Clonal Diversity and Antimicrobial Resistance of Methicillin-Resistant Staphylococcus pseudintermedius Isolated from Canine Pyoderma
Abstract
:1. Introduction
2. Materials and Methods
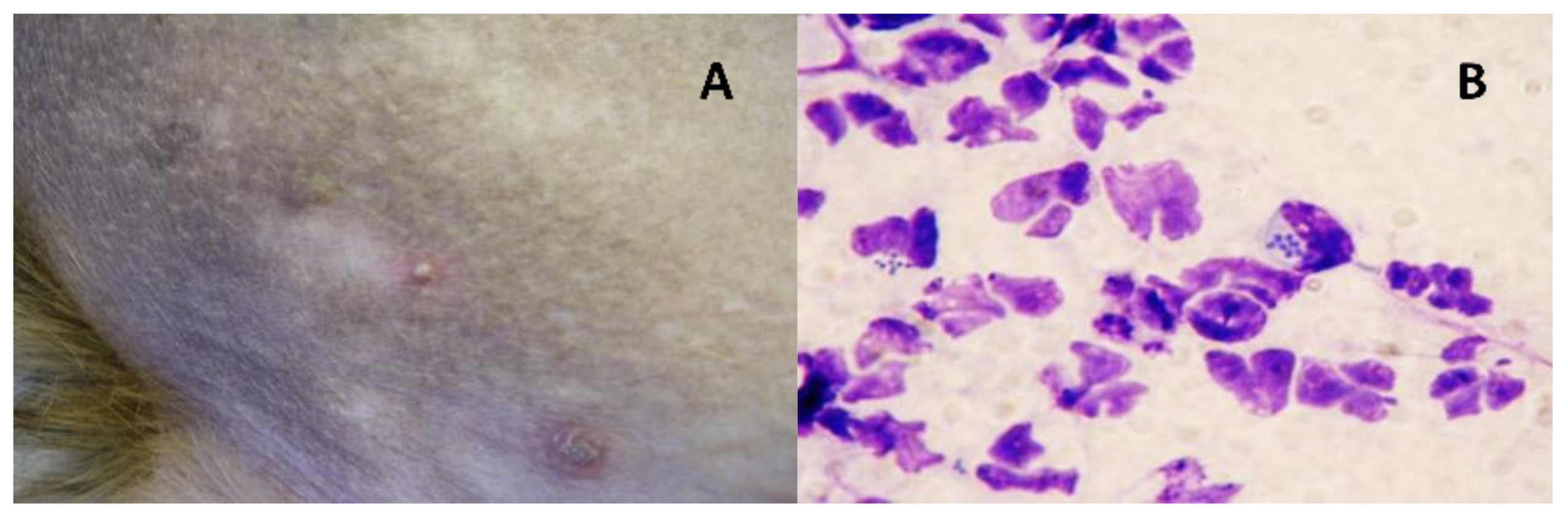
2.1. Samples and Bacterial Isolates
2.2. Antimicrobial Susceptibility Testing
2.3. Antibiotic Resistance Genes and Virulence Factors
2.4. Molecular Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Syed, A. Antibiotic Use and Resistance. Int. J. Curr. Res. Med. Sci 2019, 5, 17–23. [Google Scholar]
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial resistance in bacteria: Mechanisms, evolution, and persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Osman, K.; Alvarez-Ordóñez, A.; Ruiz, L.; Badr, J.; ElHofy, F.; Al-Maary, K.S.; Moussa, I.M.I.; Hessain, A.M.; Orabi, A.; Saad, A.; et al. Antimicrobial resistance and virulence characterization of Staphylococcus aureus and coagulase-negative staphylococci from imported beef meat. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 35. [Google Scholar] [CrossRef] [Green Version]
- Fishovitz, J.; Hermoso, J.A.; Chang, M.; Mobashery, S. Penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. IUBMB Life 2014, 66, 572–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. World Health Organization Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Smith, J.T.; Amador, S.; McGonagle, C.J.; Needle, D.; Gibson, R.; Andam, C.P. Population genomics of Staphylococcus pseudintermedius in companion animals in the United States. Commun. Biol. 2020, 3, 282. [Google Scholar] [CrossRef]
- Balachandran, M.; Bemis, D.A.; Kania, S.A. Expression and function of protein A in Staphylococcus pseudintermedius. Virulence 2018, 9, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Krapf, M.; Müller, E.; Reissig, A.; Slickers, P.; Braun, S.D.; Müller, E.; Ehricht, R.; Monecke, S. Molecular characterisation of methicillin-resistant Staphylococcus pseudintermedius from dogs and the description of their SCCmec elements. Vet. Microbiol. 2019, 233, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Somayaji, R.; Priyantha, M.A.R.; Rubin, J.E.; Church, D. Human infections due to Staphylococcus pseudintermedius, an emerging zoonosis of canine origin: Report of 24 cases. Diagn. Microbiol. Infect. Dis. 2016, 85, 471–476. [Google Scholar] [CrossRef]
- Abouelkhair, M.A.; Bemis, D.A.; Giannone, R.J.; Frank, L.A.; Kania, S.A. Characterization of a leukocidin identified in Staphylococcus pseudintermedius. PLoS ONE 2018, 13, e0204450. [Google Scholar] [CrossRef]
- Worthing, K.A.; Abraham, S.; Coombs, G.W.; Pang, S.; Saputra, S.; Jordan, D.; Trott, D.J.; Norris, J.M. Clonal diversity and geographic distribution of methicillin-resistant Staphylococcus pseudintermedius from Australian animals: Discovery of novel sequence types. Vet. Microbiol. 2018, 213, 58–65. [Google Scholar] [CrossRef]
- Kawakami, T.; Shibata, S.; Murayama, N.; Nagata, M.; Nishifuji, K.; Iwasaki, T.; Fukata, T. Antimicrobial susceptibility and methicillin resistance in Staphylococcus pseudintermedius and Staphylococcus schleiferi subsp. coagulans isolated from dogs with pyoderma in Japan. J. Vet. Med. Sci. 2010, 72, 1615–1619. [Google Scholar] [CrossRef] [Green Version]
- Gagetti, P.; Wattam, A.R.; Giacoboni, G.; De Paulis, A.; Bertona, E.; Corso, A.; Rosato, A.E. Identification and molecular epidemiology of methicillin resistant Staphylococcus pseudintermedius strains isolated from canine clinical samples in Argentina. BMC Vet. Res. 2019, 15, 264. [Google Scholar] [CrossRef]
- Maali, Y.; Badiou, C.; Martins-Simões, P.; Hodille, E.; Bes, M.; Vandenesch, F.; Lina, G.; Diot, A.; Laurent, F.; Trouillet-Assant, S. Understanding the Virulence of Staphylococcus pseudintermedius: A Major Role of Pore-Forming Toxins. Front. Cell. Infect. Microbiol. 2018, 8, 221. [Google Scholar] [CrossRef]
- Tabatabaei, S.; Najafifar, A.; Askari Badouei, M.; Zahraei Salehi, T.; Ashrafi Tamai, I.; Khaksar, E.; Abbassi, M.S.; Ghazisaeedi, F. Genetic characterisation of methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in pets and veterinary personnel in Iran: New insights into emerging methicillin-resistant S. pseudintermedius (MRSP). J. Glob. Antimicrob. Resist. 2019, 16, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Bergot, M.; Martins-Simoes, P.; Kilian, H.; Châtre, P.; Worthing, K.A.; Norris, J.M.; Madec, J.-Y.; Laurent, F.; Haenni, M. Evolution of the Population Structure of Staphylococcus pseudintermedius in France. Front. Microbiol. 2018, 9, 3055. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Belas, A.; Couto, I.; Perreten, V.; Pomba, C. Genetic Relatedness, Antimicrobial and Biocide Susceptibility Comparative Analysis of Methicillin-Resistant and -Susceptible Staphylococcus pseudintermedius from Portugal. Microb. Drug Resist. 2013, 20, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couto, N.; Belas, A.; Oliveira, M.; Almeida, P.; Clemente, C.; Pomba, C. Comparative RNA-seq-based transcriptome analysis of the virulence characteristics of methicillin-resistant and-susceptible Staphylococcus pseudintermedius strains isolated from small animals. Antimicrob. Agents Chemother. 2016, 60, 962–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannoehr, J.; Franco, A.; Iurescia, M.; Battisti, A.; Fitzgerald, J.R. Molecular diagnostic identification of Staphylococcus pseudintermedius. J. Clin. Microbiol. 2009, 47, 469–471. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Almeida, F.; Carvalho, J.A.; Castro, A.P.; Ferreira, E.; Manageiro, V.; Tejedor-Junco, M.T.; Caniça, M.; Igrejas, G.; Poeta, P. Emergence of community-acquired methicillin-resistant Staphylococcus aureus EMRSA-15 clone as the predominant cause of diabetic foot ulcer infections in Portugal. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 179–186. [Google Scholar] [CrossRef]
- Silva, V.; Almeida, F.; Silva, A.; Correia, S.; Carvalho, J.A.; Castro, A.P.; Ferreira, E.; Manageiro, V.; Caniça, M.; Igrejas, G.; et al. First report of linezolid-resistant cfr-positive methicillin-resistant Staphylococcus aureus in humans in Portugal. J. Glob. Antimicrob. Resist. 2019, 17, 323–325. [Google Scholar] [CrossRef]
- Yu, F.; Liu, Y.; Lv, J.; Qi, X.; Lu, C.; Ding, Y.; Li, D.; Liu, H.; Wang, L. Antimicrobial susceptibility, virulence determinant carriage and molecular characteristics of Staphylococcus aureus isolates associated with skin and soft tissue infections. Braz. J. Infect. Dis. 2015, 19, 614–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarraud, S.; Mougel, C.; Thioulouse, J.; Lina, G.; Meugnier, H.; Forey, F.; Etienne, J.; Vandenesch, F.; Nesme, X. Relationships between Staphylococcus aureus Genetic Background, Virulence Factors, agr Groups (Alleles), and Human Disease. Infect. Immun. 2002, 70, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Lina, G.; Piemont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.-O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine Leukocidin-Producing Staphylococcus aureus in Primary Skin Infections and Pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Futagawa-Saito, K.; Sugiyama, T.; Karube, S.; Sakurai, N.; Ba-Thein, W.; Fukuyasu, T. Prevalence and characterization of leukotoxin-producing Staphylococcus intermedius in isolates from dogs and pigeons. J. Clin. Microbiol. 2004, 42, 5324–5326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solyman, S.M.; Black, C.C.; Duim, B.; Perreten, V.; Van Duijkeren, E.; Wagenaar, J.A.; Eberlein, L.C.; Sadeghi, L.N.; Videla, R.; Bemis, D.A. Multilocus sequence typing for characterization of Staphylococcus pseudintermedius. J. Clin. Microbiol. 2013, 51, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Bannoehr, J.; Ben Zakour, N.L.; Waller, A.S.; Guardabassi, L.; Thoday, K.L.; Broek, A.H.M.V.D.; Fitzgerald, J.R. Population genetic structure of the Staphylococcus intermedius group: Insights into agr diversification and the emergence of methicillin-resistant strains. J. Bacteriol. 2007, 189, 8685–8692. [Google Scholar] [CrossRef] [Green Version]
- Nakaminami, H.; Okamura, Y.; Tanaka, S.; Wajima, T.; Murayama, N.; Noguchi, N. Prevalence of antimicrobial-resistant staphylococci in nares and affected sites of pet dogs with superficial pyoderma. J. Vet. Med. Sci. 2020. [Google Scholar] [CrossRef]
- González-Domínguez, M.S.; Carvajal, H.D.; Calle-Echeverri, D.A.; Chinchilla-Cárdenas, D. Molecular Detection and Characterization of the mecA and nuc Genes From Staphylococcus Species (S. aureus, S. pseudintermedius, and S. schleiferi) Isolated From Dogs Suffering Superficial Pyoderma and Their Antimicrobial Resistance Profiles. Front. Vet. Sci. 2020, 7, 376. [Google Scholar] [CrossRef]
- Ruzauskas, M.; Couto, M.; Pavilonis, A.; Klimiene, I.; Siugzdiniene, R.; Virgailis, M.; Vaskeviciute, L.; Anskiene, L.; Pomba, C. Characterization of Staphylococcus pseudintermedius isolated from diseased dogs in Lithuania. Pol. J. Vet. Sci. 2016, 19, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Priyantha, R.; Gaunt, M.C.; Rubin, J.E. Antimicrobial susceptibility of Staphylococcus pseudintermedius colonizing healthy dogs in Saskatoon, Canada. Can. Vet. J. 2016, 57, 65. [Google Scholar]
- Menandro, M.L.; Dotto, G.; Mondin, A.; Martini, M.; Ceglie, L.; Pasotto, D. Prevalence and characterization of methicillin-resistant Staphylococcus pseudintermedius from symptomatic companion animals in Northern Italy: Clonal diversity and novel sequence types. Comp. Immunol. Microbiol. Infect. Dis. 2019, 66, 101331. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.C.; Perreten, V.; Bemis, D.A.; Kania, S.A. Complete genome sequences of three important methicillin-resistant clinical isolates of Staphylococcus pseudintermedius. Genome Announc. 2016, 4, e01194-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onuma, K.; Tanabe, T.; Sato, H. Antimicrobial resistance of Staphylococcus pseudintermedius isolates from healthy dogs and dogs affected with pyoderma in Japan. Vet. Dermatol. 2012, 23, 17-e5. [Google Scholar] [CrossRef] [PubMed]
- Wegener, A.; Broens, E.M.; Zomer, A.; Spaninks, M.; Wagenaar, J.A.; Duim, B. Comparative genomics of phenotypic antimicrobial resistances in methicillin-resistant Staphylococcus pseudintermedius of canine origin. Vet. Microbiol. 2018, 225, 125–131. [Google Scholar] [CrossRef]
- Petersen, A.; Stegger, M.; Heltberg, O.; Christensen, J.; Zeuthen, A.; Knudsen, L.K.; Urth, T.; Sorum, M.; Schouls, L.; Larsen, J.; et al. Epidemiology of methicillin-resistant Staphylococcus aureus carrying the novel mecC gene in Denmark corroborates a zoonotic reservoir with transmission to humans. Clin. Microbiol. Infect. 2013, 19, E16–E22. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ripa, L.; Simón, C.; Ceballos, S.; Ortega, C.; Zarazaga, M.; Torres, C.; Gómez-Sanz, E.S. pseudintermedius and S. aureus lineages with transmission ability circulate as causative agents of infections in pets for years. BMC Vet. Res. 2021, 17, 42. [Google Scholar] [CrossRef]
- Gómez-Sanz, E.; Torres, C.; Lozano, C.; Zarazaga, M. High diversity of Staphylococcus aureus and Staphylococcus pseudintermedius lineages and toxigenic traits in healthy pet-owning household members. Underestimating normal household contact? Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 83–94. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Harrison, E.M.; Stanczak-Mrozek, K.; Leggett, B.; Waller, A.; Holmes, M.A.; Lloyd, D.H.; Lindsay, J.A.; Loeffler, A. Genomic insights into the rapid emergence and evolution of MDR in Staphylococcus pseudintermedius. J. Antimicrob. Chemother. 2015, 70, 997–1007. [Google Scholar] [CrossRef] [Green Version]
- Pitchenin, L.C.; Brandão, L.N.S.; Rosa, J.M.A.; Kagueyama, F.C.; da Silva Alves, A.; Rocha, Í.S.M.; Nakazato, L.; Dutra, V. Occurrence of toxin genes in Staphylococcus pseudintermedius from diseased dogs and other domestic and wild species. J. Infect. Dev. Ctries. 2018, 11, 957–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melter, O.; Svec, P.; Tkadlec, J.; Doskar, J.; Kinská, H.; Pantucek, R. Characterisation of methicillin-susceptible Staphylococcus pseudintermedius isolates from canine infections and determination of virulence factors using multiplex PCR. Vet. Med. (Praha) 2017, 62, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Damborg, P.; Moodley, A.; Aalbæk, B.; Ventrella, G.; Dos Santos, T.P.; Guardabassi, L. High genotypic diversity among methicillin-resistant Staphylococcus pseudintermedius isolated from canine infections in Denmark. BMC Vet. Res. 2016, 12, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires dos Santos, T.; Damborg, P.; Moodley, A.; Guardabassi, L. Systematic Review on Global Epidemiology of Methicillin-Resistant Staphylococcus pseudintermedius: Inference of Population Structure from Multilocus Sequence Typing Data. Front. Microbiol. 2016, 7, 1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meroni, G.; Soares Filipe, J.F.; Drago, L.; Martino, P.A. Investigation on antibiotic-resistance, biofilm formation and virulence factors in multi drug resistant and non multi drug resistant Staphylococcus pseudintermedius. Microorganisms 2019, 7, 702. [Google Scholar] [CrossRef] [Green Version]
- Soimala, T.; Lübke-Becker, A.; Hanke, D.; Eichhorn, I.; Feßler, A.T.; Schwarz, S.; Eule, J.C. Molecular and phenotypic characterization of methicillin-resistant Staphylococcus pseudintermedius from ocular surfaces of dogs and cats suffering from ophthalmological diseases. Vet. Microbiol. 2020, 244, 108687. [Google Scholar] [CrossRef]
- Duim, B.; Verstappen, K.M.; Broens, E.M.; Laarhoven, L.M.; van Duijkeren, E.; Hordijk, J.; de Heus, P.; Spaninks, M.; Timmerman, A.J.; Wagenaar, J.A. Changes in the Population of Methicillin-Resistant Staphylococcus pseudintermedius and Dissemination of Antimicrobial-Resistant Phenotypes in the Netherlands. J. Clin. Microbiol. 2016, 54, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, K.; Koizumi, A.; Saito, M.; Muramatsu, Y.; Tamura, Y. Detection of methicillin-resistant Staphylococcus pseudintermedius ST169 and novel ST354 SCCmec II–III isolates related to the worldwide ST71 clone. Epidemiol. Infect. 2016, 144, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Videla, R.; Solyman, S.M.; Brahmbhatt, A.; Sadeghi, L.; Bemis, D.A.; Kania, S.A. Clonal Complexes and Antimicrobial Susceptibility Profiles of Staphylococcus pseudintermedius Isolates from Dogs in the United States. Microb. Drug Resist. 2017, 24, 83–88. [Google Scholar] [CrossRef]
| Isolate | Age (Years) | Gender | Breed |
|---|---|---|---|
| VS2777 | 5 | F | Bull Terrier |
| VS2778 | 6 | M | Golden Retriever |
| VS2779 | 8 | F | German Shepherd |
| VS2780 | 2 | F | Yorkshire Terrier |
| VS2781 | 5 | M | Labrador Retriever |
| VS2782 | 6 | M | Boxer |
| VS2783 | 10 | M | Mixed breed |
| VS2784 | 4 | M | Estrela Mountain |
| VS2785 | 9 | F | Mixed breed |
| VS2786 | 7 | M | Boxer |
| VS2787 | 6 | M | Golden Retriever |
| VS2788 | 6 | M | Cocker Spaniel |
| VS2789 | 10 | M | Rafeiro do Alentejo |
| VS2790 | 6 | M | Yorkshire Terrier |
| VS2791 | 2 | M | Mixed breed |
| VS2792 | 9 | F | French Bulldog |
| VS2793 | 12 | F | Mixed breed |
| VS2794 | 5 | F | Bull Terrier |
| VS2795 | 7 | F | Cocker Spaniel |
| VS2796 | 3 | F | German Shepherd |
| VS2797 | 9 | M | Akita |
| VS2798 | 4 | F | French Bulldog |
| VS2799 | 6 | M | Spanish Water Dog |
| VS2800 | 8 | M | Labrador Retriever |
| VS2801 | 4 | F | Mixed breed |
| VS2802 | 5 | F | Belgian Shepherd |
| VS2803 | 5 | M | Beagle |
| VS2804 | 4 | F | Mixed breed |
| VS2805 | 4 | F | French Bulldog |
| VS2806 | 12 | F | Mixed breed |
| VS2807 | 18 | M | Poodle |
| Isolate | Antimicrobial resistance | Virulence | ST (CC) | ||
|---|---|---|---|---|---|
| Phenotype | Genotype | ||||
| VS2777 | PEN-KAN-ERY-CD | blaZ, ermB, aph(3′)-IIIa | lukF-I/lukS-I | ST2024 | |
| VS2778 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aph(3′)-IIIa, tetK, dfrG | lukF-I/lukS-I | ST2025 | |
| VS2779 | PEN-KAN-ERY-CD-TET-CHL | blaZ, ermB, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST2026 | |
| VS2780 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aph(3′)-IIIa, aac(6′)-Ie-aph(2′′)-Ia, ant(4′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST2027 | |
| VS2781 | PEN-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST2028 | |
| VS2782 | PEN-KAN-ERY-CD-TET-SXT | blaZ, ermB, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST71 (71) | |
| VS2783 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-CHL-SXT | blaZ, msr(A/B), aph(3′)-IIIa, aac(6′)-Ie-aph(2′′)-Ia, ant(4′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST1468 | |
| VS2784 | PEN-CIP-CN-TOB-KAN-ERY-CD-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2785 | PEN-CIP-KAN-ERY-CD-TET-CHL-SXT | blaZ, ermB, tetM, dfrG | lukF-I/lukS-I | ST727 | |
| VS2786 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST339 | |
| VS2787 | PEN-CIP-KAN-ERY-CD-SXT | blaZ, ermB, aph(3′)-IIIa, dfrG | lukF-I/lukS-I | ST537 | |
| VS2788 | PEN-CIP-KAN-ERY-CD-SXT | blaZ, ermB, aph(3′)-IIIa, dfrG | lukF-I/lukS-I | ST339 | |
| VS2789 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, msr(A/B), aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2790 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-CHL-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2791 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-CHL-SXT | blaZ, ermB, msr(A/B), aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST45 (45) | |
| VS2792 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2793 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2794 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetK, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2795 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2796 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-CHL-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2797 | PEN-CN-TOB-KAN-ERY-CD-TET-SXT-RD | blaZ, ermB, aph(3′)-IIIa, ant(4′)-Ia, tetK, dfrG | lukF-I/lukS-I | ST1029 | |
| VS2798 | PEN-CIP-CN-TOB-KAN-ERY-CD-FD-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2799 | PEN-CN-KAN-ERY-CD-TET-SXT | blaZ, ermB, msr(A/B), aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetK, dfrG | lukF-I/lukS-I | ST727 | |
| VS2800 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, msr(A/B), aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetK, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2801 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, msr(A/B), aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetK, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2802 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2803 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetK, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2804 | PEN-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, msr(A/B), aph(3′)-IIIa, ant(4′)-Ia, tetK, dfrG | lukF-I/lukS-I | ST118 (258) | |
| VS2805 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | ermB, msr(A/B), aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, tetK, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2806 | PEN-CIP-CN-TOB-KAN-ERY-CD-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, dfrG | lukF-I/lukS-I | ST123 (71) | |
| VS2807 | PEN-CIP-CN-TOB-KAN-ERY-CD-TET-SXT | blaZ, ermB, aac(6′)-Ie-aph(2′′)-Ia, aph(3′)-IIIa, ant(4′)-Ia, tetM, dfrG | lukF-I/lukS-I | ST123 (71) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.; Oliveira, A.; Manageiro, V.; Caniça, M.; Contente, D.; Capita, R.; Alonso-Calleja, C.; Carvalho, I.; Capelo, J.L.; Igrejas, G.; et al. Clonal Diversity and Antimicrobial Resistance of Methicillin-Resistant Staphylococcus pseudintermedius Isolated from Canine Pyoderma. Microorganisms 2021, 9, 482. https://doi.org/10.3390/microorganisms9030482
Silva V, Oliveira A, Manageiro V, Caniça M, Contente D, Capita R, Alonso-Calleja C, Carvalho I, Capelo JL, Igrejas G, et al. Clonal Diversity and Antimicrobial Resistance of Methicillin-Resistant Staphylococcus pseudintermedius Isolated from Canine Pyoderma. Microorganisms. 2021; 9(3):482. https://doi.org/10.3390/microorganisms9030482
Chicago/Turabian StyleSilva, Vanessa, Ana Oliveira, Vera Manageiro, Manuela Caniça, Diogo Contente, Rosa Capita, Carlos Alonso-Calleja, Isabel Carvalho, José L. Capelo, Gilberto Igrejas, and et al. 2021. "Clonal Diversity and Antimicrobial Resistance of Methicillin-Resistant Staphylococcus pseudintermedius Isolated from Canine Pyoderma" Microorganisms 9, no. 3: 482. https://doi.org/10.3390/microorganisms9030482
APA StyleSilva, V., Oliveira, A., Manageiro, V., Caniça, M., Contente, D., Capita, R., Alonso-Calleja, C., Carvalho, I., Capelo, J. L., Igrejas, G., & Poeta, P. (2021). Clonal Diversity and Antimicrobial Resistance of Methicillin-Resistant Staphylococcus pseudintermedius Isolated from Canine Pyoderma. Microorganisms, 9(3), 482. https://doi.org/10.3390/microorganisms9030482











