The Impact and Burden of Neurological Sequelae Following Bacterial Meningitis: A Narrative Review
Abstract
:1. Introduction
2. Global Burden of Meningitis
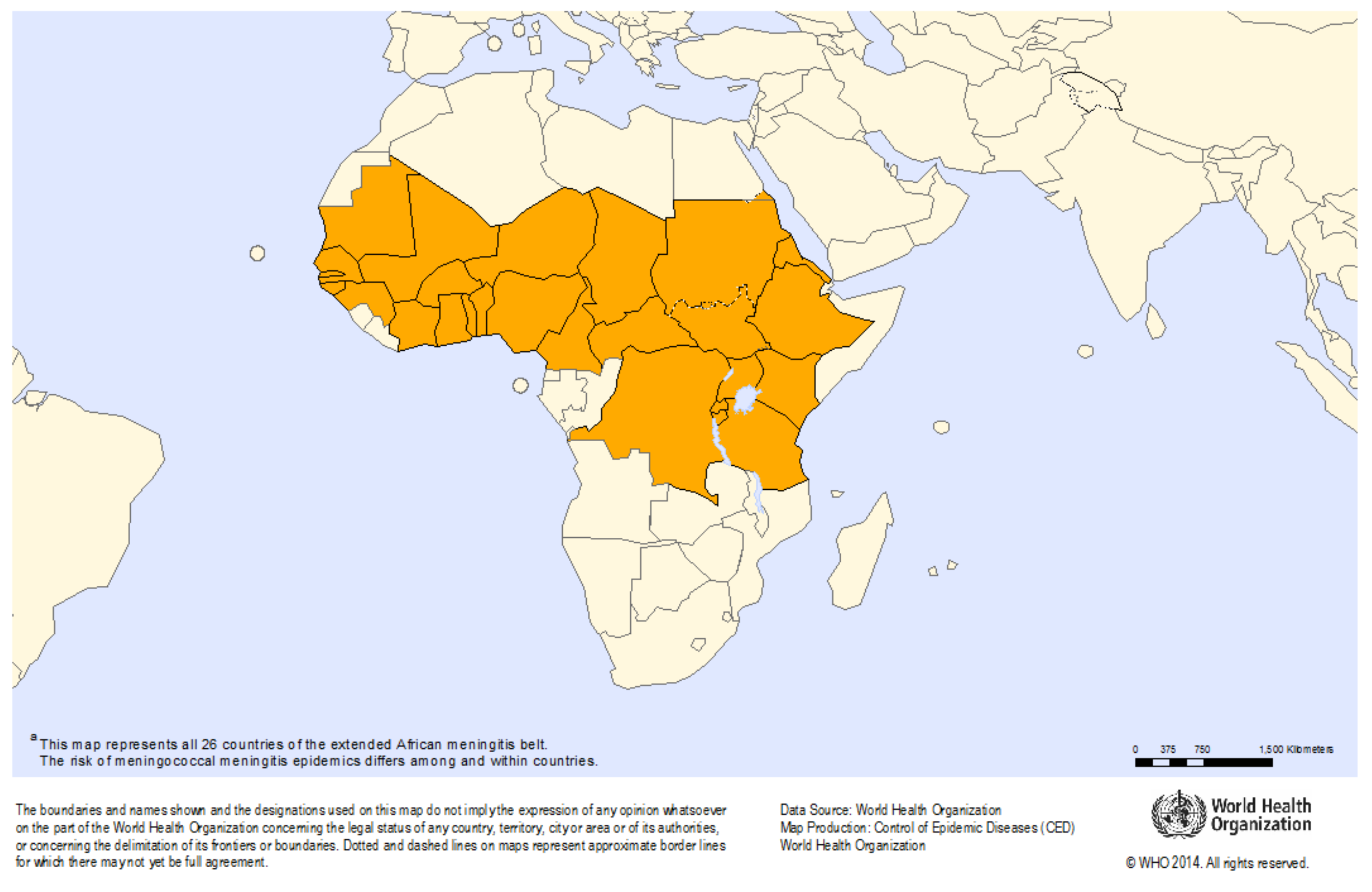
Meningitis Belt
3. Neurological Sequelae
3.1. Frequency and Types of Neurological Sequelae Following Meningitis
3.2. Persistence of Sequelae over Time
3.3. Neurological Sequelae in LMICs
4. Social and Economic Burden of Neurological Sequelae
5. Neurological Disability, Quality of Life and Access to Care
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- World Health Organization (WHO). Bacterial Meningitis. Available online: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/sentinel/meningitis_surveillance/en/ (accessed on 11 January 2020).
- Greenwood, B.M. Meningococcal meningitis in Africa. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 341–353. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Tuberculosis. 2020. Available online: https://www.who.int/health-topics/tuberculosis#tab=tab_1 (accessed on 12 March 2021).
- Phypers, M.; Harris, T.; Power, C. CNS tuberculosis: A longitudinal analysis of epidemiological and clinical features. Int. J. Tuberc. Lung Dis. 2006, 10, 99–103. [Google Scholar] [PubMed]
- Logan, C.; Mullender, C.; Mirfenderesky, M.; Feasey, N.; Cosgrove, C.; Riley, P.; Houston, A.; Harrison, T.; Bicanic, T.; Rich, P.; et al. Presentations and outcomes of central nervous system TB in a UK cohort: The high burden of neurological morbidity. J. Infect. 2021, 82, 90–97. [Google Scholar] [CrossRef]
- Koelman, D.L.H.; van Kassel, M.N.; Bijlsma, M.W.; Brouwer, M.C.; van de Beek, D.; van der Ende, A. Changing Epidemiology of Bacterial Meningitis Since Introduction of Conjugate Vaccines: Three Decades of National Meningitis Surveillance in The Netherlands. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.C.; Tunkel, A.R.; Van De Beek, D. Epidemiology, Diagnosis, and Antimicrobial Treatment of Acute Bacterial Meningitis. Clin. Microbiol. Rev. 2010, 23, 467–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuchat, A.; Robinson, K.; Wenger, J.D.; Harrison, L.H.; Farley, M.; Reingold, A.L.; Lefkowitz, L.; Perkins, B.A. Bacterial Meningitis in the United States in 1995. N. Engl. J. Med. 1997, 337, 970–976. [Google Scholar] [CrossRef]
- Cowgill, K.D.; Ndiritu, M.; Nyiro, J.; Slack, M.P.E.; Chiphatsi, S.; Ismail, A.; Kamau, T.; Mwangi, I.; English, M.; Newton, C.R.J.C.; et al. Effectiveness of Haemophilus influenzae Type b Conjugate Vaccine Introduction Into Routine Childhood Immunization in Kenya. JAMA 2006, 296, 671–678. [Google Scholar] [CrossRef]
- Adegbola, R.A.; Secka, O.; Lahai, G.; Lloyd-Evans, N.; Njie, A.; Usen, S.; Oluwalana, C.; Obaro, S.; Weber, M.; Corrah, T.; et al. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: A prospective study. Lancet 2005, 366, 144–150. [Google Scholar] [CrossRef]
- Trotter, C.L.; Lingani, C.; Fernandez, K.; Cooper, L.V.; Bita, A.; Tevi-Benissan, C.; Ronveaux, O.; Préziosi, M.-P.; Stuart, J.M. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: An analysis of surveillance data. Lancet Infect. Dis. 2017, 17, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Iwata, S.; Takata, M.; Morozumi, M.; Miyairi, I.; Matsubara, K.; Ubukata, K. Drastic reduction in pneumococcal meningitis in children owing to the introduction of pneumococcal conjugate vaccines: Longitudinal analysis from 2002 to 2016 in Japan. J. Infect. Chemother. 2021, 27, 604–612. [Google Scholar] [CrossRef]
- Klugman, K.P.; Madhi, S.A.; Huebner, R.E.; Kohberger, R.; Mbelle, N.; Pierce, N. A Trial of a 9-Valent Pneumococcal Conjugate Vaccine in Children with and Those without HIV Infection. N. Engl. J. Med. 2003, 349, 1341–1348. [Google Scholar] [CrossRef]
- El Kareh, A.; El Hage, S.; Safi, S.; Assouad, E.; Mokled, E.; Salameh, P. Epidemiology of bacterial meningitis in Lebanon from 2011 to 2019. J. Clin. Neurosci. 2020, 81, 32–36. [Google Scholar] [CrossRef]
- Global Health Data Exchanage. IHME Data. 2020. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 20 January 2021).
- Edmond, K.; Clark, A.; Korczak, V.S.; Sanderson, C.; Griffiths, U.K.; Rudan, I. Global and regional risk of disabling sequelae from bacterial meningitis: A systematic review and meta-analysis. Lancet. Infect. Dis. 2010, 10, 317–328. [Google Scholar] [CrossRef] [Green Version]
- Koomen, I.; van Furth, A.M.; Kraak, M.A.; Grobbee, D.E.; Roord, J.J.; Jennekens-Schinkel, A. Neuropsychology of academic and behavioural limitations in school-age survivors of bacterial meningitis. Dev. Med. Child. Neurol. 2004, 46, 724–732. [Google Scholar] [CrossRef]
- WHO. Defeating meningitis by 2030. Available online: https://www.who.int/initiatives/defeating-meningitis-by-2030 (accessed on 21 April 2021).
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Meningitis Weekly Bulletin 2019 (2–29 December 2019); WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Fernandez, K.; Lingani, C.; Aderinola, O.M.; Goumbi, K.; Bicaba, B.; Edea, Z.A.; Glèlè, C.; Sarkodie, B.; Tamekloe, A.; Ngomba, A.; et al. Meningococcal Meningitis Outbreaks in the African Meningitis Belt After Meningococcal Serogroup A Conjugate Vaccine Introduction, 2011–2017. J. Infect. Dis. 2019, 220, S225–S232. [Google Scholar] [CrossRef]
- Soeters, H.M.; Diallo, A.O.; Bicaba, B.W.; Kadadé, G.; Dembélé, A.Y.; Acyl, M.A.; Sadji, A.Y.; Poy, A.N.; Lingani, C.; Tall, H.; et al. Bacterial Meningitis Epidemiology in Five Countries in the Meningitis Belt of Sub-Saharan Africa, 2015–2017. J. Infect. Dis. 2019, 220 (Suppl. S4), S165–S174. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Control of Epidemic Meningococcal Disease. WHO Practical Guidelines, 2nd Edition. Available online: https://www.who.int/csr/resources/publications/meningitis/WHO_EMC_BAC_98_3_EN/en/ (accessed on 18 November 2020).
- Colombini, A.; Badolo, O.; Gessner, B.D.; Jaillard, P.; Seini, E.; Da Silva, A. Costs and impact of meningitis epidemics for the public health system in Burkina Faso. Vaccine 2011, 29, 5474–5480. [Google Scholar] [CrossRef]
- Jit, M. The risk of sequelae due to pneumococcal meningitis in high-income countries: A systematic review and meta-analysis. J. Infect. 2010, 61, 114–124. [Google Scholar] [CrossRef]
- Adil, S.M.; Hodges, S.E.; Charalambous, L.T.; Kiyani, M.; Liu, B.; Lee, H.-J.; Parente, B.A.; Perfect, J.R.; Lad, S.P. Paediatric bacterial meningitis in the USA: Outcomes and healthcare resource utilization of nosocomial versus community-acquired infection. J. Med. Microbiol. 2021, 70, 001276. [Google Scholar] [CrossRef]
- Kilpi, T.; Anttila, M.; Kallio, M.J.T.; Peltola, H. Length of prediagnostic history related to the course and sequelae of childhood bacterial meningitis. Pediatr. Infect. Dis. J. 1993, 12, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Kilpi, T.; Anttila, M.; Kallio, M.; Peltola, H. Severity of childhood bacterial meningitis and duration of illness before diagnosis. Lancet 1991, 338, 406–409. [Google Scholar] [CrossRef]
- Victora, C.G.; Wagstaff, A.; Schellenberg, J.A.; Gwatkin, D.; Claeson, M.; Habicht, J.-P. Applying an equity lens to child health and mortality: More of the same is not enough. Lancet 2003, 362, 233–241. [Google Scholar] [CrossRef]
- Edmond, K.; Dieye, Y.; Griffiths, U.K.; Fleming, J.; Ba, O.; Diallo, N.; Mulholland, K. Prospective Cohort Study of Disabling Sequelae and Quality of Life in Children With Bacterial Meningitis in Urban Senegal. Pediatr. Infect. Dis. J. 2010, 29, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Chandran, A.; Herbert, H.; Misurski, D.; Santosham, M. Long-term sequelae of childhood bacterial meningitis: An underappreciated problem. Pediatr. Infect. Dis. J. 2011, 30, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Trollfors, B.; Hugosson, S.; Fernell, E.; Svensson, E. Long-term follow-up of children with bacterial meningitis with emphasis on behavioural characteristics. Eur. J. Nucl. Med. Mol. Imaging 2002, 161, 330–336. [Google Scholar] [CrossRef]
- Halket, S.; de Louvois, J.; Holt, D.E.; Harvey, D. Long term follow up after meningitis in infancy: Behaviour of teenagers. Arch. Dis. Child. 2003, 88, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, M.; Ulland, A.J.; Steinhardt, L.C.; Moïsi, J.C.; Were, F.; Levine, O.S. Sequelae due to bacterial meningitis among African children: A systematic literature review. BMC Med. 2009, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Baraff, L.J.; Lee, S.I.; Schriger, D.L. Outcomes of bacterial meningitis in children: A meta-analysis. Pediatr. Infect. Dis. J. 1993, 12, 389–394. [Google Scholar] [CrossRef]
- Hodgson, A.; Smith, T.; Gagneux, S.; Akumah, I.; Adjuik, M.; Pluschke, G.; Binka, F.; Genton, B. Survival and sequelae of meningococcal meningitis in Ghana. Int. J. Epidemiol. 2001, 30, 1440–1446. [Google Scholar] [CrossRef] [Green Version]
- Anderson, V.; Anderson, P.; Grimwood, K.; Nolan, T. Cognitive and Executive Function 12 Years after Childhood Bacterial Meningitis: Effect of Acute Neurologic Complications and Age of Onset. J. Pediatr. Psychol. 2004, 29, 67–81. [Google Scholar] [CrossRef] [Green Version]
- Mbonda, E.; Masso-Misse, P.; Ntaplia, A.; Mefo Sile, H. Séquelles neurologiques des méningites bactériennes chez le nourrisson et l’enfant à Yaoundé. Med. Afr. Noire 1995, 42, 39–45. [Google Scholar]
- Girgis, N.I.; Farid, Z.; Mikhail, I.A.; Farrag, I.; Sultan, Y.; Kilpatrick, M.E. Dexamethasone treatment for bacterial meningitis in children and adults. Pediatr. Infect. Dis. J. 1989, 8, 848–851. [Google Scholar] [CrossRef]
- Girgis, N.I.; Farid, Z.; Kilpatrick, M.E.; Sultan, Y.; Mikhail, I.A. Dexamethasone adjunctive treatment for tuberculous meningitis. Pediatr. Infect. Dis. J. 1991, 10, 179–182. [Google Scholar] [CrossRef]
- Girgis, N.; Erian, M.W.; Farid, Z.; Mansour, M.M.; Sultan, Y.; Mateczun, A.J.; Hanna, L.S. Tuberculosis meningitis, Abbassia Fever Hospital-Naval Medical Research Unit No. 3-Cairo, Egypt, from 1976 to 1996. Am. J. Trop. Med. Hyg. 1998, 58, 28–34. [Google Scholar] [CrossRef]
- Melaku, A. Sensorineural hearing loss in children with epidemic meningococcal meningitis at Tikur Anbessa Hospital. Ethiop. Med. J. 2003, 41, 113–121. [Google Scholar]
- Bijlmer, H.A.; Van Alphen, L.; Greenwood, B.M.; Brown, J.; Schneider, G.; Hughes, A.; Menon, A.; Zanen, H.C.; Valkenburg, H.A. The Epidemiology of Haemophilus influenzae Meningitis in Children under Five Years of Age in The Gambia, West Africa. J. Infect. Dis. 1990, 161, 1210–1215. [Google Scholar] [CrossRef]
- Goetghebuer, T.; West, T.E.; Wermenbol, V.; Cadbury, A.L.; Milligan, P.; Lloyd-Evans, N.; Adegbola, R.A.; Mulholland, E.K.; Greenwood, B.M.; Weber, M.W. Outcome of meningitis caused by Streptococcus pneumoniae and Haemophilus influenzae type b in children in The Gambia. Trop. Med. Int. Health 2000, 5, 207–213. [Google Scholar] [CrossRef]
- Akpede, G.O.; Akuhwa, R.T.; Ogiji, E.O.; Ambe, J.P. Risk factors for an adverse outcome in bacterial meningitis in the tropics: A reappraisal with focus on the significance and risk of seizures. Ann. Trop. Paediatr. 1999, 19, 151–159. [Google Scholar] [CrossRef]
- Salih, M.A.M.; El Hag, A.I.; Ahmed, H.S.; Bushara, M.; Yasin, I.; Omer, M.I.A.; Hofvander, Y.; Olcen, P. Endemic bacterial meningitis in Sudanese children: Aetiology, clinical findings, treatment and short-term outcome. Ann. Trop. Paediatr. 1990, 10, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Mhirsi, A.; Boussoffara, R.; Ayadi, A.; Soua, H.; Driss, N.; Belkhir, Y.; Dalel, H.; Braham, H.; Pousse, H.; Essghairi, K. Prognosis of purulent meningitis in the infant and young child. Tunis. Med. 1992, 70, 79–85. [Google Scholar]
- Saha, S.K.; Khan, N.Z.; Ahmed, A.S.M.N.U.; Amin, M.R.; Hanif, M.; Mahbub, M.; Anwar, K.S.; Qazi, S.A.; Kilgore, P.; Baqui, A.H.; et al. Neurodevelopmental sequelae in pneumococcal meningitis cases in Bangladesh: A comprehensive follow-up study. Clin. Infect. Dis. 2009, 48 (Suppl. S2), S90–S96. [Google Scholar] [CrossRef]
- Krauss-Mars, A.H.; Lachman, P.I. Social factors associated with tuberculous meningitis. A study of children and their families in the western Cape. South Afr. Med. J. 1992, 81, 16–19. [Google Scholar]
- Ayieko, P.; Akumu, A.O.; Griffiths, U.K.; English, M. The economic burden of inpatient paediatric care in Kenya: Household and provider costs for treatment of pneumonia, malaria and meningitis. Cost Eff. Resour. Alloc. 2009, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombini, A.; Bationo, F.; Zongo, S.; Ouattara, F.; Badolo, O.; Jaillard, P.; Seini, E.; Gessner, B.D.; Da Silva, A. Costs for Households and Community Perception of Meningitis Epidemics in Burkina Faso. Clin. Infect. Dis. 2009, 49, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Groce, N.E. Global disability: An emerging issue. Lancet Glob. Health 2018, 6, e724–e725. [Google Scholar] [CrossRef]
- Scior, K.; Hamid, A.; Hastings, R.; Werner, S.; Belton, C.; Laniyan, A.; Patel, M.; Groce, N.; Kett, M. Consigned to the margins: A call for global action to challenge intellectual disability stigma. Lancet Glob. Health 2016, 4, e294–e295. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Palmer, M.; Kim, H.; Mont, D.; Groce, N. Extra costs of living with a disability: A review and agenda for research. Disabil. Health J. 2017, 10, 475–484. [Google Scholar] [CrossRef]
- Sumpter, R.; Brunklaus, A.; McWilliam, R.; Dorris, L. Health-related quality-of-life and behavioural outcome in survivors of childhood meningitis. Brain Inj. 2011, 25, 1288–1295. [Google Scholar] [CrossRef]
- Olbrich, K.J.; Müller, D.; Schumacher, S.; Beck, E.; Meszaros, K.; Koerber, F. Systematic Review of Invasive Meningococcal Disease: Sequelae and Quality of Life Impact on Patients and Their Caregivers. Infect. Dis. Ther. 2018, 7, 421–438. [Google Scholar] [CrossRef] [Green Version]
- Zelano, J.; Westman, G. Epilepsy after brain infection in adults: A register-based population-wide study. Neurology 2020, 95, e3213–e3220. [Google Scholar] [CrossRef]
- Van de Beek, D.; de Gans, J.; Spanjaard, L.; Weisfelt, M.; Reitsma, J.B.; Vermeulen, M. Clinical features and prognostic factors in adults with bacterial meningitis. N. Engl. J. Med. 2004, 351, 1849–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Beek, D.; Schmand, B.; de Gans, J.; Weisfelt, M.; Vaessen, H.; Dankert, J.; Vermeulen, M. Cognitive impairment in adults with good recovery after bacterial meningitis. J. Infect. Dis. 2002, 186, 1047–1052. [Google Scholar]
- United Nations Department of Economic and Social Affairs Disability. Convention on the Rights of Persons with Disabilities (CRPD). Available online: https://www.un.org/development/desa/disabilities/convention-on-the-rights-of-persons-with-disabilities.html (accessed on 30 January 2021).
| Country | Year Published, Reference | Total No. Assessed for Sequelae | Ave Follow Up Time (Months) | Post-Discharge Neurological Sequelae | Bacterial Pathogens |
|---|---|---|---|---|---|
| Cameroon | 1995, [39] | 67 | 14 | 25% | Spn, Hib, Nm, others |
| Egypt | 1989, [40] | 367 | 3 | 3% | Spn, Hib, Nm |
| 1991, [41] | 78 | 2–24 | 24% | Tuberculosis | |
| 1998, [42] | 289 | 12 | 32% | Tuberculosis | |
| Ethiopia | 2003, [43] | 53 | Not specified | 34% | Spn, Hib, Nm, others |
| The Gambia | 1990, [44] | 48 | 8 | 13% | Hib |
| 2000, [45] | 73 | 11 to 90 | 47% | Spn, Hib | |
| Nigeria | 1999, [46] | 47 | Not specified | 23% | Spn, Hib, Nm, Klebsiella and others |
| Sudan | 1990, [47] | 27 | 3–48 | 33% | Spn, Hib, Nm and others |
| Tunisia | 1992, [48] | 82 | 60 | 13% | Spn, Hib, Nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiess, N.; Groce, N.E.; Dua, T. The Impact and Burden of Neurological Sequelae Following Bacterial Meningitis: A Narrative Review. Microorganisms 2021, 9, 900. https://doi.org/10.3390/microorganisms9050900
Schiess N, Groce NE, Dua T. The Impact and Burden of Neurological Sequelae Following Bacterial Meningitis: A Narrative Review. Microorganisms. 2021; 9(5):900. https://doi.org/10.3390/microorganisms9050900
Chicago/Turabian StyleSchiess, Nicoline, Nora E. Groce, and Tarun Dua. 2021. "The Impact and Burden of Neurological Sequelae Following Bacterial Meningitis: A Narrative Review" Microorganisms 9, no. 5: 900. https://doi.org/10.3390/microorganisms9050900
APA StyleSchiess, N., Groce, N. E., & Dua, T. (2021). The Impact and Burden of Neurological Sequelae Following Bacterial Meningitis: A Narrative Review. Microorganisms, 9(5), 900. https://doi.org/10.3390/microorganisms9050900






