Novel Plant-Associated Acidobacteria Promotes Growth of Common Floating Aquatic Plants, Duckweeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Bacteria from Wild Duckweeds
2.2. Preparation of Bacterial Inoculants
2.3. Preparation of Aseptic Duckweeds
2.4. Co-Cultivation of Bacteria and Duckweeds
2.5. Microscopic Observations
2.6. Assays on Bacterial Plant Growth Promoting Properties
2.7. Measurement of Chlorophyll
2.8. Genome Analysis of Isolated Acidobacteria Strains
3. Results
3.1. Isolation of Novel Acidobacteria Strains
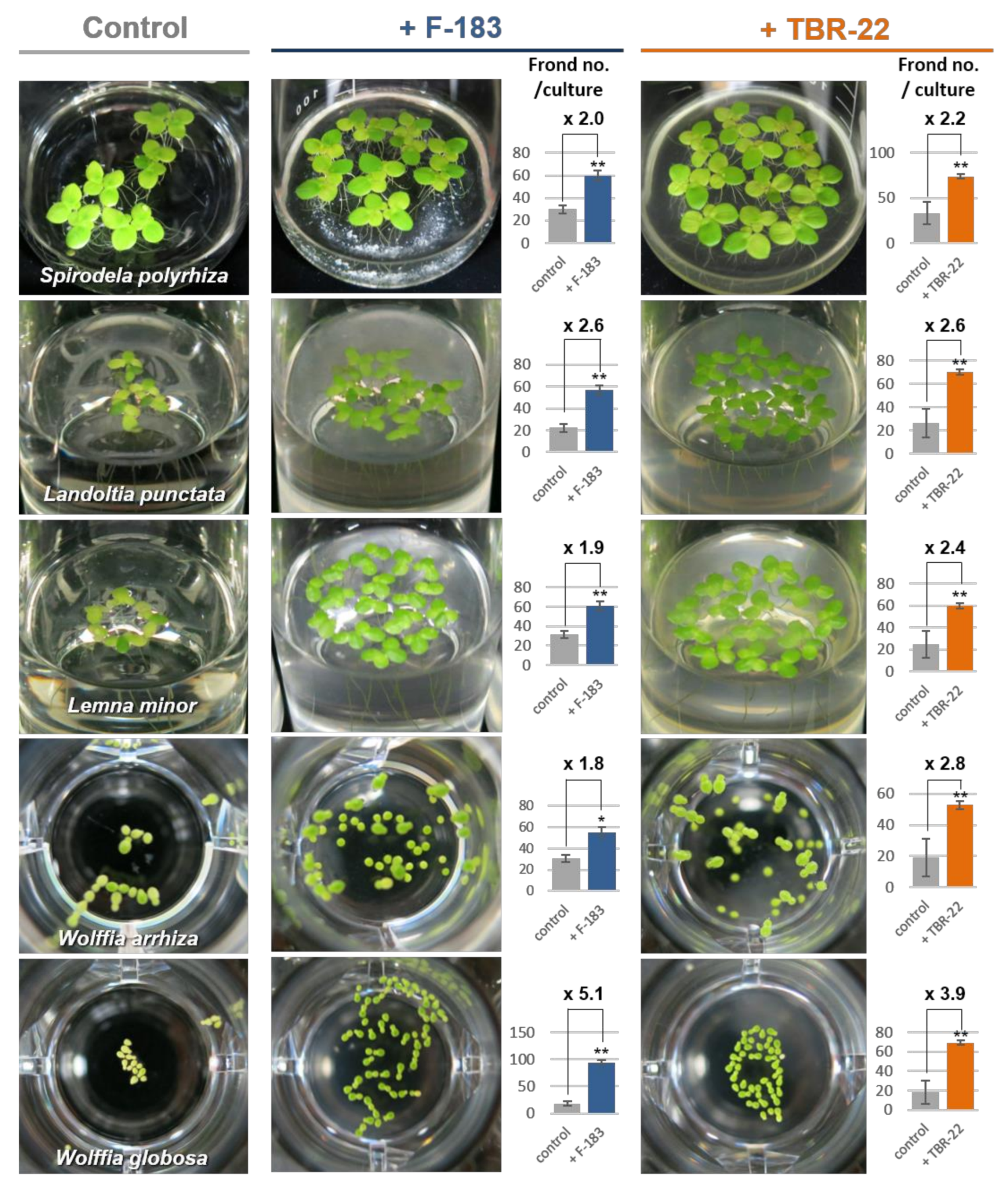
3.2. Plant Growth Promotion by the Novel Acidobacteria Strains
3.3. Acidobacteria Strains Colonization of Duckweed Plant Surface
3.4. Assays on Bacterial Plant Growth Promoting Traits
3.5. PGP-Related Genes in the Genomes of the Isolated Acidobacteria Strains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bashan, Y.; Holguin, G. Proposal for the Division of Plant Growth-Promoting Rhizobacteria into Two Classifications: Biocontrol-PGPB (Plant Growth-Promoting Bacteria) and PGPB. Soil Biol. Biochem. 1998, 30, 1225–1228. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, M.D.V.B.; Seldin, L.; de Araujo, F.F.; Mariano, R.D.L.R. Plant Growth Promoting Rhizobacteria: Fundamentals and Applications. In Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 21–43. ISBN 978-3-642-13611-5. [Google Scholar]
- Maheshwari, D.K. (Ed.) Bacteria in Agrobiology: Plant Nutrient Management; Bacteria in Agrobiology; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-21060-0. [Google Scholar]
- Leng, R. Duckweed: A Tiny Aquatic Plant with Enormous Potential for Agriculture and Environment. Available online: http://www.fao.org/ag/againfo/resources/documents/DW/Dw2.htm (accessed on 19 August 2018).
- Verma, R.; Suthar, S. Utility of Duckweeds as Source of Biomass Energy: A Review. Bioenergy Res. 2015, 8, 1589–1597. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional Value of Duckweeds (Lemnaceae) as Human Food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Borisjuk, N.; Lam, E. Telling Duckweed Apart: Genotyping Technologies for the Lemnaceae. Chin. J. Appl. Environ. Biol. 2013, 19, 1–10. [Google Scholar] [CrossRef]
- Sree, K.S.; Bog, M.; Appenroth, K.-J. Taxonomy of Duckweeds (Lemnaceae), Potential New Crop Plants. Emir. J. Food Agric. 2016, 28, 291. [Google Scholar] [CrossRef] [Green Version]
- Bradford, K.; Bewley, J. Seeds: Biology, technology and role in agriculture. In Plants, Genes and Crop Biotechnology; Chrispeels, M., Sadava, D., Eds.; Jones and Bartlett Publishers: Burlington, MA, USA, 2002; pp. 210–239. [Google Scholar]
- Yamaga, F.; Washio, K.; Morikawa, M. Sustainable Biodegradation of Phenol by Acinetobacter calcoaceticus P23 Isolated from the Rhizosphere of Duckweed Lemna Aoukikusa. Environ. Sci. Technol. 2010, 44, 6470–6474. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, W.; Sugawara, M.; Miwa, K.; Morikawa, M. Plant Growth-Promoting Bacterium Acinetobacter calcoaceticus P23 Increases the Chlorophyll Content of the Monocot Lemna minor (Duckweed) and the Dicot Lactuca Sativa (Lettuce). J. Biosci. Bioeng. 2014, 118, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, Y.; Cui, Y.; Ma, J. Effects of a Rhizobacterium on the Growth of and Chromium Remediation by Lemna minor. Environ. Sci. Pollut. Res. 2015, 22, 9686–9693. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, H.; Kuroda, M.; Morikawa, M.; Ike, M. Evaluation of Environmental Bacterial Communities as a Factor Affecting the Growth of Duckweed Lemna minor. Biotechnol. Biofuels 2017, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakawa, Y.; Jog, R.; Morikawa, M. Effects of Co-Inoculation of Two Different Plant Growth-Promoting Bacteria on Duckweed. Plant Growth Regul. 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The Ecology of Acidobacteria: Moving beyond Genes and Genomes. Front. Microbiol. 2016, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barns, S.M.; Cain, E.C.; Sommerville, L.; Kuske, C.R. Acidobacteria Phylum Sequences in Uranium-Contaminated Subsurface Sediments Greatly Expand the Known Diversity within the Phylum. Appl. Environ. Microbiol. 2007, 73, 3113–3116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugenholtz, P.; Goebel, B.M.; Pace, N.R. Impact of Culture-Independent Studies on the Emerging Phylogenetic View of Bacterial Diversity. J. Bacteriol. 1998, 180, 4765–4774. [Google Scholar] [CrossRef] [Green Version]
- Barns, S.M.; Takala, S.L.; Kuske, C.R. Wide Distribution and Diversity of Members of the Bacterial Kingdom Acidobacterium in the Environment. Appl. Environ. Microbiol. 1999, 65, 1731–1737. [Google Scholar] [CrossRef] [Green Version]
- Ciccarelli, F.D.; Doerks, T.; von Mering, C.; Creevey, C.J.; Snel, B.; Bork, P. Toward Automatic Reconstruction of a Highly Resolved Tree of Life. Science 2006, 311, 1283–1287. [Google Scholar] [CrossRef] [Green Version]
- Janssen, P.H. Identifying the Dominant Soil Bacterial Taxa in Libraries of 16S rRNA and 16S rRNA Genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef] [Green Version]
- Ross, K.A.; Feazel, L.M.; Robertson, C.E.; Fathepure, B.Z.; Wright, K.E.; Turk-MacLeod, R.M.; Chan, M.M.; Held, N.L.; Spear, J.R.; Pace, N.R. Phototrophic Phylotypes Dominate Mesothermal Microbial Mats Associated with Hot Springs in Yellowstone National Park. Microb. Ecol. 2012, 64, 162–170. [Google Scholar] [CrossRef]
- Wilhelm, R.C.; Niederberger, T.D.; Greer, C.; Whyte, L.G. Microbial Diversity of Active Layer and Permafrost in an Acidic Wetland from the Canadian High Arctic. Can. J. Microbiol. 2011, 57, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sheng, H.-F.; He, Y.; Wu, J.-Y.; Jiang, Y.-X.; Tam, N.F.-Y.; Zhou, H.-W. Comparison of the Levels of Bacterial Diversity in Freshwater, Intertidal Wetland, and Marine Sediments by Using Millions of Illumina Tags. Appl. Environ. Microbiol. 2012, 78, 8264–8271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor-Sánchez, A.; Rivera-Domínguez, A.J.; de los Santos-Briones, C.; López-Aguiar, L.K.; Peña-Ramírez, Y.J.; Prieto-Davo, A. Acidobacteria Appear to Dominate the Microbiome of Two Sympatric Caribbean Sponges and One Zoanthid. Biol. Res. 2014, 47, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Singh, D.; Lai-Hoe, A.; Go, R.; Abdul Rahim, R.; Ainuddin, A.; Chun, J.; Adams, J.M. Distinctive Phyllosphere Bacterial Communities in Tropical Trees. Microb. Ecol. 2012, 63, 674–681. [Google Scholar] [CrossRef]
- Oh, Y.M.; Kim, M.; Lee-Cruz, L.; Lai-Hoe, A.; Go, R.; Ainuddin, N.; Rahim, R.A.; Shukor, N.; Adams, J.M. Distinctive Bacterial Communities in the Rhizoplane of Four Tropical Tree Species. Microb. Ecol. 2012, 64, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Tamaki, H.; Matsuzawa, H.; Nigaya, M.; Mori, K.; Kamagata, Y. Microbial Community Analysis in the Roots of Aquatic Plants and Isolation of Novel Microbes Including an Organism of the Candidate Phylum OP10. Microbes Environ. 2012, 27, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Matsuzawa, H.; Tamaki, H.; Tagawa, M.; Toyama, T.; Kamagata, Y.; Mori, K. Isolation of Novel Bacteria Including Rarely Cultivated Phyla, Acidobacteria and Verrucomicrobia, from the Roots of Emergent Plants by Simple Culturing Method. Microbes Environ. 2017, 32, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Turner, T.R.; James, E.K.; Poole, P.S. The Plant Microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, H.; Sekiguchi, Y.; Hanada, S.; Nakamura, K.; Nomura, N.; Matsumura, M.; Kamagata, Y. Comparative Analysis of Bacterial Diversity in Freshwater Sediment of a Shallow Eutrophic Lake by Molecular and Improved Cultivation-Based Techniques. Appl. Environ. Microbiol. 2005, 71, 2162–2169. [Google Scholar] [CrossRef] [Green Version]
- Hanada, S.; Hiraish, A.; Shimada, K.; Matsuura, K. Chloroflexus aggregans sp. nov., a Filamentous Phototrophic Bacterium Which Forms Dense Cell Aggregates by Active Gliding Movement. Int. J. Syst. Evol. Microbiol. 1995, 45, 676–681. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Kawasaki, K.; Daimon, S.; Kitagawa, W.; Yamamoto, K.; Tamaki, H.; Tanaka, M.; Nakatsu, C.H.; Kamagata, Y. A Hidden Pitfall in the Preparation of Agar Media Undermines Microorganism Cultivability. Appl. Environ. Microbiol. 2014, 80, 7659–7666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gürtler, V.; Stanisich, V.A. New Approaches to Typing and Identification of Bacteria Using the 16S-23S rDNA Spacer Region. Microbiolgy 1996, 142, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzawa, H.; Tanaka, Y.; Tamaki, H.; Kamagata, Y.; Mori, K. Culture-Dependent and Independent Analyses of the Microbial Communities Inhabiting the Giant Duckweed (Spirodela Polyrrhiza) Rhizoplane and Isolation of a Variety of Rarely Cultivated Organisms within the Phylum Verrucomicrobia. Microbes Environ. 2010, 25, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, H.; Tanaka, Y.; Matsuzawa, H.; Muramatsu, M.; Meng, X.-Y.; Hanada, S.; Mori, K.; Kamagata, Y. Armatimonas rosea gen. nov., sp. nov., of a Novel Bacterial Phylum, Armatimonadetes phyl. nov., Formally Called the Candidate Phylum OP10. Int. J. Syst. Evol. Microbiol. 2011, 61, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Milagres, A.M.F.; Machuca, A.; Napoleão, D. Detection of Siderophore Production from Several Fungi and Bacteria by a Modification of Chrome Azurol S (CAS) Agar Plate Assay. J. Microbiol. Methods 1999, 37, 1–6. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida Indoleacetic Acid in Development of the Host Plant Root System. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [Green Version]
- Ueda, T.; Suga, Y.; Yahiro, N.; Matsuguchi, T. Remarkable N2-Fixing Bacterial Diversity Detected in Rice Roots by Molecular Evolutionary Analysis of nifH Gene Sequences. J. Bacteriol. 1995, 177, 1414–1417. [Google Scholar] [CrossRef] [Green Version]
- Poly, F.; Monrozier, L.J.; Bally, R. Improvement in the RFLP Procedure for Studying the Diversity of nifH Genes in Communities of Nitrogen Fixers in Soil. Res. Microbiol. 2001, 152, 95–103. [Google Scholar] [CrossRef]
- Valdés, M.; Pérez, N.-O.; Estrada-de Los Santos, P.; Caballero-Mellado, J.; Peña-Cabriales, J.J.; Normand, P.; Hirsch, A.M. Non-Frankia Actinomycetes Isolated from Surface-Sterilized Roots of Casuarina Equisetifolia Fix Nitrogen. Appl. Environ. Microbiol. 2005, 71, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yoneda, Y.; Makino, A.; Tanaka, Y.; Meng, X.-Y.; Hashimoto, J.; Shinya, K.; Satoh, N.; Fujie, M.; Toyama, T.; et al. Draft Genome Sequence of a Novel Acidobacteria, Bryobacteraceae Species Strain F-183. Microbiol. Resour. Announc. under review.
- Yamamoto, K.; Yoneda, Y.; Makino, A.; Tanaka, Y.; Meng, X.-Y.; Hashimoto, J.; Shinya, K.; Satoh, N.; Fujie, M.; Toyama, T.; et al. Draft Genome Sequence of a Novel Acidobacteria, Luteitalea Species Strain TBR-22. Microbiol. Resour. Announc. under review.
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef] [Green Version]
- NCBI Blastp. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins (accessed on 25 August 2020).
- Lalign Tools. Available online: https://embnet.vital-it.ch/software/LALIGN_form.html (accessed on 25 August 2020).
- Kulichevskaya, I.S.; Suzina, N.E.; Rijpstra, W.I.C.; Damsté, J.S.S.; Dedysh, S.N. Paludibaculum fermentans gen. nov., sp. nov., a Facultative Anaerobe Capable of Dissimilatory Iron Reduction from Subdivision 3 of the Acidobacteria. Int. J. Syst. Evol. Microbiol. 2014, 64, 2857–2864. [Google Scholar] [CrossRef] [Green Version]
- Kulichevskaya, I.S.; Suzina, N.E.; Liesack, W.; Dedysh, S.N. Bryobacter aggregatus gen. nov., sp. nov., a Peat-Inhabiting, Aerobic Chemo-Organotroph from Subdivision 3 of the Acidobacteria. Int. J. Syst. Evol. Microbiol. 2010, 60, 301–306. [Google Scholar] [CrossRef]
- Vieira, S.; Luckner, M.; Wanner, G.; Overmann, J. Luteitalea pratensis gen. nov., sp. nov. a New Member of Subdivision 6 Acidobacteria Isolated from Temperate Grassland Soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 1408–1414. [Google Scholar] [CrossRef]
- Huber, K.J.; Geppert, A.M.; Wanner, G.; Fösel, B.U.; Wüst, P.K.; Overmann, J. The First Representative of the Globally Widespread Subdivision 6 Acidobacteria, Vicinamibacter silvestris gen. nov., sp. nov., Isolated from Subtropical Savannah Soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 2971–2979. [Google Scholar] [CrossRef]
- Toyama, T.; Kuroda, M.; Ogata, Y.; Hachiya, Y.; Quach, A.; Tokura, K.; Tanaka, Y.; Mori, K.; Morikawa, M.; Ike, M. Enhanced Biomass Production of Duckweeds by Inoculating a Plant Growth-Promoting Bacterium, Acinetobacter Calcoaceticus P23, in Sterile Medium and Non-Sterile Environmental Waters. Water Sci. Technol. 2017, 76, 1418–1428. [Google Scholar] [CrossRef] [Green Version]
- Illmer, P.; Barbato, A.; Schinner, F. Solubilization of Hardly-Soluble AlPO4 with P-Solubilizing Microorganisms. Soil Biol. Biochem. 1995, 27, 265–270. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of Phosphate Solubilization and Its Potential Applications for Improving Plant Growth-Promoting Bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Parnell, J.J.; Berka, R.; Young, H.A.; Sturino, J.M.; Kang, Y.; Barnhart, D.M.; DiLeo, M.V. From the Lab to the Farm: An Industrial Perspective of Plant Beneficial Microorganisms. Front. Plant Sci. 2016, 7, 1110. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.E.; Triplett, L.R.; Argueso, C.T.; Trivedi, P. Communication in the Phytobiome. Cell 2017, 169, 587–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and Exploiting Plant Beneficial Microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Kielak, A.M.; Cipriano, M.A.P.; Kuramae, E.E. Acidobacteria Strains from Subdivision 1 Act as Plant Growth-Promoting Bacteria. Arch. Microbiol. 2016, 198, 987–993. [Google Scholar] [CrossRef] [Green Version]
- Zamioudis, C.; Pieterse, C.M.J. Modulation of Host Immunity by Beneficial Microbes. Mol. Plant Microbe Interact. 2012, 25, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Utami, D.; Kawahata, A.; Sugawara, M.; Jog, R.N.; Miwa, K.; Morikawa, M. Effect of Exogenous General Plant Growth Regulators on the Growth of the Duckweed Lemna minor. Front. Chem. 2018, 6, 251. [Google Scholar] [CrossRef]
- Gilbert, S.; Xu, J.; Acosta, K.; Poulev, A.; Lebeis, S.; Lam, E. Bacterial Production of Indole Related Compounds Reveals Their Role in Association Between Duckweeds and Endophytes. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Subba-Rao, N.S.; Mateos, P.F.; Baker, D.; Pankratz, H.S.; Palma, J.; Dazzo, F.B.; Sprent, J.I. The Unique Root-Nodule Symbiosis between Rhizobium and the Aquatic Legume, Neptunia natans (L. f.) Druce. Planta 1995, 196, 311–320. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Kumar De, T.; Maiti, T.K. Production and Metabolism of Indole Acetic Acid in Root Nodules and Symbiont (Rhizobium Undicola) Isolated from Root Nodule of Aquatic Medicinal Legume Neptunia Oleracea Lour. J. Bot. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Rang, L.; Bergman, B. Structural Characteristics of the Cyanobacterium–Azolla Symbioses. In Prokaryotic Symbionts in Plants; Pawlowski, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 235–263. ISBN 978-3-540-75459-6. [Google Scholar]
- Seneviratne, G.; Weerasekara, M.L.M.A.W.; Seneviratne, K.A.C.N.; Zavahir, J.S.; Kecskés, M.L.; Kennedy, I.R. Importance of Biofilm Formation in Plant Growth Promoting Rhizobacterial Action. In Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 81–95. [Google Scholar]
- Govind, G.; Parihar, S.S.; Singh, S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant Growth Promoting Rhizobacteria (PGPR): Current and Future Prospects for Development of Sustainable Agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, J.A.; Vilgalys, R.; Jackson, R.B. Assessment of Soil Microbial Community Structure by Use of Taxon-Specific Quantitative PCR Assays. Appl. Environ. Microbiol. 2005, 71, 4117–4120. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward An Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Kielak, A.; Pijl, A.S.; van Veen, J.A.; Kowalchuk, G.A. Phylogenetic Diversity of Acidobacteria in a Former Agricultural Soil. ISME J. 2009, 3, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A Comprehensive Survey of Soil Acidobacterial Diversity Using Pyrosequencing and Clone Library Analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Foesel, B.U.; Nägele, V.; Naether, A.; Wüst, P.K.; Weinert, J.; Bonkowski, M.; Lohaus, G.; Polle, A.; Alt, F.; Oelmann, Y.; et al. Determinants of Acidobacteria Activity Inferred from the Relative Abundances of 16S rRNA Transcripts in German Grassland and Forest Soils. Environ. Microbiol. 2014, 16, 658–675. [Google Scholar] [CrossRef]
- Schreiter, S.; Ding, G.-C.; Heuer, H.; Neumann, G.; Sandmann, M.; Grosch, R.; Kropf, S.; Smalla, K. Effect of the Soil Type on the Microbiome in the Rhizosphere of Field-Grown Lettuce. Front. Microbiol. 2014, 5, 144. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoneda, Y.; Yamamoto, K.; Makino, A.; Tanaka, Y.; Meng, X.-Y.; Hashimoto, J.; Shin-ya, K.; Satoh, N.; Fujie, M.; Toyama, T.; et al. Novel Plant-Associated Acidobacteria Promotes Growth of Common Floating Aquatic Plants, Duckweeds. Microorganisms 2021, 9, 1133. https://doi.org/10.3390/microorganisms9061133
Yoneda Y, Yamamoto K, Makino A, Tanaka Y, Meng X-Y, Hashimoto J, Shin-ya K, Satoh N, Fujie M, Toyama T, et al. Novel Plant-Associated Acidobacteria Promotes Growth of Common Floating Aquatic Plants, Duckweeds. Microorganisms. 2021; 9(6):1133. https://doi.org/10.3390/microorganisms9061133
Chicago/Turabian StyleYoneda, Yasuko, Kyosuke Yamamoto, Ayaka Makino, Yasuhiro Tanaka, Xian-Ying Meng, Junko Hashimoto, Kazuo Shin-ya, Noriyuki Satoh, Manabu Fujie, Tadashi Toyama, and et al. 2021. "Novel Plant-Associated Acidobacteria Promotes Growth of Common Floating Aquatic Plants, Duckweeds" Microorganisms 9, no. 6: 1133. https://doi.org/10.3390/microorganisms9061133
APA StyleYoneda, Y., Yamamoto, K., Makino, A., Tanaka, Y., Meng, X. -Y., Hashimoto, J., Shin-ya, K., Satoh, N., Fujie, M., Toyama, T., Mori, K., Ike, M., Morikawa, M., Kamagata, Y., & Tamaki, H. (2021). Novel Plant-Associated Acidobacteria Promotes Growth of Common Floating Aquatic Plants, Duckweeds. Microorganisms, 9(6), 1133. https://doi.org/10.3390/microorganisms9061133





