Functional Interrelationships of Microorganisms in Iron-Based Anaerobic Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
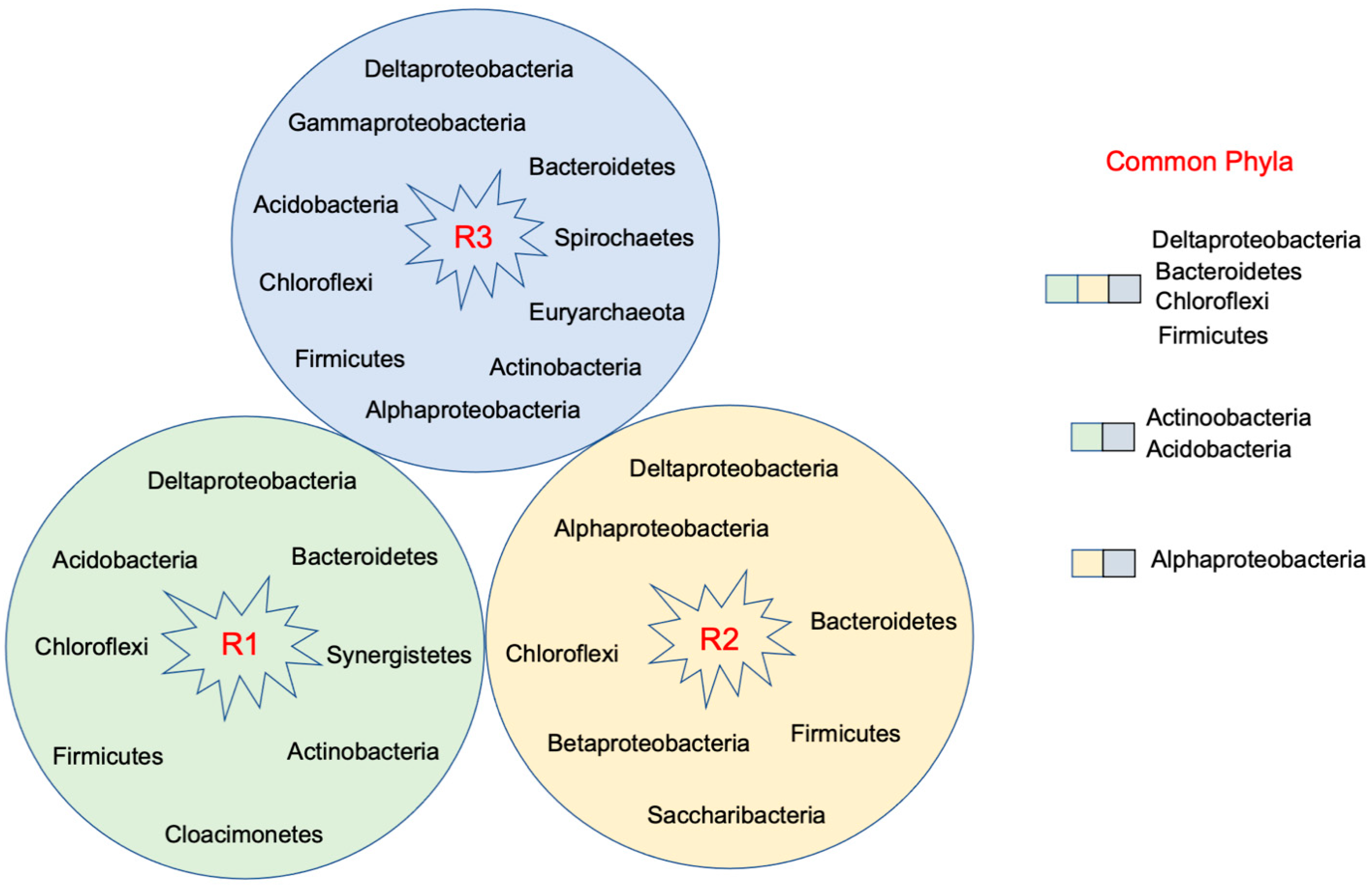
- Cotreatment of acid mine drainage and municipal wastewater (R1)
- Fe(II)-dosed anaerobic wastewater treatment system with sludge recycling (R2)
- Fe(III)-dosed anaerobic wastewater treatment system for organic removal (R3)
- Fe(III)-dosed anaerobic wastewater treatment system for both organic and nutrient removal (R4)
2.1. Cotreatment of Acid Mine Drainage and Wastewater (R1)
2.2. Fe(II)-Dosed Anaerobic Wastewater Treatment System with Sludge Recycling (R2)
2.3. Fe(III)-Dosed Anaerobic Wastewater Treatment System for Organic Removal (R3)
2.4. Fe(III)-Dosed Anaerobic Wastewater Treatment System for Both Organic and Nutrient Removal (R4)
3. Results and Discussion
3.1. Microbial Diversity
3.2. Iron-Reducing Bacteria
3.3. Sulfate-Reducing Bacteria
| Bacteria | Temperature | pH | Reference | |
|---|---|---|---|---|
| Iron Reducing Bacteria | Geobacter sp. | 4–37 °C | 6.5–7.5 | [64] |
| Ignavibacteria sp. | 30–55 °C | 6.5–8.0 | [53] | |
| Geothrix sp. | 35–40 °C | [48] | ||
| Alkaliphilus metalliredigens | 4–45 °C | 7.5–11.0 | [65] | |
| Sulfate Reducing Bacteria | Desulfovibrio sp. | 15–45 °C | 5.0–8.0 | [66,67] |
| Desulfobulbus sp. | 10–40 °C | 6.1–7.5 | [68,69] | |
| Desulfovirga sp. | 20–36 °C | 6.6–7.4 | [62] | |
| Desulfatirhabdium sp. | 15–37 °C | 6.5–8.0 | [60] | |
| Desulforhabdus sp. | 25–45 °C | 6.6–8.5 | [70] | |
| Desulfomonile sp. | 30–38 °C | 6.5–7.8 | [71] | |
| Desulfatibacillum sp. | 15–40 °C | 6.6–7.8 | [72] |
3.4. Synergistic Relationships between FeRB and SRB
3.5. Feammox and Denitrifying Bacteria
3.6. Fermentative Bacteria
3.7. Nitrogen-Fixing Bacteria
4. Functional Interrelationships among Microorganisms in Iron-Dosed Bioreactors
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Lier, J.B.; Van der Zee, F.P.; Frijters, C.T.M.J.; Ersahin, M.E. Celebrating 40 Years Anaerobic Sludge Bed Reactors for Industrial Wastewater Treatment. Rev. Environ. Sci. Biotechnol. 2015, 14, 681–702. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.J.; Chong, M.F.; Law, C.L.; Hassell, D.G. A Review on Anaerobic-Aerobic Treatment of Industrial and Municipal Wastewater. Chem. Eng. J. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- Van Lier, J.B. High-Rate Anaerobic Wastewater Treatment: Diversifying from End-of-the-Pipe Treatment to Resource-Oriented Conversion Techniques. Water Sci. Technol. 2008, 57, 1137–1148. [Google Scholar] [CrossRef]
- Manariotis, I.D.; Grigoropoulos, S.G. Low-Strength Wastewater Treatment Using an Anaerobic Baffled Reactor. Water Environ. Res. 2002, 74, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Speece, R.E. Anaerobic Biotechnology for Industrial Wastewater Treatment. Environ. Sci. Technol. 1983, 17, 416A–427A. [Google Scholar] [CrossRef]
- Damianovic, M.H.R.Z.; Foresti, E. Anaerobic Degradation of Synthetic Wastewaters at Different Levels of Sulfate and COD/Sulfate Ratios in Horizontal-Flow Anaerobic Reactors (HAIB). Environ. Eng. Sci. 2007, 24, 383–393. [Google Scholar] [CrossRef]
- Hubert, C.; Voordouw, G. Oil Field Souring Control by Nitrate-Reducing Sulfurospirillum spp. That Outcompete Sulfate-Reducing Bacteria for Organic Electron Donors. Appl. Environ. Microbiol. 2007, 73, 2644–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, T.Y.; Cha, G.C.; Seo, Y.C.; Jeon, C.; Choi, S.S. Effect of COD/Sulfate Ratios on Batch Anaerobic Digestion Using Waste Activated Sludge. J. Ind. Eng. Chem. 2008, 14, 693–697. [Google Scholar] [CrossRef]
- Strosnider, W.H.; Winfrey, B.K.; Nairn, R.W. Biochemical Oxygen Demand and Nutrient Processing in a Novel Multi-Stage Raw Municipal Wastewater and Acid Mine Drainage Passive Co-Treatment System. Water Res. 2011, 45, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Lin, L.S. Two-Stage Combined Treatment of Acid Mine Drainage and Municipal Wastewater. Water Sci. Technol. 2013, 67, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.A.; Gray, N.F. Co-Treatment of Acid Mine Drainage with Municipal Wastewater: Performance Evaluation. Environ. Sci. Pollut. Res. 2013, 20, 7863–7877. [Google Scholar] [CrossRef]
- Ahmed, M.; Saup, C.M.; Wilkins, M.J.; Lin, L.-S. Continuous Ferric Iron-Dosed Anaerobic Wastewater Treatment: Treatment Perfromance, Sludge Characteristics, and Mirobial Composition. J. Environ. Chem. Eng. 2020, 8, 103537. [Google Scholar] [CrossRef]
- Ahmed, M.; Lin, O.; Saup, C.M.; Wilkins, M.J.; Lin, L.S. Effects of Fe/S Ratio on the Kinetics and Microbial Ecology of an Fe(III)-Dosed Anaerobic Wastewater Treatment System. J. Hazard. Mater. 2019, 369, 593–600. [Google Scholar] [CrossRef]
- Ahmed, M.; Lin, L.-S. Ferric Reduction in Organic Matter Oxidation and Its Applicability for Anaerobic Wastewater Treatment: A Review and Future Aspects. Rev. Environ. Sci. Biotechnol. 2017, 16, 273–287. [Google Scholar] [CrossRef]
- Ahmed, M.; Aziziha, M.; Anwar, R.; Johnson, M.B.; Lin, L.-S. Magnetic Sludge Byproducts for Adsorptive Phosphorus Removal: Resource Recovery from Iron-Based Anaerobic Sewage Sludge. Waste Manag. 2021, 120, 269–276. [Google Scholar] [CrossRef]
- Madigan, M.; Martinko, J.; Bender, K.; Buckley, D.; Stahl, D. Brock Biology of Microorganism, 14th ed.; Pearson Education: Glenview, IL, USA, 2015. [Google Scholar]
- Esther, J.; Sukla, L.B.; Pradhan, N.; Panda, S. Fe (III) Reduction Strategies of Dissimilatory Iron Reducing Bacteria. Korean J. Chem. Eng. 2015, 32, 1–14. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Gorby, Y.A. Environmental Processes Mediated by Iron-Reducing Bacteria. Curr. Opin. Biotechnol. 1996, 7, 287–294. [Google Scholar] [CrossRef]
- Lovley, D.R.; Giovannoni, S.J.; White, D.C.; Champine, J.E.; Phillips, E.J.P.; Gorby, Y.A.; Goodwin, S. Geobacter metallireducens gen. nov. sp. nov., a Microorganism. Arch. Microbiol. 1993, 159, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Nealson, K.H.; Myers, C.R. Iron Reduction by Bacteria: A Potential Role in the Genesis of Banded Iron Formations. Am. J. Sci. 1990, 290, 35–45. [Google Scholar]
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms Pumping Iron: Anaerobic Microbial Iron Oxidation and Reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovley, D.R.; Phillips, E.J.P. Requirement for a Microbial Consortium to Completely Oxidize Glucose in Fe(III)- Reducing Sediments. Appl. Environ. Microbiol. 1989, 55, 3234–3236. [Google Scholar] [CrossRef] [Green Version]
- Lovley, D.R. Fe(LII) and Mn(LV) Reduction. In Environmentai Microbe-Metal Interactions; Lovley, D.R., Ed.; ASM Press: Washington, DC, USA, 2000; pp. 3–30. [Google Scholar]
- Lovley, D.R.; Phillips, E.J. Competitive Mechanisms for Inhibition of Sulfate Reduction and Methane Production in the Zone of Ferric Iron Reduction in Sediments. Appl. Environ. Microbiol. 1987, 53, 2636–2641. [Google Scholar] [CrossRef] [Green Version]
- Rabus, R.; Venceslau, S.S.; Wöhlbrand, L.; Voordouw, G.; Wall, J.D.; Pereira, I.A.C. A Post-Genomic View of the Ecophysiology, Catabolism and Biotechnological Relevance of Sulphate-Reducing Prokaryotes. Adv. Microb. Physiol. 2015, 66, 55–321. [Google Scholar] [PubMed]
- El Houari, A.; Ranchou-Peyruse, M.; Ranchou-Peyruse, A.; Dakdaki, A.; Guignard, M.; Idouhammou, L.; Bennisse, R.; Bouterfass, R.; Guyoneaud, R.; Qatibi, A.I. Desulfobulbus oligotrophicus sp. nov., a Sulfate-Reducing and Propionate-Oxidizing Bacterium Isolated from a Municipal Anaerobic Sewage Sludge Digester. Int. J. Syst. Evol. Microbiol. 2017, 67, 275–281. [Google Scholar] [CrossRef]
- Li, X.; Hou, L.; Liu, M.; Zheng, Y.; Yin, G.; Lin, X.; Cheng, L.; Li, Y.; Hu, X. Evidence of Nitrogen Loss from Anaerobic Ammonium Oxidation Coupled with Ferric Iron Reduction in an Intertidal Wetland. Environ. Sci. Technol. 2015, 49, 11560–11568. [Google Scholar] [CrossRef]
- Shrestha, J.; Rich, J.J.; Ehrenfeld, J.G.; Jaffe, P.R. Oxidation of Ammonium to Nitrite under Iron-Reducing Conditions in Wetland Soils: Laboratory, Field Demonstrations, and Push-Pull Rate Determination. Soil Sci. 2009, 174, 156–164. [Google Scholar] [CrossRef]
- Yang, W.H.; Weber, K.A.; Silver, W.L. Nitrogen Loss from Soil through Anaerobic Ammonium Oxidation Coupled to Iron Reduction. Nat. Geosci. 2012, 5, 538–541. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.J.; An, X.L.; Li, S.; Zhang, G.L.; Zhu, Y.G. Nitrogen Loss through Anaerobic Ammonium Oxidation Coupled to Iron Reduction from Paddy Soils in a Chronosequence. Environ. Sci. Technol. 2014, 48, 10641–10647. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Wang, F.; Wang, C.; Xu, H.; Jiang, H. Anaerobic Ammonium Oxidation Coupled to Ferric Iron Reduction in the Sediment of a Eutrophic Lake. Environ. Sci. Pollut. Res. 2019, 26, 15084–15094. [Google Scholar] [CrossRef]
- Park, W.; Nam, Y.K.; Lee, M.J.; Kim, T.H. Anaerobic Ammonia-Oxidation Coupled with Fe 3+ Reduction by an Anaerobic Culture from a Piggery Wastewater Acclimated to NH4+/Fe3+ Medium. Biotechnol. Bioprocess Eng. 2009, 14, 680–685. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, Z.; Quan, X.; Zhang, Y. Transformation of Nitrogen and Iron Species during Nitrogen Removal from Wastewater via Feammox by Adding Ferrihydrite. ACS Sustain. Chem. Eng. 2018, 6, 14394–14402. [Google Scholar] [CrossRef]
- Clément, J.C.; Shrestha, J.; Ehrenfeld, J.G.; Jaffé, P.R. Ammonium Oxidation Coupled to Dissimilatory Reduction of Iron under Anaerobic Conditions in Wetland Soils. Soil Biol. Biochem. 2005, 37, 2323–2328. [Google Scholar] [CrossRef]
- Huang, S.; Jaffé, P.R. Isolation and Characterization of an Ammonium-Oxidizing Iron Reducer: Acidimicrobiaceae sp. A6. PLoS ONE 2018, 13, e0194007. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Jaffé, P.R. Characterization of Incubation Experiments and Development of an Enrichment Culture Capable of Ammonium Oxidation under Iron-Reducing Conditions. Biogeosciences 2015, 12, 769–779. [Google Scholar] [CrossRef] [Green Version]
- Ding, B.; Li, Z.; Qin, Y. Nitrogen Loss from Anaerobic Ammonium Oxidation Coupled to Iron(III) Reduction in a Riparian Zone. Environ. Pollut. 2017, 231, 379–386. [Google Scholar] [CrossRef]
- Deng, D.; Weidhaas, J.L.; Lin, L.-S. Kinetics and Microbial Ecology of Batch Sulfidogenic Bioreactors for Co-Treatment of Municipal Wastewater and Acid Mine Drainage. J. Hazard. Mater. 2016, 305, 200–208. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Deng, D.; Lin, L.S. Continuous Sulfidogenic Wastewater Treatment with Iron Sulfide Sludge Oxidation and Recycle. Water Res. 2017, 114, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Braker, G.; Fesefeldt, A.; Witzel, K.P. Development of PCR Primer Systems for Amplification of Nitrite Reductase Genes (NirK and NirS) to Detect Denitrifying Bacteria in Environmental Samples. Appl. Environ. Microbiol. 1998, 64, 3769–3775. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Twachtmann, U.; Klein, M.; Strous, M.; Juretschko, S.; Jetten, M.; Metzger, J.W.; Schleifer, K.H.; Wagner, M. Molecular Evidence for Genus Level Diversity of Bacteria Capable of Catalyzing Anaerobic Ammonium Oxidation. Syst. Appl. Microbiol. 2000, 23, 93–106. [Google Scholar] [CrossRef]
- Roh, Y.; Chon, C.M.; Moon, J.W. Metal Reduction and Biomineralization by an Alkaliphilic Metal-Reducing Bacterium, Alkaliphilus metalliredigens (QYMF). Geosci. J. 2007, 11, 415–423. [Google Scholar] [CrossRef]
- Hwang, C.; Copeland, A.; Lucas, S.; Lapidus, A.; Barry, K.; Detter, J.C.; Glavina del Rio, T.; Hammon, N.; Israni, S.; Dalin, E.; et al. Complete Genome Sequence of Alkaliphilus metalliredigens Strain QYMF, an Alkaliphilic and Metal-Reducing Bacterium Isolated from Borax-Contaminated Leachate Ponds. Genome Announc. 2016, 4, 3–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevin, K.P.; Holmes, D.E.; Woodard, T.L.; Hinlein, E.S.; Ostendorf, D.W.; Lovley, D.R. Geobacter bemidjiensis sp. nov. and Geobacter psychrophilus sp. nov., Two Novel Fe(III)-Reducing Subsurface Isolates. Int. J. Syst. Evol. Microbiol. 2005, 55, 1667–1674. [Google Scholar] [CrossRef] [Green Version]
- Prakash, O.; Gihring, T.M.; Dalton, D.D.; Chin, K.J.; Green, S.J.; Akob, D.M.; Wanger, G.; Kostka, J.E. Geobacter daltonii sp. nov., an Fe(III)- and Uranium(VI)-Reducing Bacterium Isolated from a Shallow Subsurface Exposed to Mixed Heavy Metal and Hydrocarbon Contamination. Int. J. Syst. Evol. Microbiol. 2010, 60, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Childers, S.E.; Ciufo, S.; Lovley, D.R. Geobacter metallireducens Accesses Insoluble Fe(III) Oxide by Chemotaxis. Nature 2002, 416, 767–769. [Google Scholar] [CrossRef]
- Coates, J.D.; Ellis, D.J.; Gaw, C.V.; Lovley, D.R. Geothrix fermentans gen. nov., sp. nov., a Novel Fe(III)-Reducing Bacterium from a Hydrocarbon-Contaminated Aquifer. Int. J. Syst. Evol. Microbiol. 1999, 49, 1615–1622. [Google Scholar] [CrossRef] [Green Version]
- Coates, J.D.; Bhupathiraju, V.K.; Achenbach, L.A.; Mcinerney, M.J.; Lovley, D.R. Geobacter hydrogenophilus, Geobacter chapellei and Geobacter grbiciae, Three New, Strictly Anaerobic, Dissimilatory Fe(III)-Reducers. Int. J. Syst. Evol. Microbiol. 2001, 51, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Randall, A.A.; McCue, T. The Efficiency of Enhanced Biological Phosphorus Removal from Real Wastewater Affected by Different Ratios of Acetic to Propionic Acid. Water Res. 2004, 38, 27–36. [Google Scholar] [CrossRef]
- Van den Brand, T.P.H.; Roest, K.; Brdjanovic, D.; Chen, G.H.; van Loosdrecht, M.C.M. Influence of Acetate and Propionate on Sulphate-Reducing Bacteria Activity. J. Appl. Microbiol. 2014, 117, 1839–1847. [Google Scholar] [CrossRef]
- Fortney, N.W.; He, S.; Kulkarni, A.; Friedrich, M.W.; Holz, C.; Boyd, E.S.; Roden, E.E. Stable Isotope Probing for Microbial Iron Reduction in Chocolate Pots Hot Spring, Yellowstone National Park. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [Green Version]
- Iino, T.; Mori, K.; Uchino, Y.; Nakagawa, T.; Harayama, S.; Suzuki, K.I. Ignavibacterium album gen. nov., sp. nov., a Moderately Thermophilic Anaerobic Bacterium Isolated from Microbial Mats at a Terrestrial Hot Spring and Proposal of Ignavibacteria Classis Nov., for a Novel Lineage at the Periphery of Green Sulfur Bacteria. Int. J. Syst. Evol. Microbiol. 2010, 60, 1376–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podosokorskaya, O.A.; Kadnikov, V.V.; Gavrilov, S.N.; Mardanov, A.V.; Merkel, A.Y.; Karnachuk, O.V.; Ravin, N.V.; Bonch-Osmolovskaya, E.A.; Kublanov, I.V. Characterization of Melioribacter roseus gen. nov., sp. nov., a Novel Facultatively Anaerobic Thermophilic Cellulolytic Bacterium from the Class Ignavibacteria, and a Proposal of a Novel Bacterial Phylum Ignavibacteriae. Environ. Microbiol. 2013, 15, 1759–1771. [Google Scholar] [CrossRef]
- Liu, Z.; Frigaard, N.U.; Vogl, K.; Iino, T.; Ohkuma, M.; Overmann, J.; Bryant, D.A. Complete Genome of Ignavibacterium album, a Metabolically Versatile, Flagellated, Facultative Anaerobe from the Phylum Chlorobi. Front. Microbiol. 2012, 3, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevin, K.P.; Lovley, D.R. Mechanisms for Accessing Insoluble Fe(III) Oxide during Dissimilatory Fe(III) Reduction by Geothrix Fermentans. Appl. Environ. Microbiol. 2002, 68, 2294–2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnuson, T.S.; Isoyama, N.; Hodges-Myerson, A.L.; Davidson, G.; Maroney, M.J.; Geesey, G.G.; Lovley, D.R. Isolation, Characterization and Gene Sequence Analysis of a Membrane-Associated 89 KDa Fe(III) Reducing Cytochrome c from Geobacter sulfurreducens. Biochem. J. 2001, 359, 147–152. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular Electron Transfer via Microbial Nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zuo, J.; Cui, L.; Deng, Q.; Dang, Y. Diversity of Microbes and Potential Exoelectrogenic Bacteria on Anode Surface in Microbial Fuel Cells. J. Gen. Appl. Microbiol. 2010, 56, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Balk, M.; Altinbaş, M.; Rijpstra, W.I.C.; Damsté, J.S.S.; Stams, A.J.M. Desulfatirhabdium butyrativorans gen. nov., sp. nov., a Butyrate-Oxidizing, Sulfate-Reducing Bacterium Isolated from an Anaerobic Bioreactor. Int. J. Syst. Evol. Microbiol. 2008, 58, 110–115. [Google Scholar] [CrossRef]
- Elferink, S.J.W.H.O.; Maas, R.N.; Harmsen, H.J.M.; Stams, A.J.M. Desulforhabdus amnigenus gen. nov. sp. nov., a Sulfate Reducer Isolated from Anaerobic Granular Sludge. Arch. Microbiol. 1995, 164, 119–124. [Google Scholar] [CrossRef]
- Tanaka, K.; Stackebrandt, E.; Tohyama, S.; Eguchi, T. Desulfovirga adipica gen. nov., sp. nov., an Adipate-Degrading, Gram- Negative, Sulfate-Reducing Bacterium. Int. J. Syst. Evol. Microbiol. 2000, 50, 639–644. [Google Scholar] [CrossRef] [Green Version]
- Metcalf & Eddy, Inc.; Tchobanoglous, G.; Stensel, H.D.; Tsuchihashi, R.; Burton, F. Wastewater Engineering: Treatment and Resorce Recovery; McGraw-Hill: New York, NY, USA, 2014. [Google Scholar]
- Babauta, J.T.; Nguyen, H.D.; Harrington, T.D.; Renslow, R.; Beyenal, H. PH, Redox Potential and Local Biofilm Potential Microenvironments within Geobacter sulferreducens. Biotechnol. Bioeng. 2013, 109, 2651–2662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Roh, Y.; Carroll, S.L.; Blair, B.; Zhou, J.; Zhang, C.L.; Fields, M.W. Alkaline Anaerobic Respiration: Isolation and Characterization of a Novel Alkaliphilic and Metal-Reducing Bacterium. Appl. Environ. Microbiol. 2004, 70, 5595–5602. [Google Scholar] [CrossRef] [Green Version]
- Zellner, G.; Messner, P.; Kneifel, H.; Winter, J. Desulfovibrio simplex spec. nov., a New Sulfate-Reducing Bacterium from a Sour Whey Digester. Arch. Microbiol. 1989, 152, 329–334. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Vítězová, M. Analysis of PH Dose-Dependent Growth of Sulfate-Reducing Bacteria. Open Med. 2019, 14, 66–74. [Google Scholar] [CrossRef]
- Lien, T.; Madsen, M.; Steen, I.H.; Gjerdevik, K. Desulfobulbus rhabdoformis sp. nov., a Sulfate Reducer from a Water-Oil Separation System. Int. J. Syst. Bacteriol. 1998, 48, 469–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, D.; Ueki, A.; Amaishi, A.; Ueki, K. Desulfobulbus japonicus sp. nov., a Novel Gram-Negative Propionate-Oxidizing, Sulfate-Reducing Bacterium Isolated from an Estuarine Sediment in Japan. Int. J. Syst. Evol. Microbiol. 2007, 57, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Oude Elferink, S.J.W.H.; Visser, A.; Pol, L.W.H.; Stams, A.J. Sulfate Reduction in Methanogenic Bioreactors. FEMS Microbiol. Rev. 1994, 15, 119–136. [Google Scholar]
- DeWeerd, K.A.; Mandelco, L.; Tanner, R.S.; Woese, C.R.; Suflita, J.M. Desulfomonile tiedjei gen. nov. and sp. nov., a Novel Anaerobic, Dehalogenating, Sulfate-Reducing Bacterium. Arch. Microbiol. 1990, 154, 23–30. [Google Scholar] [CrossRef]
- Cravo-Laureau, C.; Matheron, R.; Cayol, J.L.; Joulian, C.; Hirschler-Réa, A. Desulfatibacillum aliphaticivorans gen. nov., sp. nov., an n-Alkane- and n-Alkene-Degrading, Sulfate-Reducing Bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 77–83. [Google Scholar] [CrossRef]
- Zhang, L.; Keller, J.; Yuan, Z. Inhibition of Sulfate-Reducing and Methanogenic Activities of Anaerobic Sewer Biofilms by Ferric Iron Dosing. Water Res. 2009, 43, 4123–4132. [Google Scholar] [CrossRef]
- Lovley, D.R. Organic Matter Mineralization with the Reduction of Ferric Iron: A Review. Geomicrobiol. J. 1987, 5, 375–399. [Google Scholar] [CrossRef]
- Coleman, M.L.; Hedrick, D.B.; Lovley, D.R.; White, D.C.; Pye, K. Reduction of Fe(III) in Sediments by Sulphate-Reducing Bacteria. Nature 1993, 361, 436–438. [Google Scholar] [CrossRef]
- Holmes, D.E.; Bond, D.R.; Lovley, D.R. Electron Transfer by Desulfobulbus propionicus to Fe (III) and Graphite Electrodes Electron Transfer by Desulfobulbus Propionicus to Fe (III) and Graphite Electrodes. Appl. Environ. Microbiol. 2004, 70, 1234–1237. [Google Scholar] [CrossRef] [Green Version]
- Tebo, B.M.; Obraztsova, A.Y. Sulfate-Reducing Bacterium Grows with Cr (VI), U (VI), Mn (IV), and Fe (III) as Electron Acceptors. Source 1998, 162, 193–198. [Google Scholar] [CrossRef]
- Berner, R.A. Sedimentary Pyrite Formation: An Update. Geochim. Cosmochim. Acta 1984, 48, 605–615. [Google Scholar] [CrossRef]
- Kumar, R.; Dwivedi, V.; Nayyar, N.; Verma, H.; Singh, A.K.; Rani, P.; Rao, D.L.N.; Lal, R. Parapedobacter indicus sp. nov., Isolated from Hexachlorocyclohexane-Contaminated Soil. Int. J. Syst. Evol. Microbiol. 2015, 65, 129–134. [Google Scholar] [CrossRef]
- Collet, C.; Adler, N.; Schwitzguébel, J.P.; Péringer, P. Hydrogen Production by Clostridium thermolacticum during Continuous Fermentation of Lactose. Int. J. Hydrogen Energy 2004, 29, 1479–1485. [Google Scholar] [CrossRef]
- Holmes, D.E.; Nevin, K.P.; Woodard, T.L.; Peacock, A.D.; Lovley, D.R. Prolixibacter bellariivorans gen. nov., sp. nov., a Sugar-Fermenting, Psychrotolerant Anaerobe of the Phylum Bacteroidetes, Isolated from a Marine-Sediment Fuel Cell. Int. J. Syst. Evol. Microbiol. 2007, 57, 701–707. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakagawa, Y.; Harayama, S.; Yamamoto, S. Phylogenetic Analysis of Genus Marinilabilia and Related Bacteria Based on the Amino Acid Sequences of GyrB and Emended Description of Marinilabilia salmonicolor with Marinilabilia agarovorans as Its Subjective Synonym. Int. J. Syst. Bacteriol. 1999, 49, 1551–1557. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Sekiguchi, Y.; Hanada, S.; Imachi, H.; Ohashi, A.; Harada, H.; Kamagata, Y. Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., Novel Filamentous Anaerobes, and Description of the New Classes Anaerolineae Classis Nov. and Caldilineae Classis Nov. in the bacterial phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2006, 56, 1331–1340. [Google Scholar]
- Zhu, X.; Zhou, Y.; Wang, Y.; Wu, T.; Li, X.; Li, D.; Tao, Y. Production of High-Concentration n-Caproic Acid from Lactate through Fermentation Using a Newly Isolated Ruminococcaceae Bacterium CPB6. Biotechnol. Biofuels 2017, 10, 1–12. [Google Scholar] [CrossRef]
- Imachi, H.; Sakai, S.; Kubota, T.; Miyazaki, M.; Saito, Y.; Takai, K. Sedimentibacter acidaminivorans sp. nov., an Anaerobic, Amino-Acid-Utilizing Bacterium Isolated from Marine Subsurface Sediment. Int. J. Syst. Evol. Microbiol. 2016, 66, 1293–1300. [Google Scholar] [CrossRef]
- Kindaichi, T.; Yamaoka, S.; Uehara, R.; Ozaki, N.; Ohashi, A.; Albertsen, M.; Nielsen, P.H.; Nielsen, J.L. Phylogenetic Diversity and Ecophysiology of Candidate Phylum Saccharibacteria in Activated Sludge. FEMS Microbiol. Ecol. 2016, 92, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ueki, A.; Akasaka, H.; Suzuki, D.; Ueki, K. Paludibacter propionicigenes gen. nov., sp. nov., a Novel Strictly Anaerobic, Gram-Negative, Propionate-Producing Bacterium Isolated from Plant Residue in Irrigated Rice-Field Soil in Japan. Int. J. Syst. Evol. Microbiol. 2006, 56, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Dröge, S.; Rachel, R.; Radek, R.; König, H. Treponema isoptericolens sp. nov., a Novel Spirochaete from the Hindgut of the Termite Incisitermes Tabogae. Int. J. Syst. Evol. Microbiol. 2008, 58, 1079–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobbin, P.S.; Carter, J.P.; Juan, C.G.S.S.; Von Höbe, M.; Powell, A.K.; Richardson, D.J. Dissimilatory Fe(III) Reduction by Clostridium beijerinckii Isolated from Freshwater Sediment Using Fe(III) Maltol Enrichment. FEMS Microbiol. Lett. 1999, 176, 131–138. [Google Scholar] [CrossRef]
- Shah, M. Iron Oxide Reduction by a Clostridial Consortium: Insights from Physiological and Genome Analyses. Ph.D. Thesis, Rutgers University, New Brunswick, NJ, USA, 2013. [Google Scholar]
- Xie, C.H.; Yokota, A. Pleomorphomonas oryzae gen. nov., sp. nov., a Nitrogen-Fixing Bacterium Isolated from Paddy Soil of Oryza sativa. Int. J. Syst. Evol. Microbiol. 2005, 55, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.A.; Mirza, B.; Rodrigues, J.L.M.; Passy, S.I. Iron Limitation Effects on Nitrogen-Fixing Organisms with Possible Implications for Cyanobacterial Blooms. FEMS Microbiol. Ecol. 2018, 94, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jia, R.; Qu, Z.; Li, T.; Shen, W.; Qu, D. Coupling between Nitrogen-Fixing and Iron(III)-Reducing Bacteria as Revealed by the Metabolically Active Bacterial Community in Flooded Paddy Soils Amended with Glucose. Sci. Total Environ. 2020, 716, 137056. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Shiratori, Y.; Ohba, H.; Ishida, T.; Takano, R.; Satoh, S.; Shen, W.; Gao, N.; Itoh, H.; Senoo, K. Enhancement of the Nitrogen-Fixing Activity of Paddy Soils Owing to Iron Application. Soil Sci. Plant Nutr. 2021, 1–5. [Google Scholar] [CrossRef]
- Masuda, Y.; Itoh, H.; Shiratori, Y.; Isobe, K.; Otsuka, S.; Senoo, K. Predominant but Previously-Overlooked Prokaryotic Drivers of Reductive Nitrogen Transformation in Paddy Soils, Revealed by Metatranscriptomics. Microbes Environ. 2017, 32, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Minamisawa, K.; Nishioka, K.; Miyaki, T.; Ye, B.; Miyamoto, T.; You, M.; Saito, A.; Saito, M.; Barraquio, W.L.; Teaumroong, N.; et al. Anaerobic Nitrogen-Fixing Consortia Consisting of Clostridia Isolated from Gramineous Plants. Appl. Environ. Microbiol. 2004, 70, 3096–3102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarti, A.; Zaiat, M. Anaerobic Treatment of Sulfate-Rich Wastewater in an Anaerobic Sequential Batch Reactor (AnSBR) Using Butanol as the Carbon Source. J. Environ. Manag. 2011, 92, 1537–1541. [Google Scholar] [CrossRef]
- Dries, J.; De Smul, A.; Goethals, L.; Grootaerd, H.; Verstraete, W. High Rate Biological Treatment of Sulfate-Rich Wastewater in an Acetate-Fed EGSB Reactor. Biodegradation 1998, 9, 103–111. [Google Scholar] [CrossRef]
- Lovley, D.R. Dissimilatory Metal Reduction. Annu. Rev. Microbiol. 1993, 47, 20–29. [Google Scholar] [CrossRef]
- Tan, J.; Wang, J.; Xue, J.; Liu, S.Y.; Peng, S.C.; Ma, D.; Chen, T.H.; Yue, Z. Methane Production and Microbial Community Analysis in the Goethite Facilitated Anaerobic Reactors Using Algal Biomass. Fuel 2015, 145, 196–201. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, J.; Yang, G.; Zhuang, L. Methanogenesis Affected by the Co-Occurrence of Iron(III) Oxides and Humic Substances. FEMS Microbiol. Ecol. 2014, 88, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Shin, S.G.; Lee, C. Bioaugmentation of Anaerobic Sludge Digestion with Iron-Reducing Bacteria: Process and Microbial Responses to Variations in Hydraulic Retention Time. Appl. Microbiol. Biotechnol. 2016, 100, 927–937. [Google Scholar] [CrossRef] [PubMed]




| Bacteria | Phyla | Functional Activities | Bioreactor |
|---|---|---|---|
| Clostridium sp. | Firmicutes | Ferment glucose, lactose to produce acetate and H2 | R1, R3 |
| Prolixibacter sp. | Bacteroidetes | Ferment sugar, lactose to acetate and other smaller C compounds | R1 |
| Marinilabilia salmonicolor | Bacteroidetes | Ferment lactose to smaller C compounds | R1 |
| Leptolinea tardivitalis | Chloroflexi | Ferment glucose, fructose, and sucrose to smaller C compounds | R1 |
| Ruminococcaceae bacterium | Firmicutes | Ferment lactate to smaller C compounds | R1 |
| Sedimentibacter sp. | Firmicutes | Ferment pyruvate with the presence of yeast extract to produce acetate, lactate | R1 |
| Candidatus Saccharimonas | Saccharibacteria | Ferment sugars to smaller compounds | R2 |
| Parapedobacter sp. | Bacteroidetes | Ferment glucose, lactose to smaller C compounds | R2 |
| Paludibacter sp. | Bacteroidetes | Ferment glucose to acetate | R2, R3 |
| Treponema sp. | Spirochaetes | Ferment glucose, lactose to smaller C compounds | R3 |
| Ruminiclostridium sp. | Firmicutes | Ferment glucose, cellulose to acetate, ethanol, and lactate | R3 |
| Anaerolineae sp. | Chloroflexi | Ferment glucose, lactose to smaller C compounds | R3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Anwar, R.; Deng, D.; Garner, E.; Lin, L.-S. Functional Interrelationships of Microorganisms in Iron-Based Anaerobic Wastewater Treatment. Microorganisms 2021, 9, 1039. https://doi.org/10.3390/microorganisms9051039
Ahmed M, Anwar R, Deng D, Garner E, Lin L-S. Functional Interrelationships of Microorganisms in Iron-Based Anaerobic Wastewater Treatment. Microorganisms. 2021; 9(5):1039. https://doi.org/10.3390/microorganisms9051039
Chicago/Turabian StyleAhmed, Musfique, Rifat Anwar, Dongyang Deng, Emily Garner, and Lian-Shin Lin. 2021. "Functional Interrelationships of Microorganisms in Iron-Based Anaerobic Wastewater Treatment" Microorganisms 9, no. 5: 1039. https://doi.org/10.3390/microorganisms9051039
APA StyleAhmed, M., Anwar, R., Deng, D., Garner, E., & Lin, L.-S. (2021). Functional Interrelationships of Microorganisms in Iron-Based Anaerobic Wastewater Treatment. Microorganisms, 9(5), 1039. https://doi.org/10.3390/microorganisms9051039






