The Impact of Tick-Borne Diseases on the Bone
Abstract
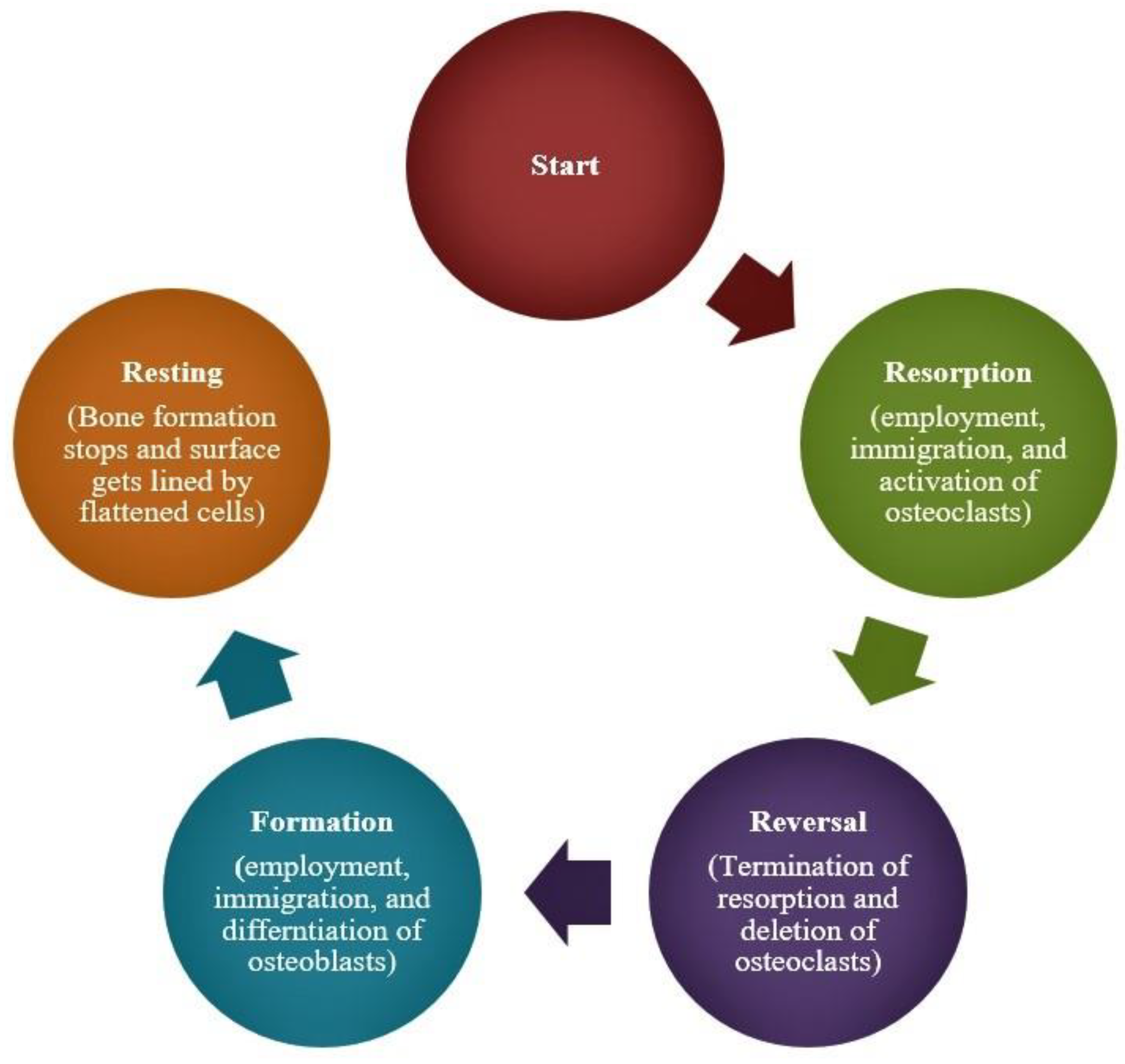
:1. Introduction
2. Anaplasmosis (Formerly Human Granulocytic Ehrlichiosis)
3. Ehrlichiosis
4. Babesiosis
5. Lyme Disease
6. Bourbon Virus Disease
7. Colorado Tick Fever Disease
8. Tick-Borne Encephalitis
9. Crimean–Congo Hemorrhagic Fever
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rochlin, I.; Toledo, A. Emerging tick-borne pathogens of public health importance: A mini-review. J. Med. Microbiol. 2020, 69, 781–791. [Google Scholar] [CrossRef]
- Rodino, K.G.; Theel, E.S.; Pritt, B.S. Tick-borne diseases in the United States. Clin. Chem. 2020, 66, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Dumler, J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. N. Am. 2015, 29, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Fillâtre, P.; Revest, M.; Tattevin, P. Crimean-Congo hemorrhagic fever: An update. Med. Mal. Infect. 2019, 49, 574–585. [Google Scholar] [CrossRef]
- Krasteva, S.; Jara, M.; Frias-De-Diego, A.; Machado, G. Nairobi Sheep Disease Virus: A Historical and Epidemiological Perspective. Front. Vet. Sci. 2020, 7, 419. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lv, X.-L.; Zhang, X.; Han, S.Z.; Wang, Z.D.; Li, L.; Sun, H.T.; Ma, L.X.; Cheng, Z.L.; Shao, J.W.; et al. Identification of a new orthocnairovirus associated with human febrile illness in China. Nat. Med. 2021, 27, 434–439. [Google Scholar] [CrossRef]
- Krause, P.J. Human babesiosis. Int. J. Parasitol. 2019, 49, 165–174. [Google Scholar] [CrossRef]
- Saito, T.B.; Walker, D.H. Ehrlichioses: An Important One Health Opportunity. Vet. Sci. 2016, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Brault, A.C.; Savage, H.M.; Duggal, N.K.; Eisen, R.J.; Staples, J.E. Heartland Virus Epidemiology, Vector Association, and Disease Potential. Viruses 2018, 10, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bopp, N.E.; Kaiser, J.A.; Strother, A.E.; Barrett, A.D.T.; Beasley, D.W.C.; Benassi, V.; Milligan, G.N.; Preziosi, M.P.; Reece, L.M. Baseline mapping of severe fever with thrombocytopenia syndrome virology, epidemiology and vaccine research and development. NPJ Vaccines 2020, 5, 111. [Google Scholar] [CrossRef]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primers 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed]
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33, e00083-18. [Google Scholar] [CrossRef] [PubMed]
- Sahni, A.; Fang, R.; Sahni, S.K.; Walker, D.H. Pathogenesis of Rickettsial Diseases: Pathogenic and Immune Mechanisms of an Endotheliotropic Infection. Annu. Rev. Pathol. 2019, 14, 127–152. [Google Scholar] [CrossRef] [Green Version]
- Piotrowski, M.; Rymaszewska, A. Expansion of Tick-Borne Rickettsioses in the World. Microorganisms 2020, 8, 1906. [Google Scholar] [CrossRef] [PubMed]
- Kemenesi, G.; Bányai, K. Tick-Borne Flaviviruses, with a Focus on Powassan Virus. Clin. Microbiol. Rev. 2019, 32, e00106-17. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, B.; Fledmann, H.; Marzi, A. Kyasanur Forest Disease and Alkhurma Hemorrhagic Fever Virus-Two Neglected Zoonotic Pathogens. Microorganisms 2020, 8, 1406. [Google Scholar] [CrossRef]
- Shah, S.Z.; Jabbar, B.; Ahmed, N.; Rehman, A.; Nasir, H.; Nadeem, S.; Jabbar, I.; Rahman, Z.U.; Azam, S. Epidemiology, Pathogenesis, and Control of a Tick-Borne Disease- Kyasanur Forest Disease: Current Status and Future Directions. Front. Cell. Infect. Microbiol. 2018, 8, 149. [Google Scholar] [CrossRef] [Green Version]
- Corrin, T.; Greig, J.; Harding, S.; Young, I.; Mascarenhas, M.; Waddell, L.A. Powassan virus, a scoping review of the global evidence. Zoonoses Public Health 2018, 65, 595–624. [Google Scholar] [CrossRef]
- Velay, A.; Paz, M.; Cesbron, M.; Gantner, P.; Solis, M.; Soulier, E.; Argemi, X.; Martinot, M.; Hansmann, Y.; Fafi-Kremer, S. Tick-borne encephalitis virus: Molecular determinants of neuropathogenesis of an emerging pathogen. Crit. Rev. Microbiol. 2019, 45, 472–493. [Google Scholar] [CrossRef]
- Talagrand-Reboul, E.; Boyer, P.H.; Bergström, S.; Vial, L.; Boulanger, N. Relapsing Fevers: Neglected Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2018, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Eisen, R.J.; Kugeler, K.J.; Eisen, L.; Beard, C.B.; Paddock, C.D. Tick-Borne Zoonoses in the United States: Persistent and Emerging Threats to Human Health. ILAR J. 2017, 58, 319–335. [Google Scholar] [CrossRef] [Green Version]
- Telford, S.R.; Goethert, H.K. Ecology of Francisella tularensis. Annu. Rev. Entomol. 2020, 65, 351–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, N.; Yang, J.; Xie, Y.; Du, X.; Chen, H.; Zhou, H.; Chen, L. Bone function, dysfunction and its role in diseases including critical illness. Int. J. Biol. Sci. 2019, 15, 776–787. [Google Scholar] [CrossRef] [Green Version]
- Boskey, A.L. Bone composition: Relationship to bone fragility and antiosteoporotic drug effects. Bonekey Rep. 2013, 2, 447. [Google Scholar] [CrossRef] [Green Version]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar]
- Travlos, G.S. Normal structure, function, and histology of the bone marrow. Toxicol. Pathol. 2006, 34, 548–565. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef]
- Rucci, N. Molecular biology of bone remodelling. Clin. Cases Miner. Bone Metab. 2008, 5, 49–56. [Google Scholar]
- Raggat, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, T.C.; Gomes, M.S.; Gomes, A.C. The crossroads between infection and bone loss. Microorganisms 2020, 8, 1765. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Romanò, D.; Logoluso, N.; Drago, L. Bone and joint infections in adults: A comprehensive classification proposal. Eur. J. Orthop. Surg. Traumatol. 2011, 1, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urish, K.L.; Cassat, J.E. Staphylococcus aureus osteomyelitis: Bone, bugs, and surgery. Infect. Immun. 2020, 88, e00932-19. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Cain, C.J.; Rueda, R.; McLelland, B.; Collette, N.M.; Loots, G.G.; Manilay, J.O. Absence of sclerostin adversely affects B-cell survival. J. Bone Miner. Res. 2012, 27, 1451–1461. [Google Scholar] [CrossRef] [Green Version]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Bravenboer, N.; Oostlander, A.E.; van Bodegraven, A.A. Bone loss in patients with inflammatory bowel disease: Cause, detection and treatment. Curr. Opin. Gastroenterol. 2021, 37, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ojogun, N.; Barnstein, B.; Huang, B.; Oskeritzian, C.A.; Homeister, J.W.; Miller, D.; Ryan, J.J.; Carlyon, J.A. Anaplasma phagocytophilum infects mast cells via alpha1,3-fucosylated but not sialylated glycans and inhibits IgE-mediated cytokine production and histamine release. Infect. Immun. 2011, 79, 2717–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granick, J.L.; Reneer, D.V.; Carlyon, J.A.; Borjesson, D.L. Anaplasma phagocytophilum infects cells of the megakaryocytic lineage through sialylated ligands but fails to alter platelet production. J. Med. Microbiol. 2018, 57, 416–423. [Google Scholar] [CrossRef]
- Herron, M.J.; Ericson, M.E.; Kurtti, T.J.; Munderloh, U.G. The interactions of Anaplasma phagocytophilum, endothelial cells, and human neutrophils. Ann. N. Y. Acad. Sci. 2005, 1063, 374–382. [Google Scholar] [CrossRef]
- Almazán, C.; Fourniol, L.; Rouxel, C.; Alberdi, P.; Gandoin, C.; Lagrée, A.C.; Boulouis, H.J.; de la Fuente, J.; Bonnet, S.I. Experimental Ixodes ricinus-Sheep Cycle of Anaplasma phagocytophilum NV2Os Propagated in Tick Cell Cultures. Front. Vet. Sci. 2020, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Johns, J.L.; Discipulo, M.L.; Koehne, A.L.; Moorhead, K.A.; Nagamine, C.M. Influence of Genetic Background on Hematologic and Histopathologic Alterations during Acute Granulocytic Anaplasmosis in 129/SvEv and C57BL/6J Mice Lacking Type I and Type II Interferon Signaling. Comp. Med. 2017, 67, 127–137. [Google Scholar]
- Tate, C.M.; Mead, D.G.; Luttrell, M.P.; Howerth, E.W.; Dugan, V.G.; Munderloh, U.G.; Davidson, W.R. Experimental infection of white-tailed deer with Anaplasma phagocytophilum, etiologic agent of human granulocytic anaplasmosis. J. Clin. Microbiol. 2005, 43, 3595–3601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annen, K.; Friedman, K.; Eshoa, C.; Horowitz, M.; Gottschall, J.; Straus, T. Two cases of transfusion-transmitted Anaplasma phagocytophilum. Am. J. Clin. Pathol. 2012, 137, 562–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, S.R.; Zimmerman, K.; Dascanio, J.J.; Pleasant, R.S.; Witonsky, S.G. Equine granulocytic anaplasmosis: A case report and review. J. Equine Vet. Sci. 2009, 29, 160–166. [Google Scholar] [CrossRef]
- Uehlinger, F.D.; Clancey, N.P.; Lofstedt, J. Granulocytic anaplasmosis in a horse from Nova Scotia caused by infection with Anaplasma phagocytophilum. Can. Vet. J. 2011, 52, 537–540. [Google Scholar] [PubMed]
- Khatat, S.E.; Culang, D.; Gara-Boivin, C. Granulocytic anaplasmosis in 2 dogs from Quebec. Can. Vet. J. 2018, 59, 663–667. [Google Scholar]
- Yi, J.; Kim, K.-H.; Ko, M.K.; Lee, E.Y.; Choi, S.J.; Oh, M.D. Human Granulocytic Anaplasmosis as a Cause of Febrile Illness in Korea Since at Least 2006. Am. J. Trop. Med. Hyg. 2017, 96, 777–782. [Google Scholar] [CrossRef] [Green Version]
- Marko, D.; Perry, A.M.; Ponnampalam, A.; Nasr, M.R. Cytopenias and clonal expansion of gamma/delta T-cells in a patient with anaplasmosis: A potential diagnostic pitfall. J. Clin. Exp. Hematop. 2017, 56, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Stokes, W.; Lisboa, L.F.; Lindsay, L.R.; Fonseca, K. Case Report: Anaplasmosis in Canada: Locally Acquired Anaplasma phagocytophilum Infection in Alberta. Am. J. Trop. Med. Hyg. 2020, 103, 2478–2480. [Google Scholar] [CrossRef]
- Jereb, M.; Pecaver, B.; Tomazic, J.; Muzlovic, I.; Avsic-Zupanc, T.; Premru-Srsen, T.; Levicnik-Stezinar, S.; Karner, P.; Strle, F. Severe human granulocytic anaplasmosis transmitted by blood transfusion. Emerg. Infect. Dis. 2012, 18, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, C.M.; Kim, D.M.; Yun, N.R. Manifestation of anaplasmosis as cerebral infarction: A case report. BMC Infect. Dis. 2018, 18, 409. [Google Scholar] [CrossRef] [Green Version]
- Parkins, M.D.; Church, D.L.; Jiang, X.Y.; Gregson, D.B. Human granulocytic anaplasmosis: First reported case in Canada. Can. J. Infect. Dis. Med. Microbiol. 2009, 20, e100–e102. [Google Scholar] [CrossRef]
- Borjesson, D.; Macnamara, K.; Johns, J.; Winslow, G. Anaplasma phagocytophilum and Ehrlichia muris induce cytopenias and global defects in hematopoiesis. Clin. Microbiol. Infect. 2009, 15, 66–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bexfield, N.H.; Villiers, E.J.; Herrtage, M.E. Immune-mediated haemolytic anaemia and thrombocytopenia associated with Anaplasma phagocytophilum in a dog. J. Small Anim. Pract. 2005, 46, 543–548. [Google Scholar] [CrossRef]
- Klein, M.B.; Miller, J.S.; Nelson, C.M.; Goodman, J.L. Primary bone marrow progenitors of both granulocytic and monocytic lineages are susceptible to infection with the agent of human granulocytic ehrlichiosis. J. Infect. Dis. 1997, 176, 1405–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, M.B.; Hu, S.; Chao, C.C.; Goodman, J.L. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J. Infect. Dis. 2000, 182, 200–205. [Google Scholar] [CrossRef]
- Rikihisa, Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin. Microbiol. Rev. 2011, 24, 469–489. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.B.; Yabsley, M.J.; Freye, J.D.; Dunlap, B.G.; Rowland, M.E.; Huang, J.; Dunn, J.R.; Jones, T.F.; Moncayo, A.C. Prevalence of Ehrlichia chaffeensis and Ehrlichia ewingii in ticks from Tennessee. Vector Borne Zoonotic Dis. 2010, 10, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Pritt, B.S.; Allerdice, M.E.J.; Sloan, L.M.; Paddock, C.D.; Munderloh, U.G.; Rikihisa, Y.; Tajima, T.; Paskewitz, S.M.; Neitzel, D.F.; Hoang Johnson, D.K.; et al. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans. Int. J. Syst. Evol. Microbiol. 2017, 67, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olano, J.P.; Masters, E.; Hogrefe, W.; Walker, D.H. Human monocytotropic ehrlichiosis, Missouri. Emerg. Infect. Dis. 2003, 9, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Dubie, T.; Mohammed, Y.; Terefe, G.; Muktar, Y.; Tesfaye, J. An insight review on canine ehrlichiosis with emphasis on its epidemiology and pathogenesity importance. Glob. J. Vet. Med. Res. 2014, 2, 59–67. [Google Scholar]
- Al-Badrani, B.A. Diagnostic study of ehrlichiosis in cattle of Mosul-Iraq. Bas. J. Vet. Res. 2013, 12, 87–97. [Google Scholar] [CrossRef]
- Saito, T.B.; Thirumalapura, N.R.; Shelite, T.R.; Rockx-Brouwer, D.; Popov, V.L.; Walker, D.H. An animal model of a newly emerging human ehrlichiosis. J. Infect. Dis. 2015, 211, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.B.; Pritt, B.S.; Sloan, L.M.; Paddock, C.D.; Musham, C.K.; Ramos, J.M.; Cetin, N.; Rosenbaum, E.R. First reported case of Ehrlichia ewingii involving human bone marrow. J. Clin. Microbiol. 2014, 52, 4102–4104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qurollo, B.A.; Buch, J.; Chandrashekar, R.; Beall, M.J.; Breitschwerdt, E.B.; Yancey, C.B.; Caudill, A.H.; Comyn, A. Clinicopathological findings in 41 dogs (2008–2018) naturally infected with Ehrlichia ewingii. J. Vet. Intern. Med. 2019, 33, 618–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.P.; Dumler, J.S.; Maley, W.R.; Klein, A.S.; Burdick, J.F.; Fred Poordad, F.; Thuluvath, P.J.; Markowitz, J.S. Human monocytic ehrlichiosis: An emerging pathogen in transplantation. Transplantation 2001, 71, 1678–1680. [Google Scholar] [CrossRef] [PubMed]
- MacNamara, K.C.; Racine, R.; Chatterjee, M.; Borjesson, D.; Winslow, G.M. Diminished hematopoietic activity associated with alterations in innate and adaptive immunity in a mouse model of human monocytic ehrlichiosis. Infect. Immun. 2009, 77, 4061–4069. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.N.P.; Zhang, Y.; Li, J.J.; McCabe, A.; Jo, H.J.; Maloney, J.; MacNamara, K.C. Type I IFNs drive hematopoietic stem and progenitor cell collapse via impaired proliferation and increased RIPK1-dependent cell death during shock-like ehrlichial infection. PLoS Pathog. 2018, 14, e1007234. [Google Scholar] [CrossRef] [Green Version]
- Dumler, J.S.; Dawson, J.E.; Walker, D.H. Human ehrlichiosis: Hematopathology and immunohistologic detection of Ehrlichia chaffeensis. Hum Pathol. 1993, 24, 391–396. [Google Scholar] [CrossRef]
- Vannier, E.G.; Diuk-Wasser, M.A.; Ben, M.C.; Krause, P.J. Babesiosis. Infect. Dis. Clin. N. Am. 2015, 29, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.T.; Roy, S.S.; Shams, N.; Visintainer, P.; Nadelman, R.B.; Hosur, S.; Nelson, J.; Wormser, G.P. Babesiosis in Lower Hudson Valley, New York, USA. Emerg. Infect. Dis. 2011, 17, 843–847. [Google Scholar] [CrossRef]
- Orinda, G.O.; Commins, M.A.; Waltisbuhl, D.J.; Goodger, B.V.; Wright, I.G. A study of autoantibodies to phosphatidyl-serine in Babesia bovis and Babesia bigemina infections in cattle. Vet. Immunol. Immunopathol. 1994, 40, 275–281. [Google Scholar] [CrossRef]
- Bhanot, P.; Parveen, N. Investigating disease severity in an animal model of concurrent babesiosis and Lyme disease. Int. J. Parasitol. 2019, 49, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.C.; Meyers, K.M.; Callan, M.B.; Bücheler, J.; Giger, U. Detection of platelet-bound and serum platelet-bindable antibodies for diagnosis of idiopathic thrombocytopenic purpura in dogs. J. Am. Vet. Med. Assoc. 1995, 206, 47–52. [Google Scholar]
- Huang, S.; Zhang, L.; Yao, L.; Li, J.; Chen, H.; Ni, Q.; Pan, C.; Jin, L. Human babesiosis in Southeast China: A case report. Int. J. Infect. Dis. 2018, 68, 36–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, S.Q.; Qiao, K.; Cui, J.; Feng, M.; Fu, Y.F.; Cheng, X.J. A case of human infection with a novel Babesia species in China. Infect. Dis. Poverty 2016, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Jiang, R.; Jia, N.; Ning, N.; Zheng, Y.; Huo, Q.; Sun, Y.; Yuan, T.; Jiang, B.; Li, T.; et al. Human Case Infected with Babesia venatorum: A 5-Year Follow-Up Study. Open Forum Infect. Dis. 2020, 7, ofaa062. [Google Scholar] [CrossRef] [Green Version]
- Clark, I.A.; Jacobson, L.S. Do babesiosis and malaria share a common disease process? Ann. Trop. Med. Parasitol. 1998, 92, 483–488. [Google Scholar] [CrossRef]
- Orf, K.; Cunnington, A.J. Infection-related hemolysis and susceptibility to Gram-negative bacterial co-infection. Front Microbiol. 2015, 6, 666. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, E.; Leisewitz, A.L.; Thompson, P.N.; Christopher, M.M. Serial haematology results in transfused and non-transfused dogs naturally infected with Babesia rossi. J. S. Afr. Vet Assoc. 2011, 82, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Akel, T.; Mobarakai, N. Hematologic manifestations of babesiosis. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 6. [Google Scholar] [CrossRef]
- Bullard, J.M.; Ahsanuddin, A.N.; Perry, A.M.; Lindsay, L.R.; Iranpour, M.; Dibernardo, A.; Van Caeseele, P.G. The first case of locally acquired tick-borne Babesia microti infection in Canada. Can. J. Infect. Dis. Med. Microbiol. 2014, 25, e87–e89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovius, K.E.; Rijpkema, S.G.; Westers, P.; van der Zeijst, B.A.; van Asten, F.J.; Houwers, D.J. A serological study of cohorts of young dogs, naturally exposed to Ixodes ricinus ticks, indicates seasonal reinfection by Borrelia burgdorferi sensu lato. Vet. Q. 1999, 21, 16–20. [Google Scholar] [CrossRef]
- Cleveland, C.A.; Swanepoel, L.; Brown, J.D.; Casalena, M.J.; Williams, L.; Yabsley, M.J. Surveillance for Borrelia spp. in Upland Game Birds in Pennsylvania, USA. Vet. Sci. 2020, 7, 82. [Google Scholar] [CrossRef]
- Tang, T.T.; Zhang, L.; Bansal, A.; Grynpas, M.; Moriarty, T.J. The Lyme Disease Pathogen Borrelia burgdorferi Infects Murine Bone and Induces Trabecular Bone Loss. Infect. Immun. 2017, 85, e00781-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksi, J.; Mertsola, J.; Reunanen, M.; Marjamäki, M.; Viljanen, M.K. Subacute multiple-site osteomyelitis caused by Borrelia burgdorferi. Clin. Infect. Dis. 1994, 19, 891–896. [Google Scholar] [CrossRef]
- Steere, A.C.; Schoen, R.T.; Taylor, E. The clinical evolution of Lyme arthritis. Ann. Intern. Med. 1987, 107, 725–731. [Google Scholar] [CrossRef]
- Schlesinger, P.A.; Duray, P.H.; Burke, B.A.; Steere, A.C.; Stillman, M.T. Maternal-fetal transmission of the Lyme disease spirochete, Borrelia burgdorferi. Ann. Intern. Med. 1985, 103, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Houtman, P.M.; Tazelaar, D.J. Joint and bone involvement in Dutch patients with Lyme borreliosis presenting with acrodermatitis chronica atrophicans. Neth. J. Med. 1999, 54, 5–9. [Google Scholar] [CrossRef]
- Hovmark, A.; Asbrink, E.; Olsson, I. Joint and bone involvement in Swedish patients with Ixodes ricinus-borne Borrelia infection. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1986, 263, 275–284. [Google Scholar] [PubMed]
- Kvasnicka, H.M.; Thiele, J.; Ahmadi, T. Bone marrow manifestation of Lyme disease (Lyme borreliosis). Br. J. Haematol. 2003, 120, 723. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.P.; Rahn, D.W. Lyme disease and radiologic findings in Lyme arthritis. AJR Am. J. Roentgenol. 1992, 158, 1065–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, J.P.; Steere, A.C. Lyme arthritis: Radiologic findings. Radiology 1985, 154, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Munson, E.; Nardelli, D.T.; Du-Chateau, B.K.; Callister, S.M.; Schell, R.F. Hamster and murine models of severe destructive Lyme arthritis. Clin. Dev. Immunol. 2012, 2012, 504215. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Vanhoenacker, F.M.; Gielen, J. Unusual musculoskeletal manifestations of Lyme disease. JBR-BTR 2004, 87, 224–228. [Google Scholar]
- Steere, A.C. Musculoskeletal manifestations of Lyme disease. Am. J. Med. 1995, 98, 44S–48S. [Google Scholar] [CrossRef]
- Zlotnikov, N.; Javid, A.; Ahmed, M.; Eshghi, A.; Tang, T.T.; Arya, A.; Bansal, A.; Matar, F.; Parikh, M.; Ebady, R.; et al. Infection with the Lyme disease pathogen suppresses innate immunity in mice with diet-induced obesity. Cell. Microbiol. 2017, 19, e12689. [Google Scholar] [CrossRef] [PubMed]
- Isogai, E.; Isogai, H.; Kimura, K.; Hayashi, S.; Kubota, T.; Nishikawa, T.; Nakane, A.; Fujii, N. Cytokines in the serum and brain in mice infected with distinct species of Lyme disease Borrelia. Microb. Pathog. 1996, 21, 413–419. [Google Scholar] [CrossRef]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Kosoy, O.I.; Lambert, A.J.; Hawkinson, D.J.; Pastula, D.M.; Goldsmith, C.S.; Hunt, D.C.; Staples, J.E. Novel thogotovirus associated with febrile illness and death, United States, 2014. Emerg. Infect. Dis. 2015, 21, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Bricker, T.L.; Shafiuddin, M.; Gounder, A.P.; Janowski, A.B.; Zhao, G.; Williams, G.D.; Jagger, B.W.; Diamond, M.S.; Bailey, T.; Kwon, J.H.; et al. Therapeutic efficacy of favipiravir against Bourbon virus in mice. PLoS Pathog. 2019, 15, e1007790. [Google Scholar] [CrossRef]
- Pace, E.J.; O’Reilly, M. Tickborne Diseases: Diagnosis and Management. Am. Fam. Physician 2020, 101, 530–540. [Google Scholar]
- Burgdorfer, W. Colorado tick fever. II. The behavior of Colorado tick fever virus in rodents. J. Infect. Dis. 1960, 107, 384–388. [Google Scholar] [CrossRef]
- Bowen, G.S.; Shriner, R.B.; Pokorny, K.S.; Kirk, L.J.; McLean, R.G. Experimental Colorado tick fever virus infection in Colorado mammals. Am. J. Trop. Med. Hyg. 1981, 30, 224–229. [Google Scholar] [CrossRef]
- Kadkhoda, K.; Semus, M.; Jelic, T.; Walkty, A. Case Report: A case of colorado tick fever acquired in Southwestern Saskatchewan. Am. J. Trop. Med. Hyg. 2018, 98, 891–893. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/coloradotickfever/faqs.html (accessed on 1 March 2021).
- Oshiro, L.S.; Dondero, D.V.; Emmons, R.W.; Lennette, E.H. The development of Colorado tick fever virus within cells of the haemopoietic system. J. Gen. Virol. 1978, 39, 73–79. [Google Scholar] [CrossRef]
- Emmons, R.W.; Oshiro, L.S.; Johnson, H.N.; Lennette, E.H. Intra-erythrocytic location of Colorado tick fever virus. J. Gen. Virol. 1972, 17, 185–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philipp, C.S.; Callaway, C.; Chu, M.C.; Huang, G.H.; Monath, T.P.; Trent, D.; Evatt, B.L. Replication of Colorado tick fever virus within human hematopoietic progenitor cells. J. Virol. 1993, 67, 2389–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbuSamra, D.B.; Aleisa, F.A.; Al-Amoodi, A.S.; Jalal Ahmed, H.M.; Chin, C.J.; Abuelela, A.F.; Bergam, P.; Sougrat, R.; Merzaban, J.S. Not just a marker: CD34 on human hematopoietic stem/progenitor cells dominates vascular selectin binding along with CD44. Blood Adv. 2017, 1, 2799–2816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmons, R.W. Colorado tick fever along the Pacific slope of North America. Jap. J. Med. Sci. Biol. 1967, 20, 166–170. [Google Scholar] [PubMed]
- Government of Canada, Diseases and Conditions. Available online: https://www.canada.ca/en/public-health/services/diseases/tick-borne-encephalitis/causes-tick-borne-encephalitis.html (accessed on 1 March 2021).
- Barp, N.; Trentini, A.; Di-Nuzzo, M.; Mondardini, V.; Francavilla, E.; Contini, C. Clinical and laboratory findings in tick-borne encephalitis virus infection. Parasite Epidemiol. Control. 2020, 10, e00160. [Google Scholar] [CrossRef] [PubMed]
- Bogovic, P.; Strle, F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases 2015, 3, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Lotric-Furlan, S.; Strle, F. Thrombocytopenia--a common finding in the initial phase of tick-borne encephalitis. Infection 1995, 23, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Lotric-Furlan, S.; Strle, F. Thrombocytopenia, leukopenia and abnormal liver function tests in the initial phase of tick-borne encephalitis. Zentralbl. Bakteriol. 1995, 282, 275–278. [Google Scholar] [CrossRef]
- Bühler, T.; Boos, N.; Leuppi-Taegtmeyer, A.B.; Berger, C.T. Febrile illness and bicytopenia within hours after tick-borne encephalitis booster vaccination. NPJ Vaccines 2019, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Ruzek, J.S.D. Tick-borne encephalitis in domestic animals. Acta Virol. 2020, 64, 223–229. [Google Scholar]
- Wilhelmsson, P.; Jaenson, T.G.T.; Olsen, B.; Waldenström, J.; Lindgren, P.E. Migratory birds as disseminators of ticks and the tick-borne pathogens Borrelia bacteria and tick-borne encephalitis (TBE) virus: A seasonal study at Ottenby Bird Observatory in South-eastern Sweden. Parasites Vectors 2020, 13, 607. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Johnson, N.; Phipps, L.P.; Stephenson, J.R.; Fooks, A.R.; Solomon, T. Tick-borne encephalitis virus—A review of an emerging zoonosis. J. Gen. Virol. 2009, 90, 1781–1794. [Google Scholar] [CrossRef]
- Růžek, D.; Dobler, G.; Mantke, O.D. Tick-borne encephalitis: Pathogenesis and clinical implications. Travel Med. Infect. Dis. 2010, 8, 223–232. [Google Scholar] [CrossRef]
- Whitehouse, C.A. Crimean-Congo hemorrhagic fever. Antivir. Res. 2004, 64, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Ergonul, O. Crimean-Congo hemorrhagic fever virus: New outbreaks, new discoveries. Curr. Opin. Virol. 2012, 2, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, D.; Barut, H.; Duygu, F.; Çevik, B.; Kurt, S.; Sümbül, O. Characteristics of headache and its relationship with disease severity in patients with Crimean-Congo hemorrhagic fever. Agri 2018, 30, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Peyrefitte, C.; Marianneau, P.; Tordo, N.; Bouloy, M. Crimean-Congo haemorrhagic fever. Rev. Sci. Tech. 2015, 34, 391–401. [Google Scholar] [CrossRef]
- Bente, D.A.; Alimonti, J.B.; Shieh, W.J.; Camus, G.; Ströher, U.; Zaki, S.; Jones, S.M. Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model. J. Virol. 2010, 84, 11089–11100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, R.W.; Prasad, A.N.; Borisevich, V.; Geisbert, J.B.; Agans, K.N.; Deer, D.J.; Fenton, K.A.; Geisbert, T.W. Crimean-Congo hemorrhagic fever virus strains Hoti and Afghanistan cause viremia and mild clinical disease in cynomolgus monkeys. PLoS Negl. Trop. Dis. 2020, 14, e0008637. [Google Scholar] [CrossRef]
- Swanepoel, R.; Gill, D.E.; Shepherd, A.J.; Leman, P.A.; Mynhardt, J.H.; Harvey, S. The clinical pathology of Crimean-Congo hemorrhagic fever. Rev. Infect. Dis. 1989, 11, S794–S800. [Google Scholar] [CrossRef]
- Cagatay, A.; Kapmaz, M.; Karadeniz, A.; Basaran, S.; Yenerel, M.; Yavuz, S.; Midilli, K.; Ozsut, H.; Eraksoy, H.; Calangu, S. Haemophagocytosis in a patient with Crimean Congo haemorrhagic fever. J. Med. Microbiol. 2007, 56, 1126–1128. [Google Scholar] [CrossRef] [Green Version]
- Fisgin, N.T.; Fisgin, T.; Tanyel, E.; Doganci, L.; Tulek, N.; Guler, N.; Duru, F. Crimean-Congo hemorrhagic fever: Five patients with hemophagocytic syndrome. Am. J. Hematol. 2008, 83, 73–76. [Google Scholar] [CrossRef]
- Morimoto, A.; Nakazawa, Y.; Ishii, E. Hemophagocytic lymphohistiocytosis: Pathogenesis, diagnosis, and management. Pediatr. Int. 2016, 58, 817–825. [Google Scholar] [CrossRef]

| Bacterial | Viral | Parasitic |
|---|---|---|
| Anaplasmosis (Anaplasma phagocytophilum) [3] | Nairoviral diseases: Crimean-Congo Hemorrhagic Fever (CCHFV) [4]; Nairobi Sheep Disease (NSDV) [5]; Songling Virus Disease (SGLV) [6] | Babesiosis (Babesia microti, B. divergens, B. duncani, B. venatorum) [7] |
| Ehrlichiosis (Ehrlichia chaffeensis, E. ewingii, E. muris) [8] | Phenuiviral diseases: Heartland Virus Disease (HRTV) [9]; Severe Fever with Thrombocytopenia Syndrome (SFTSV) [10] | |
| Lyme Disease (Borreliella afzelii, B. burgdorferi sensu stricto, B. garinii, B. mayonii) [11] | Orthomyxoviral diseases: Bourbon virus disease (BRBV) [12] | |
| Rickettsioses, including Flinders Island (R. honei), Israeli (R. conoriisubsp. israelensis), Mediterranean (R. conoriisubsp. conorii), Japanese (R. japonica) and Rocky Mountain (R. rickettsii) Spotted Fevers; Indian (R. conoriisubsp. indica), Queensland (R. australis) and Siberian Tick Typhus (R. sibiricasubsp. sibirica); Far Eastern (R. heilongjiangensis) and Lymphangitis-Associated (R. sibirica subsp. mongolitimonae) Rickettsioses; African Tick Bite (R. rickettsii) and Astrakhan (R. conorii subsp. caspia) Fevers; SENLAT (R. raoultii) [13,14] | Flaviviral diseases [15]: Alkhurma Hemorrhagic Fever (AHFV) [16]; Kyasanur Forest Disease (KFDV) [17]; Omsk Hemorrhagic Fever (OHFV) [15]; Powassan Disease (POWV) [15,18]; Tick-borne Encephalitis (TBEV) [19] | |
| Tick-borne Relapsing Fever (Borrelia crocidurae, B. duttoni, B. hermsii, B. hispanica, B. miyamotoi, B. parkeri, B. persica, B. turicatae) [20] | Reoviral diseases: Colorado Tick Fever Disease (CTFV); Eyach Virus Disease (EYAV) [21] | |
| Tularemia (Francisella tularensis) [22] |
| Tick-Borne Disease | Impact on Bone | |
|---|---|---|
| Disrupted Bone Marrow Function | Bone Loss | |
| Anaplasmosis | √ | - |
| Ehrlichiosis | √ | - |
| Babesiosis | √ | - |
| Lyme disease | - | √ |
| Bourbon virus disease | √ | - |
| Colorado tick fever disease | √ | - |
| Tick-borne encephalitis | √ | - |
| Crimean-Congo Hemorrhagic Fever | √ | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, I.; Moriarty, T.J. The Impact of Tick-Borne Diseases on the Bone. Microorganisms 2021, 9, 663. https://doi.org/10.3390/microorganisms9030663
Farooq I, Moriarty TJ. The Impact of Tick-Borne Diseases on the Bone. Microorganisms. 2021; 9(3):663. https://doi.org/10.3390/microorganisms9030663
Chicago/Turabian StyleFarooq, Imran, and Tara J. Moriarty. 2021. "The Impact of Tick-Borne Diseases on the Bone" Microorganisms 9, no. 3: 663. https://doi.org/10.3390/microorganisms9030663
APA StyleFarooq, I., & Moriarty, T. J. (2021). The Impact of Tick-Borne Diseases on the Bone. Microorganisms, 9(3), 663. https://doi.org/10.3390/microorganisms9030663







