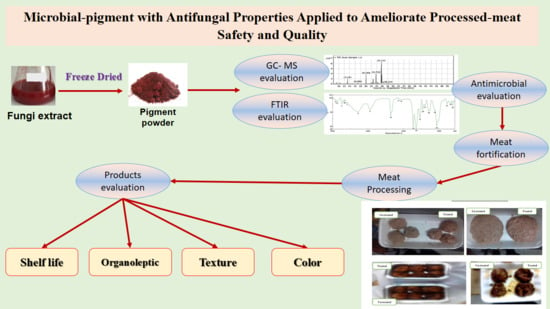
New Antifungal Microbial Pigment Applied to Improve Safety and Quality of Processed Meat-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Pigments Extraction
2.3. Determination of Pigment Antimicrobial Properties
2.4. Determination of Pigment Impact on Mycotoxin
2.5. Preparation of Pigment for Toxicity Determination
2.6. Brine Shrimp Lethality Bioassay
2.7. Determination of Citrinin Residues in Pigment
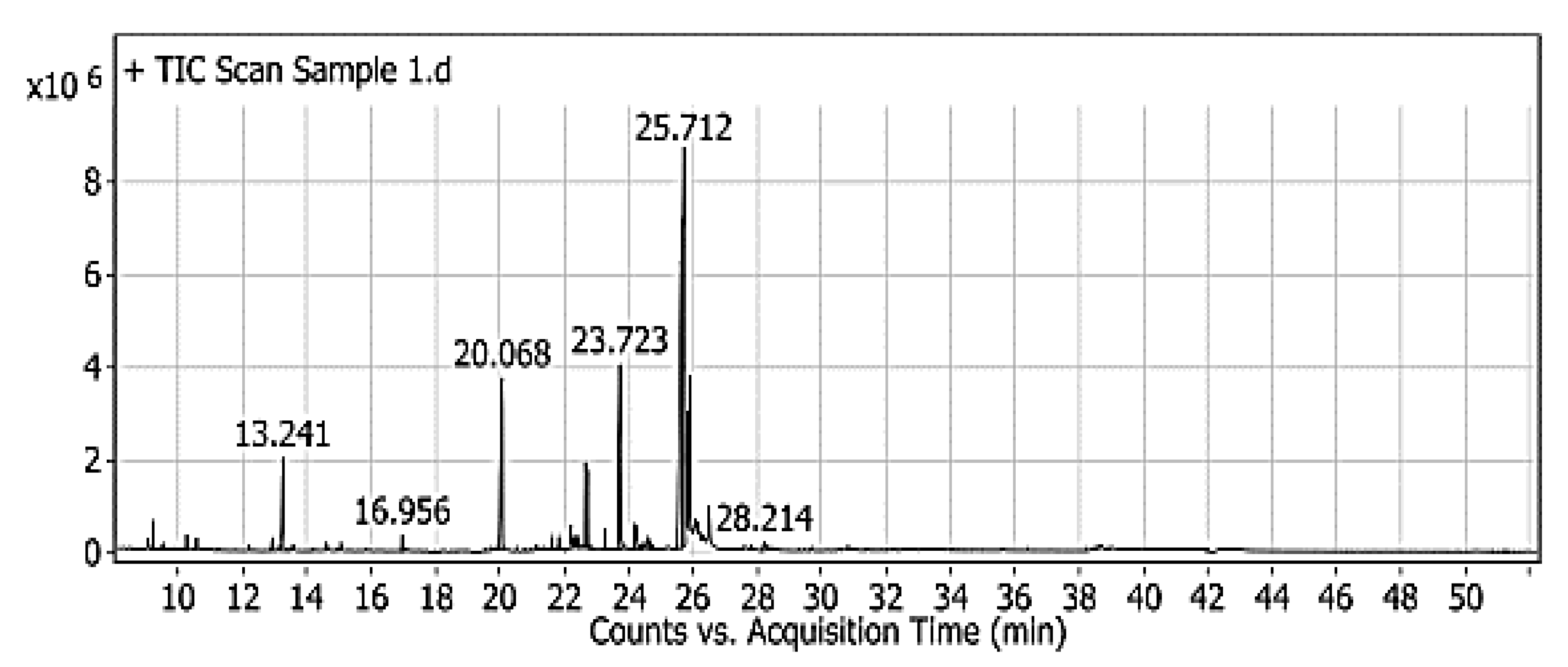
2.8. The Evaluation of Red Pigment by the GC–MS
2.9. Determination of Red-Pigment Content Using the FTIR
2.10. Manufacturing of Meat Products
2.11. Color Measurements of Meat Products
2.12. Texture Properties of Meat Products
2.13. Organoleptic Evaluation for Meat Samples
2.14. Determination of Pigment Impact on the Shelf Life
2.15. Statistical Analysis
3. Results
3.1. Antibacterial and Antifungal Properties of the Red Pigment
3.2. The Impact of Pigment on Mycotoxin Reduction
3.3. Toxicity Assay of Red Pigment
3.4. The Evaluation of Red Pigment by the GC–MS
3.5. Determination of Red-Pigment Content Using the FTIR
3.6. Color Measurements of Meat Products
3.7. Organoleptic Evaluation of Meat Samples
3.8. Determination of Pigment Impact on Meat-Products Shelf Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahid, M.; ul Shahid, I.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Poorniammal, R. Optimization of fermentation conditions for red pigment production from Penicillium sp. under submerged cultivation. Afr. J. Biotechnol. 2008, 7, 1894–1898. [Google Scholar] [CrossRef] [Green Version]
- Honikel, K. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- Mendez, A.; Pérez, C.; Montañéz, J.; Martínez, G.; Aguilar, C. Red pigment production by Penicillium purpurogenum GH2 is influenced by pH and temperature. J. Zhejiang Univ. Sci. B 2011, 12, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Suhr, K.I.; Haasum, I.; Steenstrup, L.D.; Larsen, T.O. Factors Affecting Growth and Pigmentation of Penicillium caseifulvum. J. Dairy Sci. 2002, 85, 2786–2794. [Google Scholar] [CrossRef] [Green Version]
- Dufossé, L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006, 44, 313–323. [Google Scholar]
- Badr, A.N.; Naeem, M.A. Protective efficacy using Cape- golden berry against pre-carcinogenic aflatoxins induced in rats. Toxicol. Rep. 2019, 6, 607–615. [Google Scholar] [CrossRef]
- Wang, S.; Xu, F.; Zhan, J. Introduction of Natural Pigments from Microorganisms. In Bio-Pigmentation and Biotechnological Implementations; Singh, O., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–22. [Google Scholar] [CrossRef]
- Kaynarca, H.; Hecer, C.; Ulusoy, B. Mycotoxin Hazard in Meat and Meat Products. Atatürk Üniv. Vet. Bil. Derg. 2019, 14, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Tungland, B.C.; Meyer, D. Nondigestible Oligo- and Polysaccharides (Dietary Fiber): Their Physiology and Role in Human Health and Food. Compr. Rev. Food Sci. Food Saf. 2002, 1, 90–109. [Google Scholar] [CrossRef]
- Ahmed, E.; Elkhateeb, W.; Abou Taleb, M.; Mowafi, S.; Abdelsalam, I. Wool and Silk Fabrics Dyeing by Mannitol-assisted Pigment Produced from Penicillium purpurogenum. Pharma Chem. 2018, 10, 165–176. [Google Scholar]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Dellavalle, P.D.; Cabrera, A.; Alem, D.; Larrañaga, P.; Ferreira, F.; Dalla Rizza, M. Antifungal activity of medicinal plant extracts against phytopathogenic fungus Alternaria spp. Chil. J. Agric. Res. 2011, 71, 231. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Fattah, S.M.; Badr, A.N.; Seif, F.A.H.A.; Ali, S.M.; Hassan, R.A. Antifungal and anti-mycotoxigenic impact of eco-friendly extracts of wild stevia. J. Biol. Sci. 2018, 18, 488–499. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Razek, A.G.; Badr, A.N.; El-Messery, T.M.; El-Said, M.M.; Hussein, A.M.S. Micro-nano encapsulation of black seed oil ameliorate its characteristics and its mycotoxin inhibition. Biosci. Res. 2018, 15, 2591–2601. [Google Scholar]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78. [Google Scholar] [CrossRef]
- Krishnaraju, A.; Rao, T.; Sundararaju, D.; Vanisree, M.; Tsay, H.; Subbaraju, G. Assessment of bioactivity of Indian medicinal plants using Brine shrimp (Artemia salina) lethality assay. Int. J. Appl. Sci. Eng. 2005, 3, 125–134. [Google Scholar]
- Lalisan Jeda, A.; Nuñeza Olga, M.; Uy Mylene, M. Brine shrimp (Artemia salina) bioassay of the medicinal plant Pseudelephantopus spicatus from Iligan City, Philippines. Int. Res. J. Biol. Sci. 2014, 3, 47–50. [Google Scholar]
- Sharma, N.; Gupta, P.; Singh, A.; Rao, C. Brine shrimp bioassay of Pentapetes phoenicea Linn. and Ipomoea carnea jacq. Leaves. Der Pharm. Lett. 2013, 5, 162–167. [Google Scholar]
- Ahmad, A.; Mujeeb, M.; Panda, B. An HPTLC method for the simultaneous analysis of compactin and citrinin in Penicillium citrinum fermentation broth. JPC J. Planar Chromatogr. Mod. TLC 2010, 23, 282–285. [Google Scholar] [CrossRef]
- Girija, S.; Duraipandiyan, V.; Kuppusamy, P.S.; Gajendran, H.; Rajagopal, R. Chromatographic Characterization and GC-MS Evaluation of the Bioactive Constituents with Antimicrobial Potential from the Pigmented Ink of Loligo duvauceli. Int. Sch. Res. Not. 2014, 2014, 820745. [Google Scholar] [CrossRef]
- Trivedi, N.; Tandon, S.; Dubey, A. Fourier transform infrared spectroscopy (FTIR) profiling of red pigment produced by Bacillus subtilis PD5. Afr. J. Biotechnol. 2017, 16, 1507–1512. [Google Scholar]
- Turhan, S.; Yazici, F.; Saricaoglu, F.; Mortas, M.; Genccelep, H. Evaluation of the nutritional and storage quality of meatballs formulated with bee pollen. Korean J. Food Sci. Anim. Resour. 2014, 34, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essa, R.; Mostafa, S. Utilization of Sugar Beet Pulp in Meatballs Preparation. J. Food Dairy Sci. 2018, 9, 117–119. [Google Scholar] [CrossRef]
- Hunter, G. Conditional probability amplitudes in wave mechanics. Int. J. Quantum Chem. 1975, 9, 237–242. [Google Scholar] [CrossRef]
- Su, S.I.T.; Yoshida, C.M.P.; Contreras-Castillo, C.J.; Quiñones, E.M.; Venturini, A.C. Okara, a soymilk industry by-product, as a non-meat protein source in reduced fat beef burgers. Food Sci. Technol. 2013, 33, 52–56. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, M. Sensory Evaluation of Food: Statistical Methods and Procedures, 1st ed.; Routledge: London, UK, 2017; Volume 1, Available online: https://www.taylorfrancis.com/books/9780203739884 (accessed on 20 April 2021).
- Badr, A.N.; Ali, H.S.; Ahmed, I.S.A.E.; Hussein, A.M.S.; Al-Khalifa, A.R.S. Anti-mycotoxigenic properties of “fino” using the modified zinc-yeast. CYTA J. Food 2019, 17, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A. Novel antifungal bacteriocin from Lactobacillus paracasei KC39 with anti-mycotoxigenic properties. Biosci. Res. 2018, 15, 4171–4183. [Google Scholar]
- Lagashetti, A.; Dufossé, L.; Singh, S.; Singh, P. Fungal Pigments and Their Prospects in Different Industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.; Sivanandhan, G.; Thakare, D. Effect of Physical and Chemical Parameters on the Production of RedExopigment from Penicillium purpurogenum Isolated from Spoilt Onion andStudy of its Antimicrobial Activity. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 599–609. [Google Scholar]
- Farkaš, V.; Vagabov, V.M.; Bauer, Š. Biosynthesis of yeast mannan: Diversity of mannosyltransferases in the mannan-synthesizing enzyme system from yeast. Biochim. Biophys. Acta (BBA) Gen. Subj. 1976, 428, 573–582. [Google Scholar] [CrossRef]
- Mellado-Mojica, E.; López, M.G. Identification, classification, and discrimination of agave syrups from natural sweeteners by infrared spectroscopy and HPAEC-PAD. Food Chem. 2015, 167, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Wang, J.; Yang, Y.; Zhang, Y.; Zhao, C.; Yu, Y.; Wang, S. Comparison of the thermal degradation behaviors and kinetics of palm oil waste under nitrogen and air atmosphere in TGA-FTIR with a complementary use of model-free and model-fitting approaches. J. Anal. Appl. Pyrolysis 2018, 134, 12–24. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Cheng, T.-Y.; Yang, W.-G.; Ma, P.-T.; He, H.-Z.; Yin, X.-C.; Yu, X.-X. Characteristics and environmentally friendly extraction of cellulose nanofibrils from sugarcane bagasse. Ind. Crop. Prod. 2018, 111, 285–291. [Google Scholar] [CrossRef]
- de Assis, T.; Pawlak, J.; Pal, L.; Jameel, H.; Venditti, R.; Reisinger, L.W.; Kavalew, D.; Gonzalez, R.W. Comparison of Wood and Non-Wood Market Pulps for Tissue Paper Application. BioResources 2019, 14, 30. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Tahir, P.M.; Shakeri, A.; SaifulAzry, S.; Makinejad, M.D. Physicochemical characterization of pulp and nanofibers from kenaf stem. Mater. Lett. 2011, 65, 1098–1100. [Google Scholar] [CrossRef]
- Bashir, K.; Kim, J.; An, J.; Sohn, J.; Choi, J. Natural food additives and preservatives for fish-paste products: A review of the past, present, and future states of research. J. Food Qual. 2017, 2017, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Kilcast, D. Organoleptic assessment. In Migration from Food Contact Materials; Katan, L., Ed.; Springer: Boston, MA, USA, 1996; pp. 51–76. [Google Scholar] [CrossRef]
- Heperkan, D. Detecting and controlling mycotoxin contamination of herbs and spices. In Handbook of Herbs and Spices; Peter, K., Ed.; Elsevier: Amsterdam, The Netherlands; Woodhead Publishing: Cambridge, UK, 2006; pp. 3–40. [Google Scholar]
- Shahat, M.S.; Badr, A.N.; Hegaziy, A.I.; Ramzy, S.; Samie, M.A. Reducing the histopathological and biochemical toxicity of aflatoxins contaminated soybean using ozone treatment. Annu. Res. Rev. Biol. 2017, 15. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Shehata, M.G.; Badr, A.N.; Gromadzka, K.; Stępień, L. The effect of chemical composition of wild Opuntia ficus indica byproducts on its nutritional quality, antioxidant and antifungal efficacy. Egypt. J. Chem. 2019, 62, 47–61. [Google Scholar] [CrossRef]
- Bailly, J.-D.; Guerre, P. Mycotoxins in Meat and Processed Meat Products. In Safety of Meat and Processed Meat; Toldrá, F., Ed.; Springer: New York, NY, USA, 2009; pp. 83–124. [Google Scholar] [CrossRef]
- Aziz, N.H.; Youssef, Y.A. Occurrence of aflatoxins and aflatoxin-producing moulds in fresh and processed meat in Egypt. Food Addit. Contam. 1991, 8, 321–331. [Google Scholar] [CrossRef]
- Battilani, P.; Pietri, A.; Giorni, P.; Formenti, S.; Bertuzzi, T.; Toscani, T.; Virgili, R.; Kozakiewicz, Z. Penicillium Populations in Dry-Cured Ham Manufacturing Plants. J. Food Prot. 2007, 70, 975–980. [Google Scholar] [CrossRef]
- Badr, A.N.; Logrieco, A.F.; Amra, H.A.; Hussein, T. Ochratoxin a occurrence on Egyptian wheat during seasons (2009–2014). Asian J. Sci. Res. 2017, 10, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, A.; Rodríguez, M.; Martín, A.; Nuñez, F.; Córdoba, J.J. Evaluation of hazard of aflatoxin B1, ochratoxin A and patulin production in dry-cured ham and early detection of producing moulds by qPCR. Food Control 2012, 27, 118–126. [Google Scholar] [CrossRef]
- Cvetnić, Z.; Pepeljnjak, S. Aflatoxin-producing potential of Aspergillus flavus and Aspergillus parasiticus isolated from samples of smoked-dried meat. Food Nahr. 1995, 39, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Staver, M.M.; Vahčić, N.; Kovačević, D.; Milone, S.; Saftić, L.; Scortichini, G. Survey of aflatoxin B1 and ochratoxin A occurrence in traditional meat products coming from Croatian households and markets. Food Control 2015, 52, 71–77. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Dwivedy, A.K.; Singh, V.K.; Das, S.; Singh, A.; Dubey, N.K. Essential oils and their bioactive compounds as green preservatives against fungal and mycotoxin contamination of food commodities with special reference to their nanoencapsulation. Environ. Sci. Pollut. Res. 2019, 26, 25414–25431. [Google Scholar] [CrossRef]
- Hussien, A.M.S.; Badr, A.N.; Naeem, M.A. Innovative nutritious biscuits limit aflatoxin contamination. Pak. J. Biol. Sci. 2019, 22, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains—Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Butelli, E.; De Stefano, R.; Schoonbeek, H.-j.; Magusin, A.; Pagliarani, C.; Wellner, N.; Hill, L.; Orzaez, D.; Granell, A.; et al. Anthocyanins Double the Shelf Life of Tomatoes by Delaying Overripening and Reducing Susceptibility to Gray Mold. Curr. Biol. 2013, 23, 1094–1100. [Google Scholar] [CrossRef] [Green Version]
- Abdeldaiem, M. Use of yellow pigment extracted from turmeric (Curcuma longa) rhizomes powder as natural food preservative. Am. J. Food Sci. Technol. 2014, 2, 36–47. [Google Scholar] [CrossRef]



| Microorganisms | Inhibition Zone (mm) | |||
|---|---|---|---|---|
| Ethyl Acetate Extract | Chloroform Extract | Iso-Propyl Extract | Methanol Extract | |
| Bacterial strains | ||||
| Bacillus cereus ATCC 4342 | 7.8 ± 0.7 a | 4.1 ± 0.5 d | 8.2 ± 1.0 b | 6.9 ± 1.1 c |
| Staphylococcus aureus NCTC 10788 | 6.4 ± 0.9 a | 3.7 ± 0.4 c | 6.7 ± 0.7 a | 7.1 ± 0.6 b |
| Salmonella typhi ATCC 14028 | 0.5 ± 0.01 a | 0.4 ± 0.02 b | 0.3 ± 0.01 c | 0.3 ± 0.08 c |
| Escherichia coli ATCC 11229 | 0.4 ± 0.03 a | 0.2 ± 0.01 c | 0.3 ± 0.01 b | 0.3 ± 0.01 b |
| Fungal strains | ||||
| Aspergillus flavus ITEM 698 | 1.3 ± 0.4 a | 1.5 ± 0.6 b | 1.8± 0.9 c | 1.6 ± 0.7 b |
| Aspergillus ochraceus ITEM 5010 | 1.8 ± 0.3 a | 1.8 ± 0.7 a | 1.7 ± 0.5 b | 1.9 ± 0.4 a |
| Aspergillus niger ITEM 3856 | 2.2 ± 1.0 a | 2.4 ± 0.9 b | 2.3 ± 0.6 c | 2.3 ± 0.8 c |
| Fusarium culmorum KF846 | 3.2 ± 1.2 a | 3.0 ± 0.7 b | 3.2 ± 1.0 a | 3.5 ± 0.5 c |
| Meatballs | Burgers | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Colored Steamed | Colored Steamed | Non-Colored Fried | Colored Fried | Non-Colored Steamed | Colored Steamed | Non-Colored Fried | Colored Fried | |
| Hardness (N) | 15.38 ± 1.05 a | 14.95 ± 1.48 a | 17.3 ± 1.71 b | 15.05 ± 1.37 a | 14.77 ± 1.41 a | 13.4 ± 1.36 b | 15.34 ± 1.22 a | 11.16 ± 1.27 d |
| Springiness (Mm) | 0.68 ± 0.13 a | 0.82 ± 0.14 b | 0.75 ± 0.27 c | 0.9 ± 0.21 d | 1.13 ± 0.04 a | 1.24 ± 0.03 b | 1.9 ± 0.88 c | 2.01 ± 0.93 d |
| Cohesiveness | 0.63 ± 0.11 a | 0.85 ± 0.24 b | 0.7 ± 0.06 a | 0.81 ± 0.18 b | 0.75 ± 0.37 a | 0.62 ± 0.18 a | 0.63 ± 0.17 a | 0.8 ± 0.16 b |
| Chewiness (mJ) | 7.12 ± 0.97 a | 7.3 ± 1.67 a | 7.2 ± 0.54 a | 7.91 ± 0.93 a | 7.9 ± 1.14 a | 8.45 ± 1.26 a | 9.5 ± 1.02 b | 9.7 ± 1.16 b |
| Gumminess (N) | 6.68 ± 1.09 a | 6.13 ± 1.51 a | 8.43 ± 0.87 b | 8.26 ± 1.08 b | 29.6 ± 1.64 a | 25.1 ± 1.81 b | 27.3 ± 1.48 c | 22.9 ± 1.54 d |
| L * | 45.12 ± 1.05 a | 36.33 ± 1.36 b | 45.15 ± 1.22 a | 31.36 ± 1.41 c | 40.12 ± 1.79 a | 34.16 ± 1.59 b | 36.63 ± 1.81 c | 30.88 ± 1.54 d |
| a * | 14.78 ± 1.21 a | 25.91 ± 1.15 b | 12.57 ± 1.26 a | 27.87 ± 1.84 c | 14.37 ± 1.09 a | 24.88 ± 1.37 b | 13.24 ± 1.22 a | 25.41 ± 1.37 b |
| b * | 12.27 ± 1.74 a | 27.24 ± 1.63 c | 14.82 ± 1.27 a | 19.42 ± 1.66 b | 17.31 ± 1.54 a | 27.43 ± 1.28 d | 13.27 ± 1.07 b | 20.55 ± 1.93 c |
| Samples | Color (20) | Shape (15) | Texture (15) | Aroma (15) | Taste (15) | Mouthfeel (15) |
|---|---|---|---|---|---|---|
| Meatballs | ||||||
| Steamed non-colored products | 17.4 a | 13.3 a | 13.1 a | 13.8 a | 13.2 a | 14.4 a |
| Steamed colored products | 19.6 b | 13.7 a | 13.3 b | 13.9 a | 13.2 a | 14.5 a |
| Fried non-colored products | 16.7 a | 13.4 a | 11.1 c | 14.2 b | 13.8 b | 14.2 a |
| Fried colored products | 19.5 b | 14.1 b | 11.5 d | 14.3 b | 14.4 c | 14.6 a |
| LSD at 0.05 | 1.185 | 0.701 | 0.241 | 0.522 | 0.628 | 0.819 |
| Burgers | ||||||
| Steamed non-colored products | 18.2 a | 12.8 a | 13.8 a | 13.7 a | 13.5 a | 14.2 a |
| Steamed colored products | 19.8 b | 14.3 c | 14.5 b | 13.8 a | 13.6 a | 14.6 b |
| Fried non-colored products | 18.1 a | 13.5 b | 13.9 a | 14.2 b | 14.1 b | 14.3 a |
| Fried colored products | 19.7 b | 14.5 c | 14.7 c | 14.3 b | 14.2 b | 14.5 b |
| LSD at 0.05 | 1.573 | 0.491 | 0.197 | 0.218 | 0.257 | 0.305 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, H.A.; Badr, A.N.; Elkhadragy, M.F.; Hussein, A.M.S.; Shaban, I.A.-S.; Yehia, H.M. New Antifungal Microbial Pigment Applied to Improve Safety and Quality of Processed Meat-Products. Microorganisms 2021, 9, 989. https://doi.org/10.3390/microorganisms9050989
Salama HA, Badr AN, Elkhadragy MF, Hussein AMS, Shaban IA-S, Yehia HM. New Antifungal Microbial Pigment Applied to Improve Safety and Quality of Processed Meat-Products. Microorganisms. 2021; 9(5):989. https://doi.org/10.3390/microorganisms9050989
Chicago/Turabian StyleSalama, Hatem Ali, Ahmed Noah Badr, Manal F. Elkhadragy, Ahmed Mohamed Said Hussein, Ibrahim Abdel-Salam Shaban, and Hany M. Yehia. 2021. "New Antifungal Microbial Pigment Applied to Improve Safety and Quality of Processed Meat-Products" Microorganisms 9, no. 5: 989. https://doi.org/10.3390/microorganisms9050989
APA StyleSalama, H. A., Badr, A. N., Elkhadragy, M. F., Hussein, A. M. S., Shaban, I. A.-S., & Yehia, H. M. (2021). New Antifungal Microbial Pigment Applied to Improve Safety and Quality of Processed Meat-Products. Microorganisms, 9(5), 989. https://doi.org/10.3390/microorganisms9050989