Extracellular Polymeric Substance Protects Some Cells in an Escherichia coli Biofilm from the Biomechanical Consequences of Treatment with Magainin 2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antimicrobial Peptide Preparation
2.2. Minimum Inhibitory Concentration of MAG2 for E. coli Biofilm Cells
2.3. Minimum Bactericidal Concentration of MAG2 on Established E. coli Biofilms
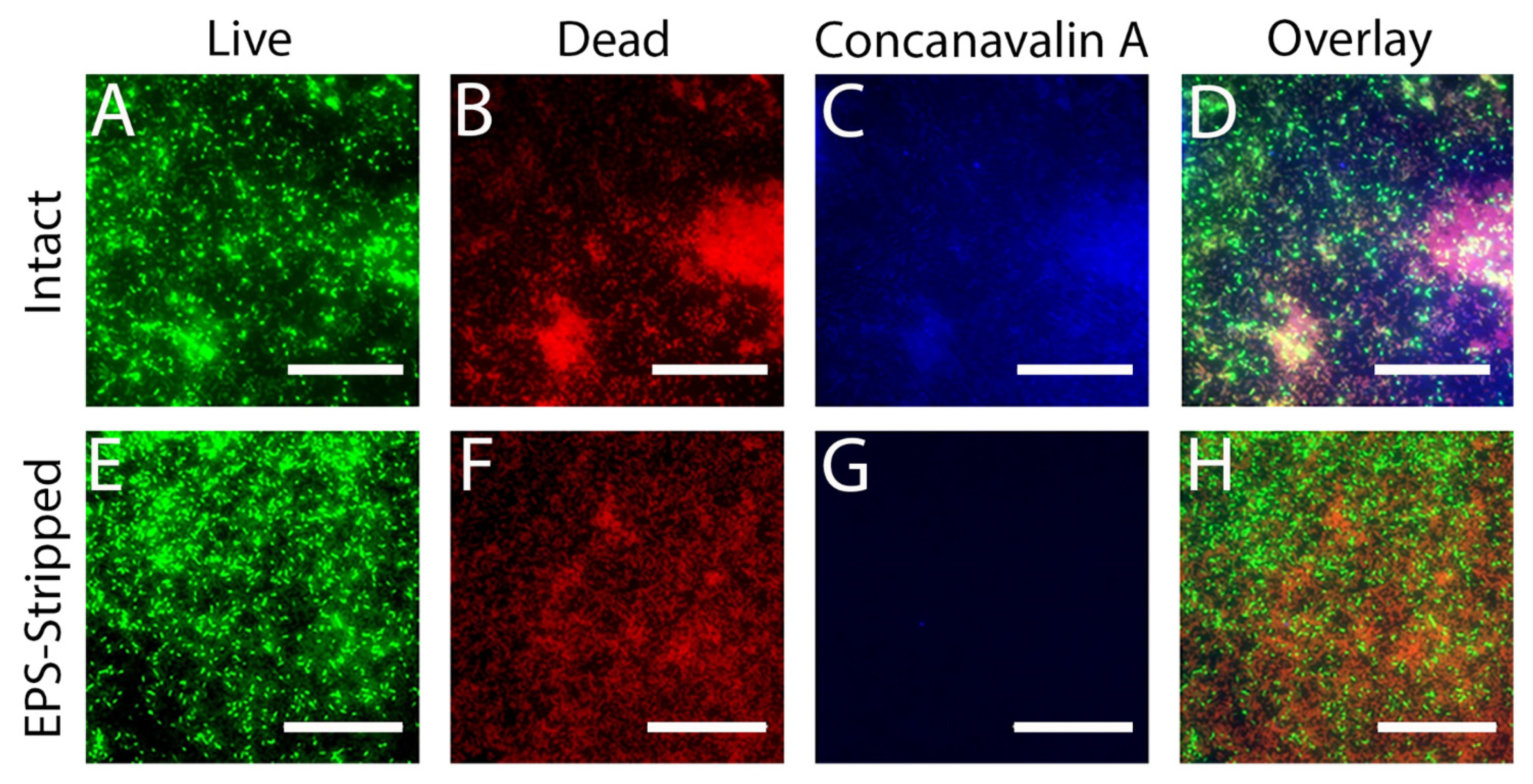
2.4. Live/Dead Cell Staining
2.5. AFM
2.6. Enzymatic Degradation of the Biofilm Extracellular Polymeric Substance (EPS)
3. Results and Discussion
3.1. MAG2 Prevents E. coli Biofilm Formation
3.2. An Established E. coli Biofilm Can Withstand High Concentrations of MAG2
3.3. MAG2 Permeabilizes Cells in the Biofilm
3.4. AFM Reveals Different Cell Stiffness for Affected and Unaffected Cells
3.5. Soft Cells Develop a Rough Outer Surface
3.6. Removing the EPS Leads to Increased Susceptibility of Cells in the Biofilm to MAG2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm Formation as Microbial Development. Ann. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Monds, R.D.; O’Toole, G.A. The Developmental Model of Microbial Biofilms: Ten Years of a Paradigm up for Review. Trend. Microbiol. 2009, 17, 73–87. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Bassler, B.L. Bacterial Quorum Sensing in Complex and Dynamically Changing Environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef] [PubMed]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should We Stay or Should We Go: Mechanisms and Ecological Consequences for Biofilm Dispersal. Nat. Rev. Microbiol. 2012, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.; Stewart, P.S.; Greenberg, E. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobretsov, S.; Abed, R.M.M.; Teplitski, M. Inhibition of Biofouling by Marine Microorganisms. Biofouling 2013, 29, 423–441. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898–909. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Peschel, A.; Sahl, H.-G.G. The Co-Evolution of Host Cationic Antimicrobial Peptides and Microbial Resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up Immunity: How Antimicrobial Peptides Have Multiple Roles in Immune Defense. Trend. Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing Antimicrobial Peptides: Form Follows Function. Nat. Rev. Drug. Discov. 2011, 11, 37–51. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a Class of Antimicrobial Peptides from Xenopus Skin: Isolation, Characterization of Two Active Forms, and Partial cDNA Sequence of a Precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soravia, E.; Martini, G.; Zasloff, M. Antimicrobial Properties of Peptides from Xenopus Granular Gland Secretions. FEBS Lett. 1988, 228, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Westerhoff, H.; Juretić, D.; Hendler, R.; Zasloff, M. Magainins and the Disruption of Membrane-Linked Free-Energy Transduction. Proc. Natl. Acad. Sci. USA 1989, 86, 6597–6601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zasloff, M.; Martin, B.; Chen, H.C. Antimicrobial Activity of Synthetic Magainin Peptides and Several Analogues. Proc. Natl. Acad. Sci. USA 1988, 85, 910–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruciani, R.A.; Barker, J.L.; Durell, S.R.; Raghunathan, G.; Guy, H.R.; Zasloff, M.; Stanley, E.F. Magainin 2, a Natural Antibiotic from Frog Skin, Forms Ion Channels in Lipid Bilayer Membranes. Eur. J. Pharmacol. 1992, 226, 287–296. [Google Scholar] [CrossRef]
- Bechinger, B. The SMART Model: Soft Membranes Adapt and Respond, Also Transiently, in the Presence of Antimicrobial Peptides. J. Pept. Sci. 2015, 21, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Wenk, M.R.; Seelig, J. Magainin 2 Amide Interaction with Lipid Membranes: Calorimetric Detection of Peptide Binding and Pore Formation. Biochemistry 1998, 37, 3909–3916. [Google Scholar] [CrossRef]
- Wieprecht, T.; Apostolov, O.; Seelig, J. Binding of the Antibacterial Peptide Magainin 2 Amide to Small and Large Unilamellar Vesicles. Biophys. Chem. 2000, 85, 187–198. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Harada, M.; Funakoshi, S.; Fujii, N.; Miyajima, K. Physicochemical Determinants for the Interactions of Magainins 1 and 2 with Acidic Lipid Bilayers. Biochim. Biophys. Acta Biomembr. 1991, 1063, 162–170. [Google Scholar] [CrossRef]
- Marquette, A.; Bechinger, B. Biophysical Investigations Elucidating the Mechanisms of Action of Antimicrobial Peptides and Their Synergism. Biomolecules 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasir, M.; Willcox, M.; Dutta, D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef] [Green Version]
- Otto, M. Bacterial Evasion of Antimicrobial Peptides by Biofilm Formation. In Antimicrobial Peptides and Human Disease; Shafer, W.M., Ed.; Springer: New York, NY, USA, 2006; pp. 251–258. ISBN 9783540299158. [Google Scholar]
- Overton, K.; Greer, H.M.; Ferguson, M.A.; Spain, E.M.; Elmore, D.E.; Núñez, M.E.; Volle, C.B. Qualitative and Quantitative Changes to Escherichia coli during Treatment with Magainin 2 Observed in Native Conditions by Atomic Force Microscopy. Langmuir 2020, 36, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Volle, C.B.; Ferguson, M.A.; Aidala, K.E.; Spain, E.M.; Núñez, M.E. Quantitative Changes in the Elasticity and Adhesive Properties of Escherichia coli ZK1056 Prey Cells During Predation by Bdellovibrio Bacteriovorus 109J. Langmuir 2008, 24, 8102–8110. [Google Scholar] [CrossRef] [PubMed]
- Volle, C.B.; Ferguson, M.A.; Aidala, K.E.; Spain, E.M.; Núñez, M.E. Spring Constants and Adhesive Properties of Native Bacterial Biofilm Cells Measured by Atomic Force Microscopy. Colloids Surf. B Biointerfaces 2008, 67, 32–40. [Google Scholar] [CrossRef]
- Goss, J.W.; Volle, C.B. Using Atomic Force Microscopy to Illuminate the Biophysical Properties of Microbes. ACS Appl. Bio Mater. 2019, 3, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, K.; Murase, O.; Tokuda, H.; Funakoshi, S.; Fujii, N.; Miyajima, K. Orientational and Aggregational States of Magainin 2 in Phospholipid Bilayers. Biochemistry 1994, 33, 3342–3349. [Google Scholar] [CrossRef]
- Wade, H.M.; Darling, L.E.O.; Elmore, D.E. Hybrids Made from Antimicrobial Peptides with Different Mechanisms of Action Show Enhanced Membrane Permeabilization. Biochim. Biophys. Acta Biomembr. 2019, 1861, 182980–182988. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Wei, G.-X.X.; Campagna, A.N.; Bobek, L.A. Effect of MUC7 Peptides on the Growth of Bacteria and on Streptococcus Mutans Biofilm. J. Antimicrob. Chemother. 2006, 57, 1100–1109. [Google Scholar] [CrossRef]
- Kim, M.K.; Kang, N.; Ko, S.J.; Park, J.; Park, E.; Shin, D.W.; Kim, S.H.; Lee, S.A.; Lee, J.I.; Lee, S.H.; et al. Antibacterial and Antibiofilm Activity and Mode of Action of Magainin 2 against Drug-Resistant Acinetobacter Baumannii. Int. J. Mol. Sci. 2018, 19, 3041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, S.N.; Bishop, B.M.; van Hoek, M.L. Natural and Synthetic Cathelicidin Peptides with Anti-Microbial and Anti-Biofilm Activity against Staphylococcus aureus. BMC Microbiol. 2011, 11, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amer, L.S.; Bishop, B.M.; van Hoek, M.L. Antimicrobial and Antibiofilm Activity of Cathelicidins and Short, Synthetic Peptides against Francisella. Biochem. Biophys. Res. 2010, 396, 246–251. [Google Scholar] [CrossRef]
- Luo, Y.; McLean, D.T.F.; Linden, G.J.; McAuley, D.F.; McMullan, R.; Lundy, F.T. The Naturally Occurring Host Defense Peptide, LL-37, and Its Truncated Mimetics KE-18 and KR-12 Have Selected Biocidal and Antibiofilm Activities against Candida albicans, Staphylococcus aureus, and Escherichia coli In Vitro. Front. Microbiol. 2017, 8, 544–555. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Parsek, M.R.; Greenberg, P.E.; Welsh, M.J. A Component of Innate Immunity Prevents Bacterial Biofilm Development. Nature 2002, 417, 552–555. [Google Scholar] [CrossRef]
- de la Fuente-Núñez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Broad-Spectrum Anti-Biofilm Peptide That Targets a Cellular Stress Response. PLoS Pathog. 2014, 10, e1004152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reffuveille, F.; de la Fuente-Núñez, C.; Mansour, S.; Hancock, R.E.W. A Broad-Spectrum Antibiofilm Peptide Enhances Antibiotic Action against Bacterial Biofilms. Antimicrob. Agents Chemother. 2014, 58, 5363–5371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, S.C.; de la Fuente-Núñez, C.; Hancock, R.E.W. Peptide IDR-1018: Modulating the Immune System and Targeting Bacterial Biofilms to Treat Antibiotic-Resistant Bacterial Infections. J. Pept. Sci. 2014, 21, 323–329. [Google Scholar] [CrossRef]
- Cao, P.; Yuan, C.; Xiao, J.; He, X.; Bai, X. A Biofilm Resistance Surface Yielded by Grafting of Antimicrobial Peptides on Stainless Steel Surface. Surf. Interface Anal. 2018, 50, 516–521. [Google Scholar] [CrossRef]
- Cao, P.; Liu, K.; Liu, X.; Sun, W.; Wu, D.; Yuan, C.; Bai, X.; Zhang, C. Antibacterial Properties of Magainin II Peptide onto 304 Stainless Steel Surfaces: A Comparison Study of Two Dopamine Modification Methods. Colloids Surf. B Biointerfaces 2020, 194, 111198–111206. [Google Scholar] [CrossRef] [PubMed]
- Héquet, A.; Humblot, V.; Berjeaud, J.-M.; Pradier, C.-M. Optimized Grafting of Antimicrobial Peptides on Stainless Steel Surface and Biofilm Resistance Tests. Colloids Surf. B Biointerfaces 2011, 84, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Stocks, S. Mechanism and Use of the Commercially Available Viability Stain, BacLight. Cytometry A 2004, 61A, 189–195. [Google Scholar] [CrossRef]
- Gh., M.; Wilhelm, M.J.; Dai, H.-L. Label-Free Optical Method for Quantifying Molecular Transport across Cellular Membranes in Vitro. J. Phys. Chem. Lett. 2016, 7, 3406–3411. [Google Scholar] [CrossRef]
- Rosenberg, M.; Azevedo, N.F.; Ivask, A. Propidium Iodide Staining Underestimates Viability of Adherent Bacterial Cells. Sci. Rep. 2019, 9, 6483–6495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dosler, S.; Karaaslan, E. Inhibition and Destruction of Pseudomonas aeruginosa Biofilms by Antibiotics and Antimicrobial Peptides. Peptides 2014, 62, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Golla, R.M.; Lau, K.; Lushnikova, T.; Wang, G. Anti-Staphylococcal Biofilm Effects of Human Cathelicidin Peptides. ACS Med. Chem. Lett. 2015, 7, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.F.; Abdelkhalek, A.; Seleem, M.N. Evaluation of Short Synthetic Antimicrobial Peptides for Treatment of Drug-Resistant and Intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 29707–29721. [Google Scholar] [CrossRef]
- Kang, J.; Dietz, M.J.; Li, B. Antimicrobial Peptide LL-37 is Bactericidal against Staphylococcus aureus Biofilms. PLoS ONE 2019, 14, e0216676. [Google Scholar] [CrossRef] [Green Version]
- Luca, V.; Stringaro, A.; Colone, M.; Pini, A.; Mangoni, M.L. Esculentin(1–21), an Amphibian Skin Membrane-Active Peptide with Potent Activity on Both Planktonic and Biofilm Cells of the Bacterial Pathogen Pseudomonas aeruginosa. Cell Mol. Life Sci. 2013, 70, 2773–2786. [Google Scholar] [CrossRef]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of Biofilm Resistance to Antimicrobial Agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Anderson, G.; O’Toole, G. Innate and Induced Resistance Mechanisms of Bacterial Biofilms. In Bacterial Biofilms; Romeo, T., Ed.; Springer: New York, NY, USA, 2008; Volume 322, pp. 85–105. ISBN 9783540754176. [Google Scholar]
- Davies, D. Understanding Biofilm Resistance to Antibacterial Agents. Nat. Rev. Drug. Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Gunn, J.S.; Bakaletz, L.O.; Wozniak, D.J. What’s on the Outside Matters: The Role of the Extracellular Polymeric Substance of Gram-Negative Biofilms in Evading Host Immunity and as a Target for Therapeutic Intervention. J. Biol. Chem. 2016, 291, 12538–12546. [Google Scholar] [CrossRef] [Green Version]
- Arnoldi, M.; Fritz, M.; Bäuerlein, E.; Radmacher, M.; Sackmann, E.; Boulbitch, A. Bacterial Turgor Pressure Can Be Measured by Atomic Force Microscopy. Phys. Rev. E 2000, 62, 1034–1044. [Google Scholar] [CrossRef]
- Yao, X.; Walter, J.; Burke, S.; Stewart, S.; Jericho, M.H.; Pink, D.; Hunter, R.; Beveridge, T.J. Atomic Force Microscopy and Theoretical Considerations of Surface Properties and Turgor Pressures of Bacteria. Colloids Surf. B Biointerfaces 2002, 23, 213–230. [Google Scholar] [CrossRef]
- Mularski, A.; Wilksch, J.J.; Wang, H.; Hossain, M.A.; Wade, J.D.; Separovic, F.; Strugnell, R.A.; Gee, M.L. Atomic Force Microscopy Reveals the Mechanobiology of Lytic Peptide Action on Bacteria. Langmuir 2015, 31, 6164–6171. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-N.; Chen, Z.-H.; Song, X.-Y.; Chen, X.-L.; Shi, M.; Zhou, B.-C.; Zhao, X.; Zhang, Y.-Z. Antimicrobial Peptide Trichokonin VI-Induced Alterations in the Morphological and Nanomechanical Properties of Bacillus subtilis. PLoS ONE 2012, 7, e45818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freudenthal, O.; Quilès, F.; Francius, G. Discrepancies between Cyclic and Linear Antimicrobial Peptide Actions on the Spectrochemical and Nanomechanical Fingerprints of a Young Biofilm. ACS Omega 2017, 2, 5861–5872. [Google Scholar] [CrossRef] [PubMed]
- Quilès, F.; Saadi, S.; Francius, G.; Bacharouche, J.; Humbert, F. In Situ and Real Time Investigation of the Evolution of a Pseudomonas fluorescens Nascent Biofilm in the Presence of an Antimicrobial Peptide. Biochim. Biophys. Acta Biomembr. 2016, 1858, 75–84. [Google Scholar] [CrossRef]
- Dufrêne, Y.F.; Ando, T.; Garcia, R.; Alsteens, D.; Martinez-Martin, D.; Engel, A.; Gerber, C.; Müller, D.J. Imaging Modes of Atomic Force Microscopy for Application in Molecular and Cell Biology. Nat. Nanotechnol. 2017, 12, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Arce, F.T.; Carlson, R.; Monds, J.; Veeh, R.; Hu, F.Z.; Stewart, P.S.; Lal, R.; Ehrlich, G.D.; Avci, R. Nanoscale Structural and Mechanical Properties of Nontypeable Haemophilus Influenzae Biofilms. J. Bacteriol. 2009, 191, 2512–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torelli, R.; Cacaci, M.; Papi, M.; Sterbini, F.P.; Martini, C.; Posteraro, B.; Palmieri, V.; Spirito, M.D.; Sanguinetti, M.; Bugli, F. Different Effects of Matrix Degrading Enzymes towards Biofilms Formed by E. faecalis and E. faecium Clinical Isolates. Colloid Surf. B Biointerfaces 2017, 158, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Baidamshina, D.R.; Trizna, E.Y.; Holyavka, M.G.; Bogachev, M.I.; Artyukhov, V.G.; Akhatova, F.S.; Rozhina, E.V.; Fakhrullin, R.F.; Kayumov, A.R. Targeting Microbial Biofilms Using Ficin, a Nonspecific Plant Protease. Sci. Rep. 2017, 7, 46068–46080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugli, F.; Palmieri, V.; Torelli, R.; Papi, M.; Spirito, M.D.; Cacaci, M.; Galgano, S.; Masucci, L.; Sterbini, F.P.; Vella, A.; et al. In Vitro Effect of Clarithromycin and Alginate Lyase against Helicobacter Pylori Biofilm. Biotechnol. Progr. 2016, 32, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Fantner, G.E.; Barbero, R.J.; Gray, D.S.; Belcher, A.M. Kinetics of Antimicrobial Peptide Activity Measured on Individual Bacterial Cells Using High-Speed Atomic Force Microscopy. Nat. Nanotechnol. 2010, 5, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Fleming, D.; Rumbaugh, K.P. Approaches to Dispersing Medical Biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef]
- Bales, P.M.; Renke, E.M.; May, S.L.; Shen, Y.; Nelson, D.C. Purification and Characterization of Biofilm-Associated EPS Exopolysaccharides from ESKAPE Organisms and Other Pathogens. PLoS ONE 2013, 8, e67950. [Google Scholar] [CrossRef] [Green Version]
- Stiefel, P.; Mauerhofer, S.; Schneider, J.; Maniura-Weber, K.; Rosenberg, U.; Ren, Q. Enzymes Enhance Biofilm Removal Efficiency of Cleaners. Antimicrob. Agents Chemother. 2016, 60, 3647–3652. [Google Scholar] [CrossRef] [Green Version]
- Libardo, M.D.J.; Bahar, A.A.; Ma, B.; Fu, R.; McCormick, L.E.; Zhao, J.; McCallum, S.A.; Nussinov, R.; Ren, D.; Angeles-Boza, A.M.; et al. Nuclease Activity Gives an Edge to Host-Defense Peptide Piscidin 3 over Piscidin 1, Rendering It More Effective against Persisters and Biofilms. FEBS J. 2017, 284, 3662–3683. [Google Scholar] [CrossRef] [Green Version]
- Hayden, R.M.; Goldberg, G.K.; Ferguson, B.M.; Schoeneck, M.W.; Libardo, M.D.J.; Mayeux, S.E.; Shrestha, A.; Bogardus, K.A.; Hammer, J.; Pryshchep, S.; et al. Complementary Effects of Host Defense Peptides Piscidin 1 and Piscidin 3 on DNA and Lipid Membranes: Biophysical Insights into Contrasting Biological Activities. J. Phys. Chem. B 2015, 119, 15235–15246. [Google Scholar] [CrossRef] [PubMed]
- Harford, C.; Sarkar, B. Amino Terminal Cu(II)- and Ni(II)-Binding (ATCUN) Motif of Proteins and Peptides: Metal Binding, DNA Cleavage, and Other Properties. Accounts Chem. Res. 1997, 30, 123–130. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greer, H.M.; Overton, K.; Ferguson, M.A.; Spain, E.M.; Darling, L.E.O.; Núñez, M.E.; Volle, C.B. Extracellular Polymeric Substance Protects Some Cells in an Escherichia coli Biofilm from the Biomechanical Consequences of Treatment with Magainin 2. Microorganisms 2021, 9, 976. https://doi.org/10.3390/microorganisms9050976
Greer HM, Overton K, Ferguson MA, Spain EM, Darling LEO, Núñez ME, Volle CB. Extracellular Polymeric Substance Protects Some Cells in an Escherichia coli Biofilm from the Biomechanical Consequences of Treatment with Magainin 2. Microorganisms. 2021; 9(5):976. https://doi.org/10.3390/microorganisms9050976
Chicago/Turabian StyleGreer, Helen M., Kanesha Overton, Megan A. Ferguson, Eileen M. Spain, Louise E. O. Darling, Megan E. Núñez, and Catherine B. Volle. 2021. "Extracellular Polymeric Substance Protects Some Cells in an Escherichia coli Biofilm from the Biomechanical Consequences of Treatment with Magainin 2" Microorganisms 9, no. 5: 976. https://doi.org/10.3390/microorganisms9050976





