The Role of Mesenchymal Stem Cells in the Treatment of a Chronic Rhinosinusitis—An In Vivo Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Animals and Housing
2.1.2. Procedure and Data Collection
CRS Mouse Model
MSC Isolation and Culture
MSC Allograft in the CRS Mouse Model
Histological Analysis–CRS Mouse Model
Histological Analysis after MSC Exposure
Confocal Scanning Laser Microscopy (CSLM) Analysis
Statistical Analysis
3. Results
3.1. CRS Mouse Model: Histological Analysis
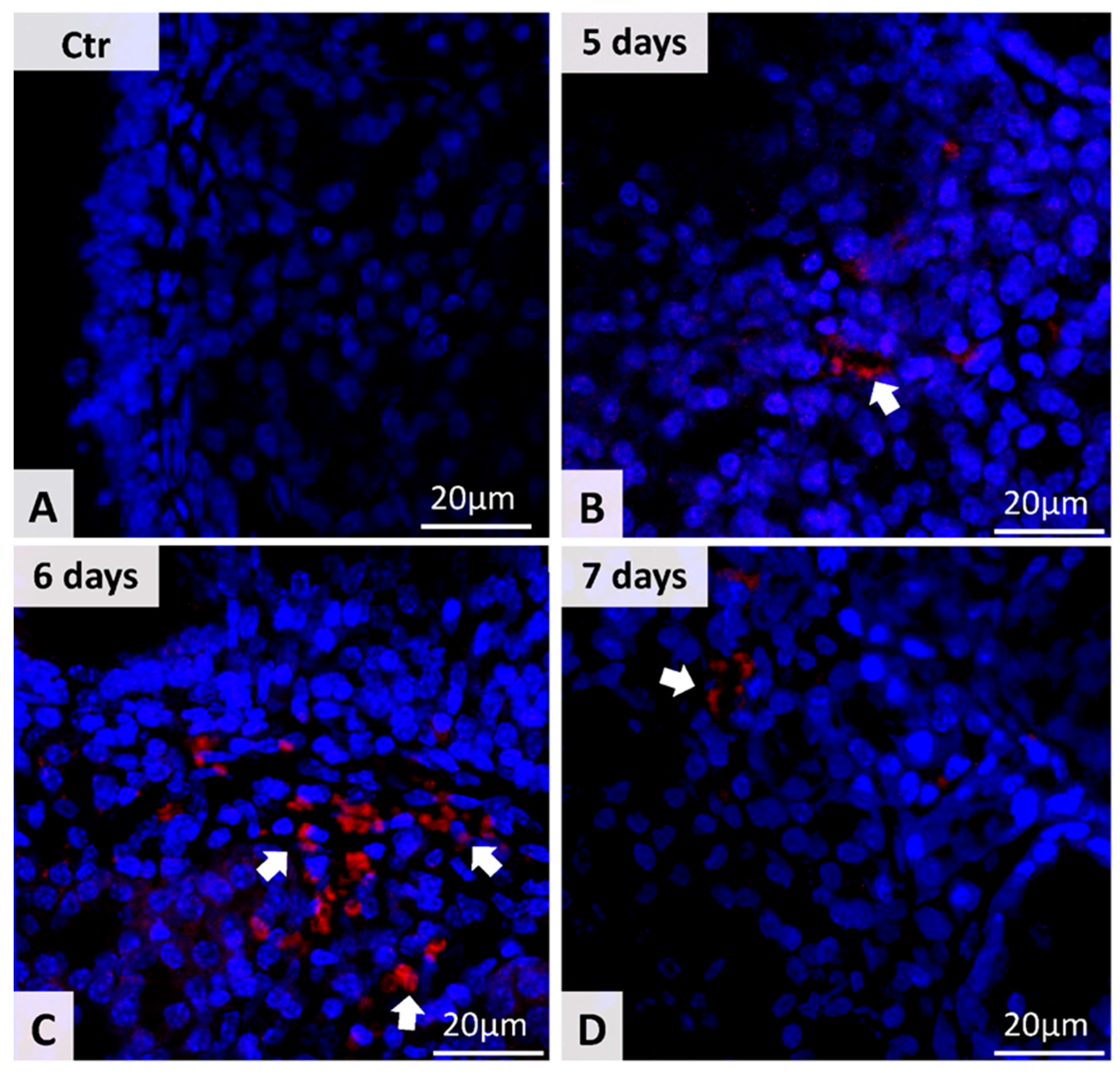
3.2. Detection of MSC in Nasal Mucosa
3.3. CRS Evolution after the MSC Administration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zomer, H.D.; Vidane, A.S.; Gonçalves, N.N.; Ambrósio, C.E. Mesenchymal and induced pluripotent stem cells: General insights and clinical perspectives. Stem Cells Cloning 2015, 28, 125–134. [Google Scholar] [CrossRef]
- Webster, R.A.; Blaber, S.P.; Herbert, B.R.; Wilkins, M.R.; Vesey, G. The role of mesenchymal stem cells in veterinary therapeutics—A review. N. Z. Vet. J. 2012, 60, 265–272. [Google Scholar] [CrossRef]
- Lindsay, R.; Slaughter, T.; Britton-Webb, J.; Mog, S.R.; Conran, R.; Tadros, M.; Earl, N.; Fox, D.; Roberts, J.; Bolger, W.E. Development of a murine model of chronic rhinosinusitis. Otolaryngol. Head Neck Surg. 2006, 134, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Tansavatdi, K.P.; McGill, L.; Riggs, S.; Orlandi, R.R. Development of an animal model for wound healing in chronic rhinosinusitis. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Park, H.K.; Park, H.Y.; Jung, J.S.; Jeon, S.G.; Kim, Y.K.; Roh, H.J. IFATS collection, immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells 2009, 27, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Carol, K.; William, B.; Innes, C.C.; Michael, E.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar]
- Park, S.C.; Kim, S.I.; Hwang, C.S.; Cho, H.J.; Yoon, J.H.; Kim, C.H. Multiple airborne allergen-induced eosinophilic chronic rhinosinusitis murine model. Eur. Arch. Otorhinolaryngol. 2019, 276, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.N.; Woodworth, B.A.; Prince, A.; Quraishi, S.A.; Antunes, M.B.; Long, F.H.; Bolger, W.E.; Chiu, A.G.; Palmer, J.N.; Cohen, N.A. Physiologic alterations in the murine model after nasal fungal antigenic exposure. Otolaryngol. Head Neck Surg. 2008, 139, 695–701. [Google Scholar] [CrossRef]
- Schieker, M.; Pautke, C.; Reitz, K.; Hemraj, I.; Neth, P.; Mutschler, W.; Milz, S. The use of four-colour immunofluorescence techniques to identify mesenchymal stem cells. J. Anat. 2004, 204, 133–139. [Google Scholar] [CrossRef]
- Yardeni, T.; Eckhaus, M.; Morris, D.; Huizing, M.; Hoogstraten-Miller, S. Retro-orbital injections in mice. Lab. Anim. 2011, 40, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Gocea, A.; Taulescu, M.; Trombitas, V.; Albu, S. Effects of cryotherapy on the maxillary antrostomy patency in a rabbit model of chronic rhinosinusitis. BioMed Res. Int. 2013, 2013, 101534. [Google Scholar] [CrossRef]
- Trombitaș, V.; Zolog, A.; Toader, M.; Albu, S. Maxillary antrostomy patency following intraoperative use of spray cryotherapy. J. Clin. Med. 2019, 9, 88. [Google Scholar] [CrossRef]
- Renne, R.; Brix, A.; Harkema, J.; Herbert, R.; Kittel, B.; Lewis, D.; March, T.; Nagano, K.; Pino, M.; Rittinghausen, S.; et al. Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicol. Pathol. 2009, 37, 5S–73S. [Google Scholar] [CrossRef]
- Ramos, M.F.; Baker, J.; Atzpodien, E.A.; Bach, U.; Brassard, J.; Cartwright, J.; Farman, C.; Fishman, C.; Jacobsen, M.; Junker-Walker, U.; et al. Nonproliferative and proliferative lesions of the ratand mouse special sense organs (Ocular [eye and glands], olfactory and otic). J. Toxicol. Pathol. 2018, 31 (Suppl. 3), 97S–214S. [Google Scholar] [CrossRef]
- Ocampo, C.J.; Peters, A.T. Antibody deficiency in chronic rhinosinusitis: Epidemiology and burden of illness. Am. J. Rhinol. Allergy 2013, 27, 34–38. [Google Scholar] [CrossRef]
- Wang, D.Y.; Li, Y.; Yan, Y.; Li, C.; Shi, L. Upper airway stem cells: Understanding the nose and role for future cell therapy. Curr. Allergy Asthma Rep. 2015, 15, 490. [Google Scholar] [CrossRef]
- Yu, F.; Zhao, X.; Li, C.; Li, Y.; Yan, Y.; Shi, L.; Gordon, B.R.; Wang, D.Y. Airway stem cells: Review of potential impact on understanding of upper airway diseases. Laryngoscope 2012, 122, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020, 20 (Suppl. S29), 1–464. [Google Scholar] [CrossRef]
- Ba, L.; Du, J.; Liu, F.; Yang, F.; Han, M.; Liu, S.; Lin, P.; Li, H. Distinct inflammatory profiles in atopic and nonatopic patients with chronic rhinosinustis accompanied by nasal polyps in Western china. Allergy Asthma Immunol. Res. 2015, 7, 346–358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kosugi, E.M.; Moussalem, G.F.; Simões, J.C.; de Souza, R.P.; Chen, V.G.; Saraceni Neto, P.; Mendes Neto, J.A. Topical therapy with high-volume budesonide nasal irrigations in difficult-to-treat chronic rhinosinusitis. Braz. J. Otorhinolaryngol. 2016, 82, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Palade, O.D.; Severin, F.; Toader, M.; Cobzeanu, M.D.; Toader, C. combined approach for large tumors of the nose and paranasal sinuses—Case report. Rev. Med. Chir. Soc. Med. Nat. Iasi 2016, 120, 380–383. [Google Scholar]
- Albu, S.; Trombitas, V.; Vlad, D.; Emanuelli, E. The influence of spray cryotherapy on wound healing following endoscopic sinus surgery in chronic rhinosinusitis. Laryngoscope 2016, 126, 25–32. [Google Scholar] [CrossRef]
- Van Drunen, C.M.; Reinartz, S.; Wigman, J.; Fokkens, W.J. Inflammation in chronic rhinosinusitis and nasal polyposis. Immunol. Allergy Clin. N. Am. 2009, 29, 621–629. [Google Scholar] [CrossRef]
- Van Zele, T.; Claeys, S.; Gevaert, P.; Van Maele, G.; Holtappels, G.; Van Cauwenberge, P.; Bachert, C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 2006, 61, 1280–1289. [Google Scholar] [CrossRef]
- Huvenne, W.; van Bruaene, N.; Zhang, N.; van Zele, T.; Patou, J.; Gevaert, P.; Claeys, S.; Van Cauwenberge, P.; Bacher, C. Chronic rhinosinusitis with and without nasal polyps: What is the difference? Curr. Allergy Asthma Rep. 2009, 9, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Van Zele, T.; Perez-Novo, C.; Van Bruaene, N.; Holtappels, G.; DeRuyck, N.; Van Cauwenberge, P.; Bachert, C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J. Allergy Clin. Immunol. 2008, 122, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.S. Allergic fungal sinusitis. Med. Mycol. 2009, 47, S324–S330. [Google Scholar] [CrossRef] [PubMed]
- Monroe, M.M.; McLean, M.; Sautter, N.; Wax, M.K.; Andersen, P.E.; Smith, T.L.; Gross, N.D. Invasive fungal rhinosinusitis: A 15-year experience with 29 patients. Laryngoscope 2013, 123, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhao, Z.; Wan, H.; Wu, R.; Fang, J.; Liu, H. A novel fungus concentration-dependent rat model for acute invasive fungal rhinosinusitis: An experimental study. BMC Infect Dis. 2014, 20, 3856. [Google Scholar] [CrossRef]
- Gupta, R.P.; Bahadur, S.; Thakar, A.; Handa, K.K.; Sarkaar, C. Management protocols of allergic fungal sinusitis. Indian J. Otolaryngol. Head Neck Surg. 2007, 59, 35–40. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Griffin, M.D.; Ryan, A.E.; Alagesan, S.; Lohan, P.; Treacy, O.; Ritter, T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: What have we learned so far? Immunol. Cell Biol. 2013, 91, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Oh, J.Y. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef]
- Pezato, R.; de Almeida, D.C.; Bezerra, T.F.; Silva Fde, S.; Perez-Novo, C.; Gregorio, L.C. Immunoregulatory effects of bone marrow-derived mesenchymal stem cells in the nasal polypmicroenvironment. Mediat. Inflamm. 2014, 2014, 583409. [Google Scholar] [CrossRef]
- Cho, K.S.; Kim, Y.W.; Kang, M.J.; Park, H.Y.; Hong, S.L.; Roh, H.J. Immunomodulatory effect of mesenchymal stem cells on t lymphocyte and cytokine expression in nasal polyps. Otolaryngol. Head Neck Surg. 2014, 150, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Vlastos, I.; Gkouskou, K.; Doulaptsi, M.; Karatzanis, A.; Prokopakis, E.P. Precision Medicine in Rhinosinusitis. Curr. Allergy Asthma Rep. 2019, 19, 12. [Google Scholar] [CrossRef]
- Black, L.L.; Gaynor, J.; Gahring, D.; Adams, C.; Aron, D.; Harman, S.; Gingerich, D.A.; Harman, R. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: A randomized, double-blinded, multicenter, controlled trial. Vet. Ther. 2007, 8, 272–284. [Google Scholar]
- Xu, Y.; Fu, M.; Li, Z.; Fan, Z.; Li, X.; Liu, Y.; Anderson, P.M.; Xie, X.; Liu, Z.; Guan, J. A prosurvival and proangiogenic stem cell delivery system to promote ischemic limb regeneration. Acta Biomater. 2016, 31, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Hubschman, J.P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic stem cell trials for macular degeneration:a preliminary report. Lancet 2012, 25, 713–720. [Google Scholar] [CrossRef]
- Bermudez, M.A.; Sendon-Lago, J.; Eiro, N.; Treviño, M.; Gonzalez, F.; Yebra-Pimentel, E.; Giraldez, M.J.; Macia, M.; Lamelas, M.L.; Saa, J.; et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Invest. Ophthalmol. Vis. Sci. 2015, 22, 983–992. [Google Scholar] [CrossRef]
- Isakson, M.; de Blacam, C.; Whelan, D.; McArdle, A.; Clover, A.J. Mesenchymal stem cells and cutaneous wound healing: Current evidence and future potential. Stem. Cells Int. 2015, 2015, 831095. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Song, Y.; Zhao, R.C.; Han, Q.; Lin, Q. Using human adipose tissue derived mesenchymal stem cells as salvage therapy for hepatic graft-versus-host disease resembling acute hepatitis. Transplant. Proc. 2007, 39, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Baksh, D.; Song, L.; Tuan, R.S. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J. Cell Mol. Med. 2004, 8, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, J.; Lin, H.; Zhao, K.; Zheng, C. Nasal mucosa derived-mesenchymal stem cells from mice reduce inflammation via modulating immune responses. PLoS ONE 2015, 10, e0118849. [Google Scholar] [CrossRef] [PubMed]


| Inflammatory Markers | Grade | Description |
|---|---|---|
| Mononuclear (inflammatory) cell infiltrate | 0 | Normal aspect |
| 0–10 cells/HPF (high-pass filter, 40×) | ||
| 1 | Discrete inflammation | |
| 11–30 cells/HPF | ||
| 2 | Moderate inflammation | |
| 31–50 cells/HPF | ||
| 3 | Severe inflammation | |
| >50 cells/ HPF | ||
| Goblet cells | 0 | Normal aspect |
| 0–10 cells/HPF (high-pass filter, 40×) | ||
| 1 | Discrete inflammation | |
| 11–30 cells/HPF | ||
| 2 | Moderate inflammation | |
| 31–50 cells/HPF | ||
| 3 | Severe inflammation | |
| >50 cells/ HPF | ||
| Edema | 0 | No edema |
| 1 | Focal subepithelial edema | |
| 2 | Diffuse subepithelial edema | |
| 3 | Diffuse subepithelial and intraglandular edema | |
| Cilia | 0 | Normal aspect |
| 1 | Shortened cilia | |
| 2 | Dotted cilia disappearance | |
| 3 | Lack of cilia | |
| Fibrosis | 0 | No fibrosis |
| 1 | Subepithelial fibrosis | |
| 2 | Subepithelial and interglandular fibrosis | |
| 3 | Diffuse fibrosis (subepithelial and interglandular) with compression atrophy of glands and capillaries | |
| Epithelial hyperplasia | 0 | No epithelial hyperplasia |
| 1 | Dotted hyperplasia | |
| 2 | Diffuse hyperplasia | |
| Squamous metaplasia | 0 | No squamous metaplasia |
| 1 | Immature squamous metaplasia | |
| 2 | Mature squamous metaplasia/diffuse | |
| Eosinophilic cells | 0 | Normal aspect |
| 0–5 cells/HPF (high-pass filter, 40×) | ||
| 1 | Discrete inflammation | |
| 6–10 cells/HPF | ||
| 2 | Moderate inflammation | |
| 11–15 cells/HPF | ||
| 3 | Severe inflammation | |
| >15 cells/HPF |
| Variable | Week 2 | Week 4 | Week 8 | Week 12 | p | |
|---|---|---|---|---|---|---|
| Mononuclear cell infiltrate * | 0 (0; 0.5) | 1 (0.5; 1.5) | 3 (2; 3) | 3 (2.5; 3) | 0.001 | |
| Fibrosis * | 1 (0; 1) | 1 (1; 1.5) | 3 (3; 3) | 3 (2.5; 3) | 0.001 | |
| Edema * | 0 (0; 0) | 0 (0; 1) | 3 (3; 3) | 3 (2; 3) | 0.001 | |
| Cilia * | 1 (1; 1) | 1 (1; 1) | 2 (1.5; 2.5) | 2 (2.5; 2) | 0.002 | |
| Goblet cells * | 0 (0; 0) | 0 (0; 0) | 2 (1.5; 3) | 2 (2; 2.5) | 0.001 | |
| Eosinophilic cells | 0 (0; 0) | 1 (1; 1) | 2 (1; 2) | 2 (2; 2) | 0.001 | |
| Epithelial Hyperplasia ** | Grad 0 | 5 (100%) | 5 (100%) | - | - | <0.001 |
| Grad 1 | - | 5 (100%) | 5 (100%) | |||
| Squamous metaplasia ** | Grad 0 | 5 (100%) | 5 (100%) | - | - | <0.001 |
| Grad 1 | - | 5 (100%) | 5 (100%) | |||
| Variable | Control Group | Week 8 | Week 12 | |
|---|---|---|---|---|
| Mononuclear cell infiltrate * | 0 (0; 0.5) | 3 (2; 3) | 3 (2.5; 3) ++ | |
| Fibrosis * | 0 (0; 0) | 3 (3; 3) + | 3 (2.5; 3) + | |
| Edema * | 0 (0; 0) | 3 (3; 3) + | 3 (2.5; 3) + | |
| Cilia * | 0 (0; 0) | 2 (1.5; 2.5) + | 2 (2.5; 2) + | |
| Goblet cells * | 0 (0; 0) | 2 (1.5; 3) + | 2 (2; 2.5) + | |
| Eosinophilic cells | 0 (0; 0) | 2 (1; 2) | 2 (2; 2) | |
| Epithelial Hyperplasia ** | Grad 0 | 5 (100%) | - + | - ++ |
| Grad 1 | - | 5 (100%) + | 5 (100%) ++ | |
| Squamous metaplasia** | Grad 0 | 5 (100%) | - | - |
| Grad 1 | - | 5 (100%) + | 5 (100%) ++ | |
| Group | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| Number of stem cells | Control | 12 | 0.00 | 0.000 | 0.000 |
| Stem | 12 | 4.58 | 3.942 | 1.138 |
| Variable | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|
| Mononuclear cell infiltrate * | 3 (3; 3) | 3 (2; -) | 2 (1; -) | 1 (1; 1) | |
| Fibrosis ** | 2 | - | - | - | 1 (33.3%) |
| 3 | 3 (100%) | 3 (100%) | 3 (100%) | 2 (66.7%) | |
| Edema * | 3 (2; -) | 1 (1; -) | 1 (1; 1) | 0 (0; -) | |
| Cilia * | 3 (3; 3) | 2 (1; -) | 1 (1; 1) | 0 (0; -) | |
| Goblet cells * | 3 (3; 3) | 2 (2; -) | 1 (1; -) | 1 (0; -) | |
| Eosinophilic cells | 2 (2; 2) | 1 (1; 1) | 1 (1; 1) | 1 (0; 1) | |
| Epithelial Hyperplasia ** | Grad 0 | - | - | - | - |
| Grad 1 | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | |
| Squamous metaplasia ** | Grad 0 | - | - | - | - |
| Grad 1 | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trombitaș, V.-E.; Nagy, A.A.; Berce, C.; Pall, E.; Tăbăran, F.; Ilea, A.; Albu, S. The Role of Mesenchymal Stem Cells in the Treatment of a Chronic Rhinosinusitis—An In Vivo Mouse Model. Microorganisms 2021, 9, 1182. https://doi.org/10.3390/microorganisms9061182
Trombitaș V-E, Nagy AA, Berce C, Pall E, Tăbăran F, Ilea A, Albu S. The Role of Mesenchymal Stem Cells in the Treatment of a Chronic Rhinosinusitis—An In Vivo Mouse Model. Microorganisms. 2021; 9(6):1182. https://doi.org/10.3390/microorganisms9061182
Chicago/Turabian StyleTrombitaș, Veronica-Elena, Alina Anda Nagy, Cristian Berce, Emoke Pall, Flaviu Tăbăran, Aranka Ilea, and Silviu Albu. 2021. "The Role of Mesenchymal Stem Cells in the Treatment of a Chronic Rhinosinusitis—An In Vivo Mouse Model" Microorganisms 9, no. 6: 1182. https://doi.org/10.3390/microorganisms9061182
APA StyleTrombitaș, V.-E., Nagy, A. A., Berce, C., Pall, E., Tăbăran, F., Ilea, A., & Albu, S. (2021). The Role of Mesenchymal Stem Cells in the Treatment of a Chronic Rhinosinusitis—An In Vivo Mouse Model. Microorganisms, 9(6), 1182. https://doi.org/10.3390/microorganisms9061182










