arcobacter, Helicobacter and Wolinella spp. Int. J. Syst. Evol. Microbiol. 2017, 67, 5296–5311. [Google Scholar] [CrossRef]
- Thibodeau, A.; Fravalo, P.; Laurent-Lewandowski, S.; Guévremont, E.; Quessy, S.; Letellier, A. Presence and characterization of Campylobacter jejuni in organically raised chickens in Quebec. Can. J. Vet. Res. 2011, 75, 298–307. [Google Scholar]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q. Antibiotic resistance in Campylobacter: Emergence, transmission and persistence. Future Microbiol. 2009, 4, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajada, P.; Gomez-Graces, J.L.; Alós, J.I.; Balas, D.; Cogollos, R. Antimicrobial susceptibilities of Campylobacter jejuni and Campylobacter coli to 12 beta-lactam agents and combinations with beta-lactamase inhibitors. Antimicrob. Agents Chemother. 1996, 40, 1924–1925. [Google Scholar] [CrossRef] [Green Version]
- Singer, R.S.; Patterson, S.K.; Wallace, R.L. Effects of therapeutic ceftiofur administration to dairy cattle on Escherichia coli dynamics in the intestinal tract. Appl. Environ. Microbiol. 2008, 74, 6956–6962. [Google Scholar] [CrossRef] [Green Version]
- Lowrance, T.C.; Loneragan, G.H.; Kunze, D.J.; Platt, T.M.; Ives, S.E.; Scott, H.M.; Norby, B.; Echeverry, A.; Brashears, M.M. Changes in antimicrobial susceptibility in a population of Escherichia coli isolated from feedlot cattle administered ceftiofur crystalline-free acid. Am. J. Vet. Res. 2007, 68, 501–507. [Google Scholar] [CrossRef]
- Cody, A.J.; McCarthy, N.D.; Jansen van Rensburg, M.; Isinkaye, T.; Bentley, S.D.; Parkhill, J.; Dingle, K.E.; Bowler, I.C.; Jolley, K.A.; Maiden, M.C. Real-time genomic epidemiological evaluation of human Campylobacter isolates by use of whole-genome multilocus sequence typing. J. Clin. Microbiol. 2013, 51, 2526–2534. [Google Scholar] [CrossRef] [Green Version]
- De Haan, C.P.; Llarena, A.K.; Revez, J.; Hänninen, M.L. Association of Campylobacter jejuni metabolic traits with multilocus sequence types. Appl. Environ. Microbiol. 2012, 78, 5550–5554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gripp, E.; Hlahla, D.; Didelot, X.; Kops, F.; Maurischat, S.; Tedin, K.; Alter, T.; Ellerbroek, L.; Schreiber, K.; Schomburg, D.; et al. Closely related Campylobacter jejuni strains from different sources reveal a generalist rather than a specialist lifestyle. BMC Genomics 2011, 12, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revez, J.; Zhang, J.; Schott, T.; Kivistö, R.; Rossi, M.; Hänninen, M.L. Genomic variation between Campylobacter jejuni isolates associated with milk-borne-disease outbreaks. J. Clin. Microbiol. 2014, 52, 2782–2786. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Foster, D.; Miller, W.G.; Osborne, J.; Kathariou, S. Impact of Ceftiofur Administration in Steers on the Prevalence and Antimicrobial Resistance of Campylobacter spp. Microorganisms 2021, 9, 318. https://doi.org/10.3390/microorganisms9020318
Fan S, Foster D, Miller WG, Osborne J, Kathariou S. Impact of Ceftiofur Administration in Steers on the Prevalence and Antimicrobial Resistance of Campylobacter spp. Microorganisms. 2021; 9(2):318. https://doi.org/10.3390/microorganisms9020318
Chicago/Turabian StyleFan, Sicun, Derek Foster, William G. Miller, Jason Osborne, and Sophia Kathariou. 2021. "Impact of Ceftiofur Administration in Steers on the Prevalence and Antimicrobial Resistance of Campylobacter spp." Microorganisms 9, no. 2: 318. https://doi.org/10.3390/microorganisms9020318
APA StyleFan, S., Foster, D., Miller, W. G., Osborne, J., & Kathariou, S. (2021). Impact of Ceftiofur Administration in Steers on the Prevalence and Antimicrobial Resistance of Campylobacter spp. Microorganisms, 9(2), 318. https://doi.org/10.3390/microorganisms9020318






