Improving Human Health with Milk Fat Globule Membrane, Lactic Acid Bacteria, and Bifidobacteria
Abstract
:1. Introduction
1.1. The Milk Fat Globule Membrane
1.1.1. Structure and Origin
1.1.2. Composition
1.2. Lactic Acid Bacteria (LAB) and Bifidobacteria
2. Interactions of Bifidobacteria, LAB, and MFGM in Dairy Food Matrixes
3. Evidence of Improved Health with Combined Probiotic and MFGM Supplementation
3.1. Bacterial Survival and Adhesion
3.2. Nutrient Absorption, Mucosal Immunity, and Gut Barrier Function
3.3. Neurodevelopment and Cognitive Function
4. Current Applications and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anto, L.; Warykas, S.W.; Torres-Gonzalez, M.; Blesso, C.N. Milk Polar Lipids: Underappreciated Lipids with Emerging Health Benefits. Nutrients 2020, 12, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snow, D.R.; Ward, R.E.; Olsen, A.; Jimenez-Flores, R.; Hintze, K.J. Membrane-Rich Milk Fat Diet Provides Protection against Gastrointestinal Leakiness in Mice Treated with Lipopolysaccharide. J. Dairy Sci. 2011, 94, 2201–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, Production, and Clinical Treatments of Milk Fat Globule Membrane for Infant Nutrition and Well-Being. Nutrients 2020, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Heid, H.W.; Keenan, T.W. Intracellular Origin and Secretion of Milk Fat Globules. Eur. J. Cell Biol. 2005, 84, 245–258. [Google Scholar] [CrossRef]
- Keenan, T.W.; Mather, I.H. Intracellular Origin of Milk Fat Globules and Nature of the Milk Fat Globule Membrane. In Advanced Dairy Chemistry Volume 2 Lipids; Springer: Boston, MA, USA, 2006; pp. 137–171. ISBN 978-0-387-28813-0. [Google Scholar]
- Silanikove, N.; Shapiro, F. Distribution of Xanthine Oxidase and Xanthine Dehydrogenase Activity in Bovine Milk: Physiological and Technological Implications. Int. Dairy J. 2007, 17, 1188–1194. [Google Scholar] [CrossRef]
- Ortega-Anaya, J.; Marciniak, A.; Jiménez-Flores, R. Milk Lipids|Milk Fat Globule Membrane. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-08-100596-5. [Google Scholar]
- McManaman, J.L.; Palmer, C.A.; Wright, R.M.; Neville, M.C. Functional Regulation of Xanthine Oxidoreductase Expression and Localization in the Mouse Mammary Gland: Evidence of a Role in Lipid Secretion. J. Physiol. 2002, 545, 567–579. [Google Scholar] [CrossRef]
- Gallier, S.; Laubscher, A.; Jiménez-Flores, R. The milk fat globule membrane. In Food Structures, Digestion and Health; Elsevier: Amsterdam, The Netherlands, 2014; pp. 107–142. ISBN 978-0-12-404610-8. [Google Scholar]
- Ortega-Anaya, J.; Jiménez-Flores, R. Symposium Review: The Relevance of Bovine Milk Phospholipids in Human Nutrition—Evidence of the Effect on Infant Gut and Brain Development. J. Dairy Sci. 2019, 102, 2738–2748. [Google Scholar] [CrossRef]
- Martel, M.B.; Dubois, P.; Got, R. Human milk fat globule membranes. Preparation, morphological studies and chemical composition. Biochim. Biophys. Acta 1973, 311, 565–575. [Google Scholar] [CrossRef]
- Patton, S.; Keenan, T.W. The Milk Fat Globule Membrane. Biochim. Biophys. Acta 1975, 415, 27309. [Google Scholar] [CrossRef]
- Fong, B.Y.; Norris, C.S.; MacGibbon, A.K.H. Protein and Lipid Composition of Bovine Milk-Fat-Globule Membrane. Int. Dairy J. 2007, 17, 275–288. [Google Scholar] [CrossRef]
- Sánchez-Juanes, F.; Alonso, J.M.; Zancada, L.; Hueso, P. Distribution and Fatty Acid Content of Phospholipids from Bovine Milk and Bovine Milk Fat Globule Membranes. Int. Dairy J. 2009, 19, 273–278. [Google Scholar] [CrossRef]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and Technological Aspects of Milk Fat Globule Membrane Material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- Jiménez-Flores, R.; Higuera-Ciapara, I.; Pouliot, Y. Beverages based on milk fat globule membrane (MFGM) and other novel concepts for dairy-based functional beverages. In Functional and Speciality Beverage Technology; Paquin, P., Ed.; Woodhead Publishing: Sawston, UK, 2009; pp. 281–296. ISBN 978-1-84569-342-8. [Google Scholar]
- Deeth, H.C. The Role of Phospholipids in the Stability of Milk Fat Globules. Aust. J. Dairy Technol. 1997, 52. [Google Scholar]
- Bourlieu, C.; Bouzerzour, K.; Ferret-Bernard, S.; Bourgot, C.L.; Chever, S.; Ménard, O.; Deglaire, A.; Cuinet, I.; Ruyet, P.L.; Bonhomme, C.; et al. Infant Formula Interface and Fat Source Impact on Neonatal Digestion and Gut Microbiota: Infant Formula Structure Affects Neonatal Health. Eur. J. Lipid Sci. Technol. 2015, 117, 1500–1512. [Google Scholar] [CrossRef]
- Claumarchirant, L.; Cilla, A.; Matencio, E.; Sanchez-Siles, L.M.; Castro-Gomez, P.; Fontecha, J.; Alegría, A.; Lagarda, M.J. Addition of Milk Fat Globule Membrane as an Ingredient of Infant Formulas for Resembling the Polar Lipids of Human Milk. Int. Dairy J. 2016, 61, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Murthy, A.V.R.; Guyomarc’h, F.; Lopez, C. Cholesterol Decreases the Size and the Mechanical Resistance to Rupture of Sphingomyelin Rich Domains in Lipid Bilayers Studied as a Model of the Milk Fat Globule Membrane. Langmuir 2016, 32, 6757–6765. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Madec, M.-N.; Jimenez-Flores, R. Lipid Rafts in the Bovine Milk Fat Globule Membrane Revealed by the Lateral Segregation of Phospholipids and Heterogeneous Distribution of Glycoproteins. Food Chem. 2010, 120, 22–33. [Google Scholar] [CrossRef] [Green Version]
- Guyomarc’h, F.; Zou, S.; Chen, M.; Milhiet, P.-E.; Godefroy, C.; Vié, V.; Lopez, C. Milk Sphingomyelin Domains in Biomimetic Membranes and the Role of Cholesterol: Morphology and Nanomechanical Properties Investigated Using AFM and Force Spectroscopy. Langmuir 2014, 30, 6516–6524. [Google Scholar] [CrossRef]
- Guyomarc’h, F.; Chen, M.; Et-Thakafy, O.; Zou, S.; Lopez, C. Gel-Gel Phase Separation within Milk Sphingomyelin Domains Revealed at the Nanoscale Using Atomic Force Microscopy. Biochim. Biophys. Acta 2017, 1859, 949–958. [Google Scholar] [CrossRef]
- Georgi, G.; Bartke, N.; Wiens, F.; Stahl, B. Functional Glycans and Glycoconjugates in Human Milk. Am. J. Clin. Nutr. 2013, 98, 578S–585S. [Google Scholar] [CrossRef] [Green Version]
- Iwamori, M.; Takamizawa, K.; Momoeda, M.; Iwamori, Y.; Taketani, Y. Gangliosides in Human, Cow and Goat Milk, and Their Abilities as to Neutralization of Cholera Toxin and Botulinum Type A Neurotoxin. Glycoconj. J. 2008, 25, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Lane, J.A.; Kilcoyne, M.; Joshi, L.; Hickey, R.M. Defatted Bovine Milk Fat Globule Membrane Inhibits Association of Enterohaemorrhagic Escherichia Coli O157:H7 with Human HT-29 Cells. Int. Dairy J. 2016, 59, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Lopez, C.; Cauty, C.; Guyomarc’h, F. Unraveling the Complexity of Milk Fat Globules to Tailor Bioinspired Emulsions Providing Health Benefits: The Key Role Played by the Biological Membrane. Eur. J. Lipid Sci. Tech. 2019, 121, 1800201. [Google Scholar] [CrossRef] [Green Version]
- Andreotti, G.; Trivellone, E.; Motta, A. Characterization of Buffalo Milk by 31P-Nuclear Magnetic Resonance Spectroscopy. J. Food Compost. Anal. 2006, 19, 843–849. [Google Scholar] [CrossRef]
- Lamothe, S.; Robitaille, G.; St-Gelais, D.; Britten, M. Butter Making from Caprine Creams: Effect of Washing Treatment on Phospholipids and Milk Fat Globule Membrane Proteins Distribution. J. Dairy Res. 2008, 75, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alcalá, L.M.; Fontecha, J. Major Lipid Classes Separation of Buttermilk, and Cows, Goats and Ewes Milk by High Performance Liquid Chromatography with an Evaporative Light Scattering Detector Focused on the Phospholipid Fraction. J. Chromatogr. A 2010, 1217, 3063–3066. [Google Scholar] [CrossRef] [PubMed]
- Donato, P.; Cacciola, F.; Cichello, F.; Russo, M.; Dugo, P.; Mondello, L. Determination of Phospholipids in Milk Samples by Means of Hydrophilic Interaction Liquid Chromatography Coupled to Evaporative Light Scattering and Mass Spectrometry Detection. J. Chromatogr. A 2011, 1218, 6476–6482. [Google Scholar] [CrossRef]
- Contarini, G.; Povolo, M. Phospholipids in Milk Fat: Composition, Biological and Technological Significance, and Analytical Strategies. Int. J. Mol. Sci. 2013, 14, 2808–2831. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, L.; Gomes, A.; Pintado, M.; Rodríguez-Alcalá, L.M. Isolation and Analysis of Phospholipids in Dairy Foods. J. Anal. Methods Chem. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhang, L.; Wu, Y.; Zhou, P. Changes in Milk Fat Globule Membrane Proteome after Pasteurization in Human, Bovine and Caprine Species. Food Chem. 2019, 279, 209–215. [Google Scholar] [CrossRef]
- Riccio, P. The Proteins of the Milk Fat Globule Membrane in the Balance. Trends Food Sci. Technol. 2004, 15, 458–461. [Google Scholar] [CrossRef]
- Michalski, M.-C.; Januel, C. Does Homogenization Affect the Human Health Properties of Cow’s Milk? Trends Food Sci. Technol. 2006, 17, 423–437. [Google Scholar] [CrossRef]
- Pan, Y.; Lee, A.; Wan, J.; Coventry, M.J.; Michalski, W.P.; Shiell, B.; Roginski, H. Antiviral Properties of Milk Proteins and Peptides. Int. Dairy J. 2006, 16, 1252–1261. [Google Scholar] [CrossRef]
- Ross, S.A.; Lane, J.; Kilcoyne, M.; Joshi, L.; Hickey, R.M. The milk fat globule membrane: A potential source of health-promoting glycans. In Biotechnology of Bioactive Compounds; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 631–668. ISBN 978-1-118-73310-3. [Google Scholar]
- Kamińska, A.; Enguita, F.J.; Stępień, E.Ł. Lactadherin: An Unappreciated Haemostasis Regulator and Potential Therapeutic Agent. Vascul. Pharmacol. 2018, 101, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Parrón, J.A.; Ripollés, D.; Sánchez, A.C.; Pérez, M.D.; Calvo, M.; López, S.; Arias, C.F.; Sánchez, L. Antirotaviral Activity of Bovine Milk Components: Extending the List of Inhibitory Proteins and Seeking a Better Understanding of Their Neutralization Mechanism. J. Funct. Foods 2018, 44, 103–111. [Google Scholar] [CrossRef]
- Bär, C.; Mathis, D.; Neuhaus, P.; Dürr, D.; Bisig, W.; Egger, L.; Portmann, R. Protein Profile of Dairy Products: Simultaneous Quantification of Twenty Bovine Milk Proteins. Int. Dairy J. 2019, 97, 167–175. [Google Scholar] [CrossRef]
- Boone, D.R.; Castenholz, R.W.; Garrity, G.M. (Eds.) Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA, 2001; Volume 3, ISBN 978-0-387-98771-2. [Google Scholar]
- Bintsis, T. Lactic Acid Bacteria as Starter Cultures: An Update in Their Metabolism and Genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Cui, Y.; Hu, T.; Qu, X.; Zhang, L.; Ding, Z.; Dong, A. Plasmids from Food Lactic Acid Bacteria: Diversity, Similarity, and New Developments. Int. J. Mol. Sci. 2015, 16, 13172–13202. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, P.; Carvalho, A.L.; Vinga, S.; Santos, H.; Neves, A.R. From Physiology to Systems Metabolic Engineering for the Production of Biochemicals by Lactic Acid Bacteria. Biotechnol. Adv. 2013, 31, 764–788. [Google Scholar] [CrossRef]
- Bondue, P.; Delcenserie, V. Genome of Bifidobacteria and Carbohydrate Metabolism. Korean J. Food Sci. Anim. Resour. 2015, 35, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Felis, G.E.; Dellaglio, F. Taxonomy of Lactobacilli and Bifidobacteria. Curr. Issues Intest. Microbiol. 2007, 8, 44–61. [Google Scholar] [PubMed]
- Katayama, T. Host-Derived Glycans Serve as Selected Nutrients for the Gut Microbe: Human Milk Oligosaccharides and Bifidobacteria. Biosci. Biotechnol. Biochem. 2016, 80, 621–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gasterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasanna, P.H.P.; Grandison, A.S.; Charalampopoulos, D. Bifidobacteria in Milk Products: An Overview of Physiological and Biochemical Properties, Exopolysaccharide Production, Selection Criteria of Milk Products and Health Benefits. Food Res. Int. 2014, 55, 247–262. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Genes and Molecules of Lactobacilli Supporting Probiotic Action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [Green Version]
- Dupont, A.; Heinbockel, L.; Brandenburg, K.; Hornef, M.W. Antimicrobial Peptides and the Enteric Mucus Layer Act in Concert to Protect the Intestinal Mucosa. Gut Microbes 2014, 5, 761–765. [Google Scholar] [CrossRef] [Green Version]
- Tavares, L.M.; de Jesus, L.C.L.; da Silva, T.F.; Barroso, F.A.L.; Batista, V.L.; Coelho-Rocha, N.D.; Azevedo, V.; Drumond, M.M.; Mancha-Agresti, P. Novel Strategies for Efficient Production and Delivery of Live Biotherapeutics and Biotechnological Uses of Lactococcus Lactis: The Lactic Acid Bacterium Model. Front. Bioeng. Biotechnol. 2020, 8, 517166. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; García-Carral, C.; Rodriguez, J.M. Unfolding the Human Milk Microbiome Landscape in the Omics Era. Front. Microbiol. 2019, 10, 1378. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, J.; Gil-Campos, M.; Maldonado-Lobón, J.A.; Benavides, M.R.; Flores-Rojas, K.; Jaldo, R.; Jiménez del Barco, I.; Bolívar, V.; Valero, A.D.; Prados, E.; et al. Evaluation of the Safety, Tolerance and Efficacy of 1-Year Consumption of Infant Formula Supplemented with Lactobacillus fermentum CECT5716 Lc40 or Bifidobacterium breve CECT7263: A Randomized Controlled Trial. BMC Pediatr. 2019, 19, 361. [Google Scholar] [CrossRef] [PubMed]
- Sung, V.; D’Amico, F.; Cabana, M.D.; Chau, K.; Koren, G.; Savino, F.; Szajewska, H.; Deshpande, G.; Dupont, C.; Indrio, F.; et al. Lactobacillus reuteri to Treat Infant Colic: A Meta-Analysis. Pediatrics 2018, 141, e20171811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagato, E.; Mileti, E.; Massimiliano, L.; Fasano, F.; Budelli, A.; Penna, G.; Rescigno, M. Lactobacillus Paracasei CBA L74 Metabolic Products and Fermented Milk for Infant Formula Have Anti-Inflammatory Activity on Dendritic Cells In Vitro and Protective Effects against Colitis and an Enteric Pathogen In Vivo. PLoS ONE 2014, 9, e87615. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Durso, L.; Hutkins, R. Starter Cultures. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 5583–5593. ISBN 978-0-12-227055-0. [Google Scholar]
- Zhang, L.; García-Cano, I.; Jiménez-Flores, R. Effect of Milk Phospholipids on the Growth and Cryotolerance of Lactic Acid Bacteria Cultured and Stored in Acid Whey-Based Media. JDS Commun. 2020, S2666910220300351. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Sousa, M.J. Biochemical Pathways for the Production of Flavour Compounds in Cheeses during Ripening: A Review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Smit, G.; Smit, B.A.; Engels, W.J.M. Flavour Formation by Lactic Acid Bacteria and Biochemical Flavour Profiling of Cheese Products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef]
- Busscher, H.J. Specific and Non-Specific Interactions in Bacterial Adhesion to Solid Substrata. FEMS Microbio. Rev. 1987, 46, 9. [Google Scholar] [CrossRef]
- An, Y.H.; Friedman, R.J. Concise Review of Mechanisms of Bacterial Adhesion to Biomaterial Surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Laloy, E.; Vuillemard, J.-C.; El Soda, M.; Simard, R.E. Influence of the Fat Content of Cheddar Cheese on Retention and Localization of Starters. Int. Dairy J. 1996, 6, 729–740. [Google Scholar] [CrossRef]
- Auty, M.A.E.; Gardiner, G.E.; McBrearty, S.J.; O’Sullivan, E.O.; Mulvihill, D.M.; Collins, J.K.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Direct in Situ Viability Assessment of Bacteria in Probiotic Dairy Products Using Viability Staining in Conjunction with Confocal Scanning Laser Microscopy. Appl. Environ. Microbiol. 2001, 67, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Lopez, C.; Maillard, M.-B.; Briard-Bion, V.; Camier, B.; Hannon, J.A. Lipolysis during Ripening of Emmental Cheese Considering Organization of Fat and Preferential Localization of Bacteria. J. Agric. Food Chem. 2006, 54, 5855–5867. [Google Scholar] [CrossRef]
- Lopez, C.; Camier, B.; Gassi, J.-Y. Development of the Milk Fat Microstructure during the Manufacture and Ripening of Emmental Cheese Observed by Confocal Laser Scanning Microscopy. Int. Dairy J. 2007, 17, 235–247. [Google Scholar] [CrossRef]
- García-Cano, I.; Rocha-Mendoza, D.; Kosmerl, E.; Jiménez-Flores, R. Purification and Characterization of a Phospholipid-Hydrolyzing Phosphoesterase Produced by Pediococcus Acidilactici Isolated from Gouda Cheese. J. Dairy Sci. 2020, S0022030220301818. [Google Scholar] [CrossRef] [PubMed]
- Moe, K.M.; Faye, T.; Abrahamsen, R.K.; Østlie, H.M.; Skeie, S. Growth and Survival of Cheese Ripening Bacteria on Milk Fat Globule Membrane Isolated from Bovine Milk and Its Monosaccharides. Int. Dairy J. 2012, 25, 29–35. [Google Scholar] [CrossRef]
- Martinovic, A.; Moe, K.M.; Romeih, E.; Aideh, B.; Vogensen, F.K.; Østlie, H.; Skeie, S. Growth of Adjunct Lactobacillus Casei in Cheddar Cheese Differing in Milk Fat Globule Membrane Components. Int. Dairy J. 2013, 31, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Flores, R.; Brisson, G. The Milk Fat Globule Membrane as an Ingredient: Why, How, When? Dairy Sci. Technol. 2008, 88, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Ly, M.H.; Vo, N.H.; Le, T.M.; Belin, J.-M.; Waché, Y. Diversity of the Surface Properties of Lactococci and Consequences on Adhesion to Food Components. Colloids Surf. B 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Brisson, G.; Payken, H.F.; Sharpe, J.P.; Jiménez-Flores, R. Characterization of Lactobacillus Reuteri Interaction with Milk Fat Globule Membrane Components in Dairy Products. J. Agric. Food Chem. 2010, 58, 5612–5619. [Google Scholar] [CrossRef] [PubMed]
- Guerin, J.; Soligot, C.; Burgain, J.; Huguet, M.; Francius, G.; El-Kirat-Chatel, S.; Gomand, F.; Lebeer, S.; Le Roux, Y.; Borges, F.; et al. Adhesive Interactions between Milk Fat Globule Membrane and Lactobacillus Rhamnosus GG Inhibit Bacterial Attachment to Caco-2 TC7 Intestinal Cell. Colloids Surf. B 2018, 167, 44–53. [Google Scholar] [CrossRef]
- Patton, S.; Gendler, S.J.; Spicer, A.P. The Epithelial Mucin, MUC1, of Milk, Mammary Gland and Other Tissues. Biochim. Biophys. Acta 1995, 1241, 407–423. [Google Scholar] [CrossRef]
- Recio, I.; Moreno, F.J.; López-Fandiño, R. Glycosylated dairy components: Their roles in nature and ways to make use of their biofunctionality in dairy products. In Dairy-Derived Ingredients; Elsevier: Amsterdam, The Netherlands, 2009; pp. 170–211. ISBN 978-1-84569-465-4. [Google Scholar]
- Gupta, V.K.; Tuohy, M.G.; O’Donovan, A.; Lohani, M. (Eds.) Biotechnology of Bioactive Compounds: Sources and Applications; Wiley-Blackwell: Chichester, UK; Hoboken, NJ, USA, 2015; ISBN 978-1-118-73344-8. [Google Scholar]
- Verma, A.; Ghosh, T.; Bhushan, B.; Packirisamy, G.; Navani, N.K.; Sarangi, P.P.; Ambatipudi, K. Characterization of Difference in Structure and Function of Fresh and Mastitic Bovine Milk Fat Globules. PLoS ONE 2019, 14, e0221830. [Google Scholar] [CrossRef]
- Shahriar, F.; Ngeleka, M.; Gordon, J.R.; Simko, E. Identification by Mass Spectroscopy of F4ac-Fimbrial-Binding Proteins in Porcine Milk and Characterization of Lactadherin as an Inhibitor of F4ac-Positive Escherichia Coli Attachment to Intestinal Villi in Vitro. Dev. Comp. Immunol. 2006, 30, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; García-Cano, I.; Jiménez-Flores, R. Characterization of Adhesion between Limosilactobacillus Reuteri and Milk Phospholipids by Density Gradient and Gene Expression. JDS Commun. 2020, S2666910220300326. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Okada, S.; Uchimura, T.; Satoh, E. A Mucus Adhesion Promoting Protein, MapA, Mediates the Adhesion of Lactobacillus Reuteri to Caco-2 Human Intestinal Epithelial Cells. Biosci. Biotechnol. Biochem. 2006, 70, 1622–1628. [Google Scholar] [CrossRef]
- Hsueh, H.-Y.; Yueh, P.-Y.; Yu, B.; Zhao, X.; Liu, J.-R. Expression of Lactobacillus Reuteri Pg4 Collagen-Binding Protein Gene in Lactobacillus Casei ATCC 393 Increases Its Adhesion Ability to Caco-2 Cells. J. Agric. Food Chem. 2010, 58, 12182–12191. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.; Roos, S.; Jonsson, H.; Rud, I.; Grimmer, S.; van Pijkeren, J.-P.; Britton, R.A.; Axelsson, L. Role of Lactobacillus Reuteri Cell and Mucus-Binding Protein A (CmbA) in Adhesion to Intestinal Epithelial Cells and Mucus in Vitro. Microbiology 2014, 160, 671–681. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Mendoza, D.; Kosmerl, E.; Miyagusuku-Cruzado, G.; Giusti, M.M.; Jiménez-Flores, R.; García-Cano, I. Growth of Lactic Acid Bacteria in Milk Phospholipids Enhances Their Adhesion to Caco-2 Cells. J. Dairy Sci. 2020, S0022030220305385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chichlowski, M.; Gross, G.; Holle, M.; lbarra-Sánchez, L.A.; Wang, S.; Miller, M.J. Milk Fat Globule Membrane Protects Lactobacillus Rhamnosus GG from Bile Stress by Regulating Exopolysaccharide Production and Biofilm Formation. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Anaya, J.; Marciniak, A.; Jimenez-Flores, R. Milk Fat Globule Membrane Phospholipids Modify Adhesion of Lactobacillus to Mucus-Producing Caco-2/Goblet Cells by Altering the Cell Envelope. Manuscript in Preparation.
- Quinn, E.M.; Slattery, H.; Thompson, A.P.; Kilcoyne, M.; Joshi, L.; Hickey, R.M. Mining Milk for Factors Which Increase the Adherence of Bifidobacterium Longum Subsp. Infantis to Intestinal Cells. Foods 2018, 7, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morifuji, M.; Kitade, M.; Oba, C.; Fukasawa, T.; Kawahata, K.; Yamaji, T.; Manabe, Y.; Sugawara, T. Milk Fermented by Lactic Acid Bacteria Enhances the Absorption of Dietary Sphingomyelin in Rats. Lipids 2017, 52, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, Y.; Li, Z.; Christensen, B.; Heckmann, A.B.; Stenlund, H.; Lönnerdal, B.; Hernell, O. Feeding Infants Formula With Probiotics or Milk Fat Globule Membrane: A Double-Blind, Randomized Controlled Trial. Front. Pediatr. 2019, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Benyacoub, J.; Blum-Sperisen, S.; Bosco, M.N.; Bovetto, L.; Jean, R.; Bureau-Franz, I.; Donnet-Hughes, A.; Schiffrin, E.; Favre, L. Infant Formula with Probiotics and Milk Fat Globule Membrane Components. U.S. Patent WO2011069987A1, 16 June 2011. [Google Scholar]
- Nieto-Ruiz, A.; García-Santos, J.A.; Bermúdez, M.G.; Herrmann, F.; Diéguez, E.; Sepúlveda-Valbuena, N.; García, S.; Miranda, M.T.; De-Castellar, R.; Rodríguez-Palmero, M.; et al. Cortical Visual Evoked Potentials and Growth in Infants Fed with Bioactive Compounds-Enriched Infant Formula: Results from COGNIS Randomized Clinical Trial. Nutrients 2019, 11, 2456. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Ruiz, A.; Diéguez, E.; Sepúlveda-Valbuena, N.; Catena, E.; Jiménez, J.; Rodríguez-Palmero, M.; Catena, A.; Miranda, M.T.; García-Santos, J.A.G.; Bermúdez, M.; et al. Influence of a Functional Nutrients-Enriched Infant Formula on Language Development in Healthy Children at Four Years Old. Nutrients 2020, 12, 535. [Google Scholar] [CrossRef] [Green Version]
- Martinez, R.C.R.; Aynaou, A.-E.; Albrecht, S.; Schols, H.A.; De Martinis, E.C.P.; Zoetendal, E.G.; Venema, K.; Saad, S.M.I.; Smidt, H. In Vitro Evaluation of Gastrointestinal Survival of Lactobacillus Amylovorus DSM 16698 Alone and Combined with Galactooligosaccharides, Milk and/or Bifidobacterium Animalis Subsp. Lactis Bb. Int. J. Food Microbiol. 2011, 149, 152–158. [Google Scholar] [CrossRef]
- Bezkorovainy, A. Probiotics: Determinants of Survival and Growth in the Gut. Am. J. Clin. Nutr. 2001, 73, 399s–405s. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Henriksson, A.; Mitchell, H. Prebiotics Enhance Survival and Prolong the Retention Period of Specific Probiotic Inocula in an in Vivo Murine Model. J. Appl. Microbiol. 2007, 103, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Z.; Zhang, Q.; Yan, L.; Chen, W.; Liu, X.-M.; Zhang, H.-P. Fermentation Characteristics and Transit Tolerance of Probiotic Lactobacillus Casei Zhang in Soymilk and Bovine Milk during Storage. J. Dairy Sci. 2009, 92, 2468–2476. [Google Scholar] [CrossRef] [Green Version]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectrum 2015, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Fanning, S.; Hall, L.J.; Cronin, M.; Zomer, A.; MacSharry, J.; Goulding, D.; O’Connell Motherway, M.; Shanahan, F.; Nally, K.; Dougan, G.; et al. Bifidobacterial Surface-Exopolysaccharide Facilitates Commensal-Host Interaction through Immune Modulation and Pathogen Protection. Proc. Natl. Acad. Sci. USA 2012, 109, 2108–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Anaya, J.; Rocha-Mendoza, D.; García-Cano, I.; Jimenez-Flores, R. Effect of Milk Fat Globule Membrane Phospholipids in the Adherence of Probiotic Lactic Acid Bacteria- Modeling Interactions in the Human Gut. In Proceedings of the American Dairy Science Association Annual Meeting, Cincinnati, OH, USA, 23–26 June 2019. [Google Scholar]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Huo, G.; Li, X.; Yang, L.; Duan, C.; Wang, T.; Chen, J. Diversity of the Intestinal Microbiota in Different Patterns of Feeding Infants by Illumina High-Throughput Sequencing. World J. Microbiol. Biotechnol. 2013, 29, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Tokuhara, D.; Kurashima, Y.; Kamioka, M.; Nakayama, T.; Ernst, P.; Kiyono, H. A Comprehensive Understanding of the Gut Mucosal Immune System in Allergic Inflammation. Allergol. Int. 2019, 68, 17–25. [Google Scholar] [CrossRef]
- Norris, G.H.; Porter, C.M.; Jiang, C.; Millar, C.L.; Blesso, C.N. Dietary Sphingomyelin Attenuates Hepatic Steatosis and Adipose Tissue Inflammation in High-Fat-Diet-Induced Obese Mice. J. Nutr. Biochem. 2017, 40, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The Pilot Study: Sphingomyelin-Fortified Milk Has a Positive Association with the Neurobehavioural Development of Very Low Birth Weight Infants during Infancy, Randomized Control Trial. Brain Dev. 2013, 35, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Oshida, K.; Shimizu, T.; Takase, M.; Tamura, Y.; Shimizu, T.; Yamashiro, Y. Effects of Dietary Sphingomyelin on Central Nervous System Myelination in Developing Rats. Pediatr. Res. 2003, 53, 589–593. [Google Scholar] [CrossRef] [Green Version]
- Oba, C.; Morifuji, M.; Ichikawa, S.; Ito, K.; Kawahata, K.; Yamaji, T.; Asami, Y.; Itou, H.; Sugawara, T. Dietary Milk Sphingomyelin Prevents Disruption of Skin Barrier Function in Hairless Mice after UV-B Irradiation. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Le Huërou-Luron, I.; Bouzerzour, K.; Ferret-Bernard, S.; Ménard, O.; Le Normand, L.; Perrier, C.; Le Bourgot, C.; Jardin, J.; Bourlieu, C.; Carton, T.; et al. A Mixture of Milk and Vegetable Lipids in Infant Formula Changes Gut Digestion, Mucosal Immunity and Microbiota Composition in Neonatal Piglets. Eur. J. Nutr. 2018, 57, 463–476. [Google Scholar] [CrossRef]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Slupsky, C.M.; Heckmann, A.B.; Christensen, B.; Peng, Y.; Li, X.; Hernell, O.; Lönnerdal, B.; Li, Z. Milk Fat Globule Membrane as a Modulator of Infant Metabolism and Gut Microbiota: A Formula Supplement Narrowing the Metabolic Differences between Breastfed and Formula-Fed Infants. Mol. Nutr. Food Res. 2020, 2000603. [Google Scholar] [CrossRef]
- He, X.; Parenti, M.; Grip, T.; Lönnerdal, B.; Timby, N.; Domellöf, M.; Hernell, O.; Slupsky, C.M. Fecal Microbiome and Metabolome of Infants Fed Bovine MFGM Supplemented Formula or Standard Formula with Breast-Fed Infants as Reference: A Randomized Controlled Trial. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodenas, C.L.; Bergonzelli, G.; Rochat, F.; Turini, M.E.; Corthesy-Theulaz, I.; Cherbut, C. Nutritional Formula for Optimal Gut Barrier Function. U.S. Patent US2004112509A2, 29 December 2004. [Google Scholar]
- Mikulic, J.; Longet, S.; Favre, L.; Benyacoub, J.; Corthesy, B. Secretory IgA in Complex with Lactobacillus Rhamnosus Potentiates Mucosal Dendritic Cell-Mediated Treg Cell Differentiation via TLR Regulatory Proteins, RALDH2 and Secretion of IL-10 and TGF-β. Cell Mol. Immunol. 2017, 14, 546–556. [Google Scholar] [CrossRef] [Green Version]
- Holland, D.; Chang, L.; Ernst, T.M.; Curran, M.; Buchthal, S.D.; Alicata, D.; Skranes, J.; Johansen, H.; Hernandez, A.; Yamakawa, R.; et al. Structural Growth Trajectories and Rates of Change in the First 3 Months of Infant Brain Development. JAMA Neurol. 2014, 71, 1266. [Google Scholar] [CrossRef]
- Stiles, J.; Jernigan, T.L. The Basics of Brain Development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef] [Green Version]
- Schneider, N.; Garcia-Rodenas, C.L. Early Nutritional Interventions for Brain and Cognitive Development in Preterm Infants: A Review of the Literature. Nutrients 2017, 9, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deoni, S.; Dean, D.; Joelson, S.; O’Regan, J.; Schneider, N. Early Nutrition Influences Developmental Myelination and Cognition in Infants and Young Children. NeuroImage 2018, 178, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, S.S.; Berseth, C.L.; Harris, C.L.; Richards, J.D.; Wampler, J.L.; Zhuang, W.; Cleghorn, G.; Rudolph, C.D.; Liu, B.; et al. Improved Neurodevelopmental Outcomes Associated with Bovine Milk Fat Globule Membrane and Lactoferrin in Infant Formula: A Randomized, Controlled Trial. J. Pediatr. 2019, S0022347619310881. [Google Scholar] [CrossRef]
- Schipper, L.; van Dijk, G.; Broersen, L.M.; Loos, M.; Bartke, N.; Scheurink, A.J.; van der Beek, E.M. A Postnatal Diet Containing Phospholipids, Processed to Yield Large, Phospholipid-Coated Lipid Droplets, Affects Specific Cognitive Behaviors in Healthy Male Mice. J. Nutr. 2016, 146, 1155–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moukarzel, S.; Dyer, R.A.; Garcia, C.; Wiedeman, A.; Boyce, G.; Weinberg, J.; Keller, B.O.; Elango, R.; Innis, S.M. Milk Fat Globule Membrane Supplementation in Formula-Fed Rat Pups Improves Reflex Development and May Alter Brain Lipid Composition. Sci. Rep. 2018, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Brink, L.R.; Gueniot, J.P.; Lönnerdal, B. Effects of Milk Fat Globule Membrane and Its Various Components on Neurologic Development in a Postnatal Growth Restriction Rat Model. J. Nutr. Biochem. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.S.; Roller, R.; Mika, A.; Greenwood, B.N.; Knight, R.; Chichlowski, M.; Berg, B.M.; Fleshner, M. Dietary Prebiotics and Bioactive Milk Fractions Improve NREM Sleep, Enhance REM Sleep Rebound and Attenuate the Stress-Induced Decrease in Diurnal Temperature and Gut Microbial Alpha Diversity. Front. Behav. Neurosci. 2017, 10. [Google Scholar] [CrossRef]
- Timby, N.; Domellöf, E.; Hernell, O.; Lönnerdal, B.; Domellöf, M. Neurodevelopment, Nutrition, and Growth until 12 Mo of Age in Infants Fed a Low-Energy, Low-Protein Formula Supplemented with Bovine Milk Fat Globule Membranes: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2014, 99, 860–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tognini, P. Gut Microbiota: A Potential Regulator of Neurodevelopment. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- O’Mahony, S.M.; Neufeld, K.-A.M.; Waworuntu, R.V.; Pusceddu, M.M.; Manurung, S.; Murphy, K.; Strain, C.; Laguna, M.C.; Peterson, V.L.; Stanton, C.; et al. The Enduring Effects of Early-Life Stress on the Microbiota–Gut–Brain Axis Are Buffered by Dietary Supplementation with Milk Fat Globule Membrane and a Prebiotic Blend. Eur. J. Neurosci. 2020, 51, 1042–1058. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, J.; Du, M.; Song, J.; Mao, X. Milk Fat Globule Membrane Attenuates High-Fat Diet-Induced Obesity by Inhibiting Adipogenesis and Increasing Uncoupling Protein 1 Expression in White Adipose Tissue of Mice. Nutrients 2018, 10, 331. [Google Scholar] [CrossRef] [Green Version]
- Nicolson, G.L.; Ash, M.E. Membrane Lipid Replacement for Chronic Illnesses, Aging and Cancer Using Oral Glycerolphospholipid Formulations with Fructooligosaccharides to Restore Phospholipid Function in Cellular Membranes, Organelles, Cells and Tissues. Biochim. Biophys. Acta 2017, 1859, 1704–1724. [Google Scholar] [CrossRef]
- García-Cano, I.; Rocha-Mendoza, D.; Ortega-Anaya, J.; Wang, K.; Kosmerl, E.; Jiménez-Flores, R. Lactic Acid Bacteria Isolated from Dairy Products as Potential Producers of Lipolytic, Proteolytic and Antibacterial Proteins. Appl. Microbiol. Biotechnol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manasian, P.; Bustos, A.-S.; Pålsson, B.; Håkansson, A.; Peñarrieta, J.M.; Nilsson, L.; Linares-Pastén, J.A. First Evidence of Acyl-Hydrolase/Lipase Activity from Human Probiotic Bacteria: Lactobacillus Rhamnosus GG and Bifidobacterium Longum NCC. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Soda, M.E.; Wahab, H.A.E.; Ezzat, N.; Desmazeaud, M.J.; Ismail, A. The Esterolytic and Lipolytic Activities of the Lactobacilli. II. Detection of the Esterase System of Lactobacillus Helveticus, Lactobacillus Bulgaricus. Lactobacillus Lactis and Lactobacillus Acidophilus. Lait 1986, 66, 431–443. [Google Scholar] [CrossRef] [Green Version]

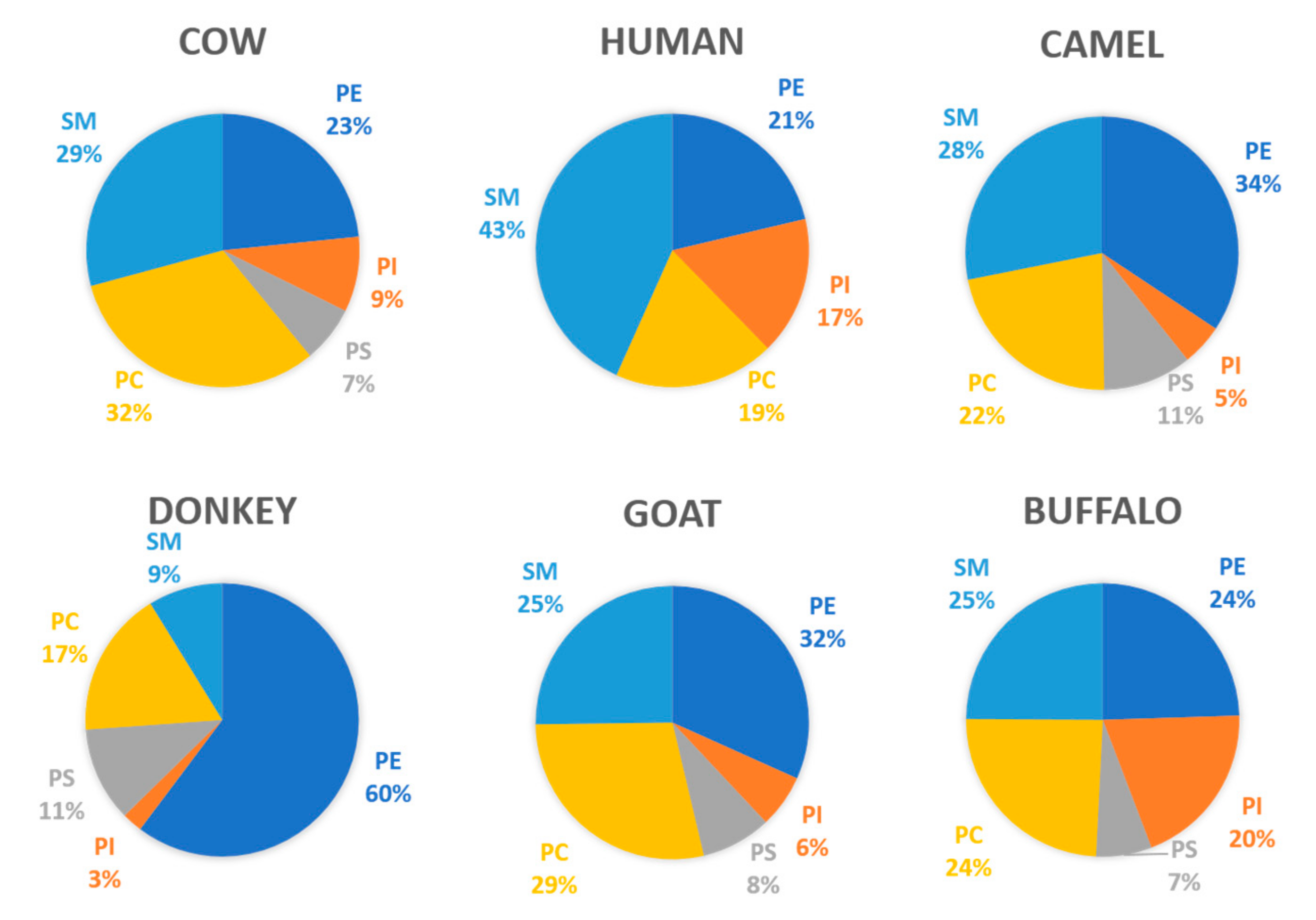
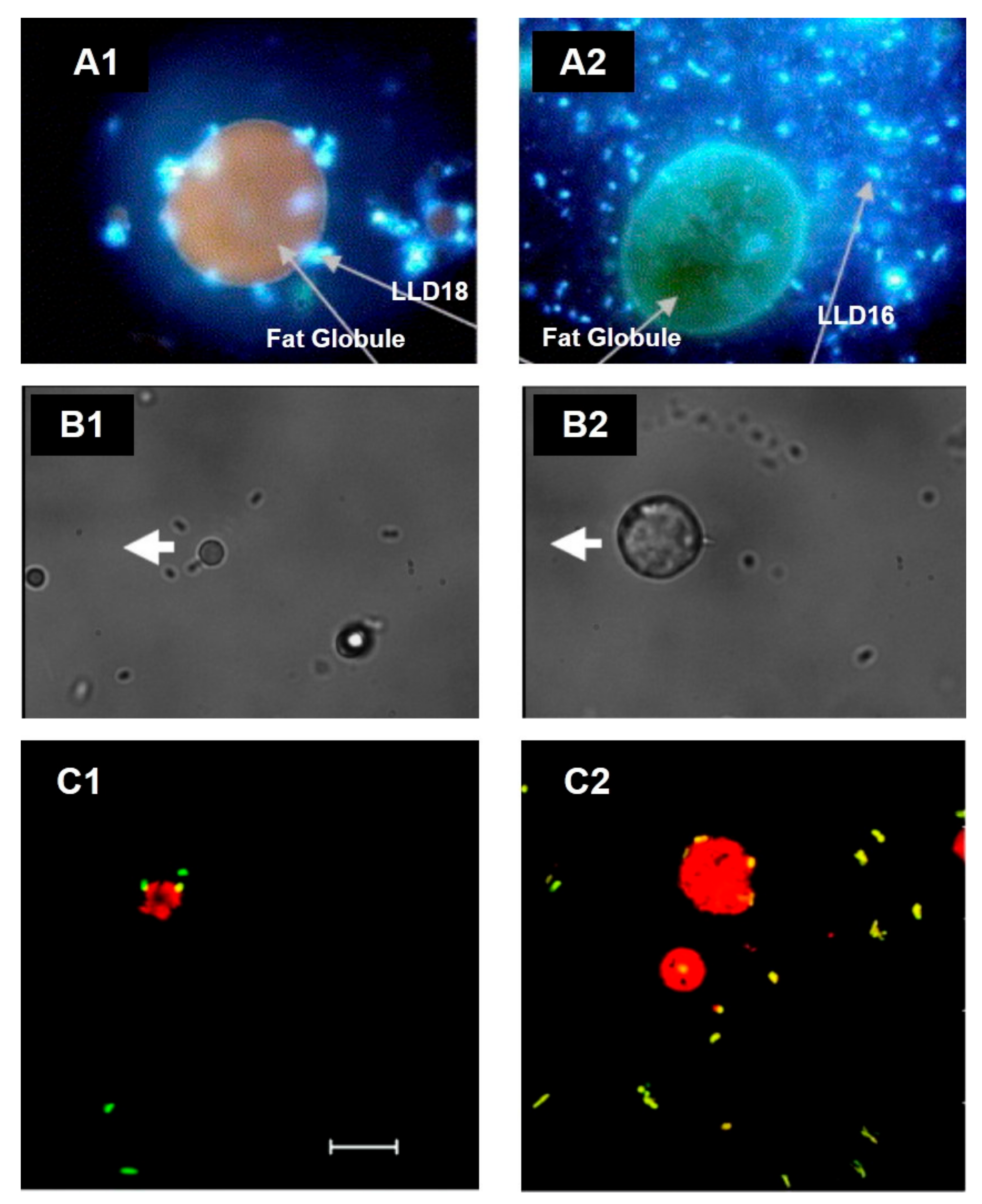
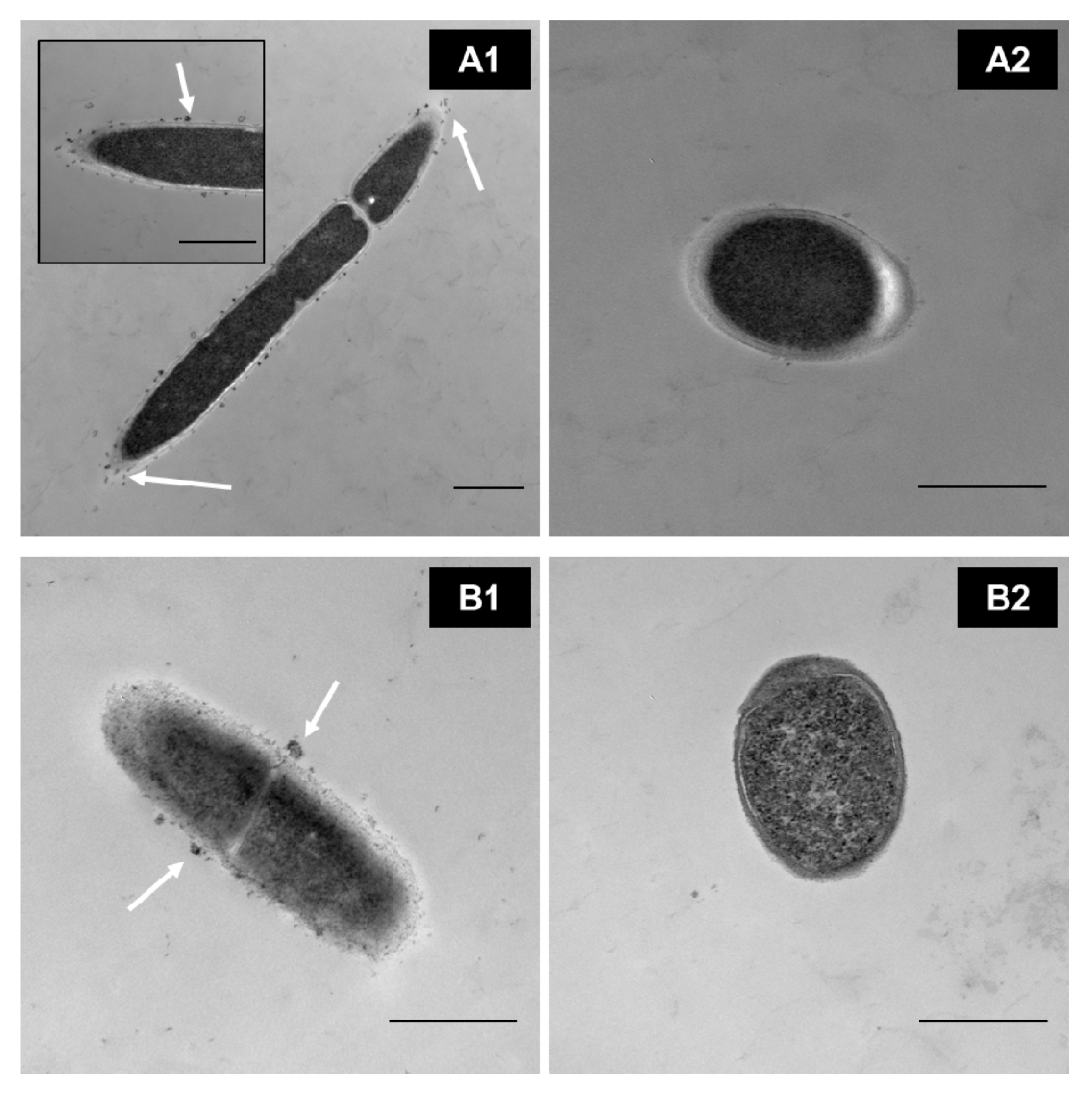
| Component | Concentration Range (%) |
|---|---|
| Lipids | 64–71.8 |
| Protein | 22.3–28 |
| Glycoconjugates | 10 |
| RNA | Traces |
| Lipid Class | Fraction Content In % | Subclass |
|---|---|---|
| Polar Lipids | ||
| Phospholipids | 65 | Glycerophospholipids
|
| Gangliosides or glycolipids | 5 |
|
| Neutral Lipids | ||
| Di-acylglycerols | 10 | |
| Mono-acylglycerols | Traces | |
| Free fatty acids | 10 | |
| Sterols | ||
| Cholesterol | 10 | |
| Liposoluble molecules | ||
| Vitamins A, D, E and K | 5 | |
| Protein | Content in g/100 g of Total Protein * | Molecular Mass (kDa) | Reported Effect on Human Health |
|---|---|---|---|
| Mucin 1 (MUC-1) | NA | 250–450 | Antiviral and antibacterial by preventing binding of pathogens to intestinal cells |
| Mucin 15 (MUC-15) | NA | 94–100 | Antiviral action |
| Xanthine dehydrogenase/oxidase (XDH/XO) | 0.58 | 150–155 | Bactericidal action by production of hydrogen peroxide and nitric oxide |
| Cluster of differentiation 36 (CD36) | 0.18 | 77–78 | Receptor for collagen and thrombospondin. Scavenger receptor for apoptotic cells |
| Butyrophilin (BTN) | 3.35 | 66 | Member of the immunoglobulin superfamily, adhesive protein, acts as a receptor and has a positive effect in the immune system. Co-inhibitor of T-cell activation |
| Adipophilin (ADPH) | 0.007 | 52 | Facilitates transport of triglycerides and fatty acids during fat globule synthesis |
| PAS 6/7 or Lactadherin | 0.93 | 48–54 | Adhesive properties with effect in the regulation of epithelial coagulation. Role in synaptic activity in the central nervous system (CNS) and protection against viral infection in the gut |
| FABP family | 0.17 | 14–15 | Transport of fatty acids |
| Ingredient | Microorganism(s) | Model | Experimental Design | Key Findings | Ref. |
|---|---|---|---|---|---|
| Bacterial Survival and Adhesion | |||||
| Whey-derived MFGM (MFGM-10 Lacprodan®) | L. rhamnosus GG (LGG) | Male, 6-week-old BALB/c mice | Oral gavage of 0.1 mL MRSC media, MRSC with 5 g/L MFGM-10, MRSC with LGG (5 × 107 CFU/mL) or MRSC with 5 g/L MFGM-10 and LGG (5 × 107 CFU/mL) for 3 days |
| [87] |
| MFGM-derived MPL concentrate | Lacticaseibacillus casei OSU-PECh-C; Lactobacillus acidophilus Musallam2; L. plantarum subsp. plantarum TW14-1; L. delbruekii OSU-PECh-3 | Gold (Au) Sensor; Caco-2/HT29-MTX | Examined the adhesion phenomena of 4 strains in the presence or absence of 0.5% (w/v) MPL to A) a gold sensor using a Quartz Crystal Micrograph with Dissipation (QCM-D); B) TEM; and C) intestinal cell culture |
| [88] |
| MFGM-derived MPL concentrate | P. acidilactici OSU-PECh-L; P. acidilactici OSU-PECh-3A; L. plantarum OSU-PECh-BB, L. reuteri OSUPECh-48; L. casei OSU-PECh-C, L. paracasei OSU-PECh-BA; L. paracasei OSU-PECh-3B | Caco-2 | LAB strains grown with or without 0.5% (w/v) MPL were characterized by functional properties and their adhesive ability to fully differentiated Caco-2 cells |
| [86] |
| MFGM extract from butter serum | L. rhamnosus GG (LGG) | Caco-2 TC7 | LGG was exposed to 5 mg/mL MFGM extract for 1 h and applied to intestinal cells (1 × 109 CFU/mL) |
| [76] |
| MPL-rich milk protein concentrate (Lacprodan® PL-20) | B. longum subsp. infantis ATCC 15697 | HT-29 | Exposed bifidobacteria to MFGM ingredients for 1 h and measured adherence of bacteria to fully confluent cells after 2 h incubation using plate count method |
| [89] |
| Whey-derived MFGM (MFGM-10 Lacprodan®) | |||||
| Buttermilk fraction (BF) | |||||
| Nutrient Absorption, Mucosal Immunity, and Gut Barrier Function | |||||
| MFGM-derived MPL concentrate | L. delbruekii subsp. bulgaricus 2038; S. thermophilus 1131 | Male Sprague-Dawley rats | Orally supplemented rats with SM, MPLs alone or either of these in fermented milk |
| [90] |
| Whey-derived MFGM (MFGM-10 Lacprodan®) | L. paracasei subsp. paracasei F19 (F19) | Infants (21-days−4-months old) | Double-blind RCT for an infant formula supplemented with MFGM (5 g/L prepared formula) or F19 (1 × 108 CFU/L) |
| [91] |
| Unspecified MFGM fraction | B. animalis subsp. lactis BB-12 (BB-12) | HT-29Cl34 (NF-κB reporter cell line); 28-day-old mice | Used reporter cell line to measure NF-κB activation in response to BB-12 (1 × 106 or 1 × 107 CFU/mL) and/or MFGM (50 μg/mL or 100 μg/mL) and LPS challenge (100 ng/mL); For in vivo mouse study, administered BB-12 (1 × 108 CFU/day), MFGM (0.6 mg/g of body weight/day), or both BB-12 and MFGM orally for 1 or 4 weeks |
| [92] |
| Neurodevelopment and Cognitive Function | |||||
| MFGM components | B. infantis IM1; L. rhamnosus LCS-742 | 12-month-old infants | Double-blind RCT (COGNIS study) for a novel infant formula containing bioactive ingredients, including MFGM [10% of total protein content (w/w)] and probiotics |
| [93] |
| MFGM components | B. infantis IM1; L. rhamnosus LCS-742 | 4-year-old infants | Double-blind RCT (COGNIS study) for a novel infant formula containing bioactive ingredients, including MFGM [10% of total protein content (w/w)] and probiotics |
| [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmerl, E.; Rocha-Mendoza, D.; Ortega-Anaya, J.; Jiménez-Flores, R.; García-Cano, I. Improving Human Health with Milk Fat Globule Membrane, Lactic Acid Bacteria, and Bifidobacteria. Microorganisms 2021, 9, 341. https://doi.org/10.3390/microorganisms9020341
Kosmerl E, Rocha-Mendoza D, Ortega-Anaya J, Jiménez-Flores R, García-Cano I. Improving Human Health with Milk Fat Globule Membrane, Lactic Acid Bacteria, and Bifidobacteria. Microorganisms. 2021; 9(2):341. https://doi.org/10.3390/microorganisms9020341
Chicago/Turabian StyleKosmerl, Erica, Diana Rocha-Mendoza, Joana Ortega-Anaya, Rafael Jiménez-Flores, and Israel García-Cano. 2021. "Improving Human Health with Milk Fat Globule Membrane, Lactic Acid Bacteria, and Bifidobacteria" Microorganisms 9, no. 2: 341. https://doi.org/10.3390/microorganisms9020341
APA StyleKosmerl, E., Rocha-Mendoza, D., Ortega-Anaya, J., Jiménez-Flores, R., & García-Cano, I. (2021). Improving Human Health with Milk Fat Globule Membrane, Lactic Acid Bacteria, and Bifidobacteria. Microorganisms, 9(2), 341. https://doi.org/10.3390/microorganisms9020341






