Mineral Phosphorus Supply in Piglets Impacts the Microbial Composition and Phytate Utilization in the Large Intestine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Trial and Sample Preparation
2.2. 16S rRNA Profiling
2.3. Inositolphosphate Analysis
2.4. Data Analysis
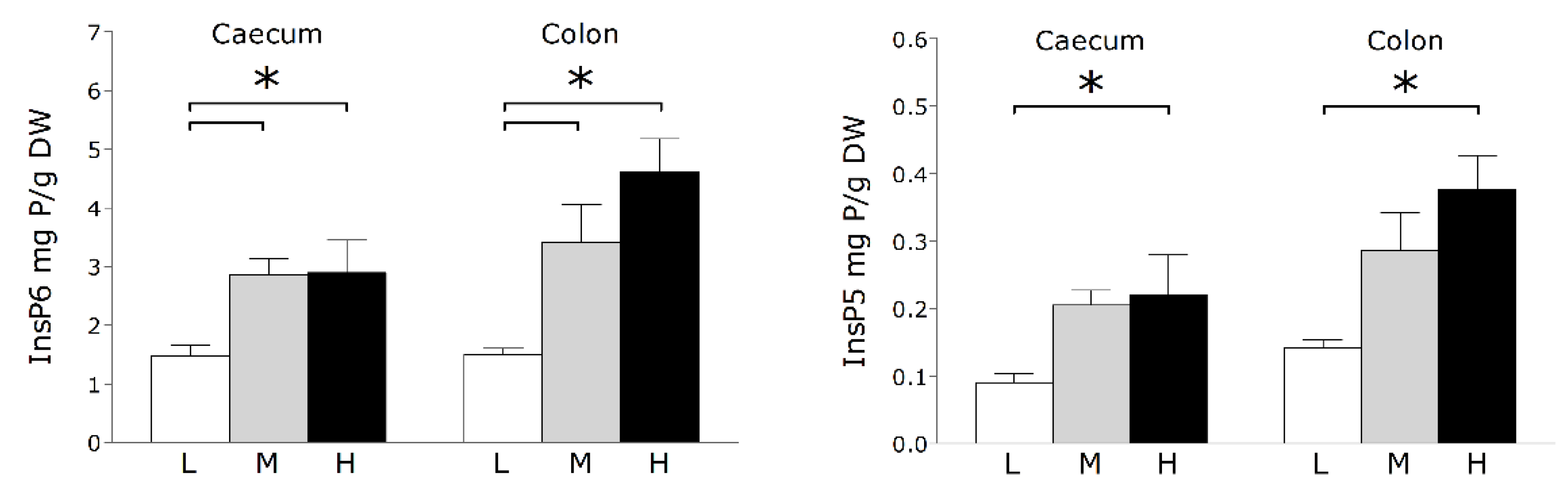
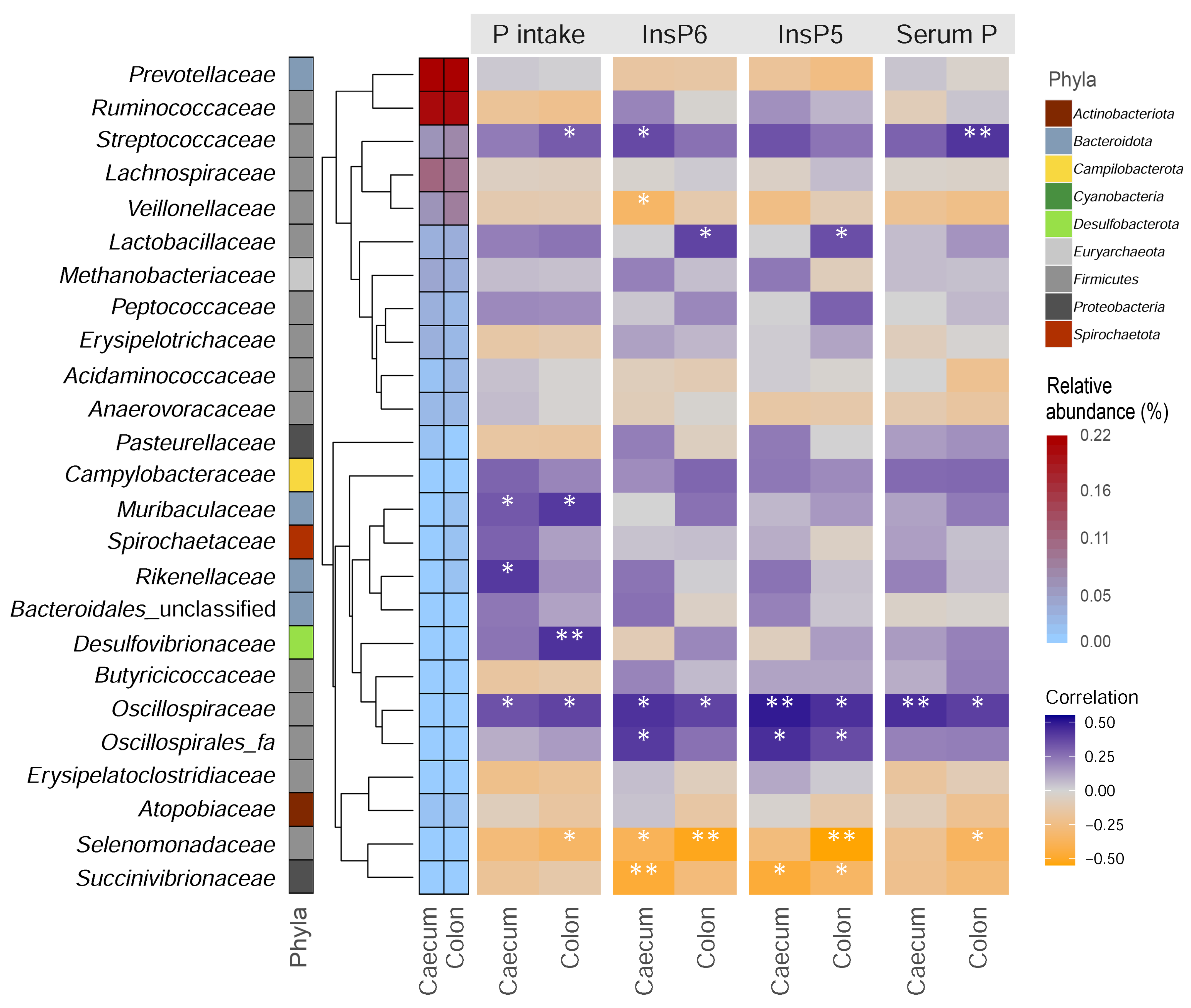
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Oster, M.; Reyer, H.; Ball, E.; Fornara, D.; McKillen, J.; Sørensen, K.U.; Poulsen, H.D.; Andersson, K.; Ddiba, D.; Rosemarin, A. Bridging gaps in the agricultural phosphorus cycle from an animal husbandry perspective—The case of pigs and poultry. Sustainability 2018, 10, 1825. [Google Scholar] [CrossRef] [Green Version]
- Humer, E.; Schwarz, C.; Schedle, K. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 605–625. [Google Scholar] [CrossRef]
- Schlemmer, U.; Jany, K.D.; Berk, A.; Schulz, E.; Rechkemmer, G. Degradation of phytate in the gut of pigs-pathway of gastrointestinal inositol phosphate hydrolysis and enzymes involved. Arch. Anim. Nutr. 2001, 55, 255–280. [Google Scholar] [CrossRef]
- Haros, M.; Bielecka, M.; Sanz, Y. Phytase activity as a novel metabolic feature in bifidobacterium. FEMS Microbiol. Lett. 2005, 247, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-Y.; Kim, Y.-H.; Rhee, M.-H.; Song, J.-C.; Lee, K.-W.; Kim, K.-S.; Lee, S.-P.; Lee, I.-S.; Park, S.-C. Selection of Lactobacillus sp. Psc101 that produces active dietary enzymes such as amylase, lipase, phytase and protease in pigs. J. Gen. Appl. Microbiol. 2007, 53, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Asghar, S.; Arif, M.; Nawaz, M.; Muhammad, K.; Ali, M.; Ahmad, M.; Iqbal, S.; Anjum, A.; Khan, M.; Nazir, J. Selection, characterisation and evaluation of potential probiotic lactobacillus spp. isolated from poultry droppings. Benef. Microbes 2016, 7, 35–44. [Google Scholar] [CrossRef]
- Raghavendra, P.; Halami, P.M. Screening, selection and characterization of phytic acid degrading lactic acid bacteria from chicken intestine. Int. J. Food Microbiol. 2009, 133, 129–134. [Google Scholar] [CrossRef]
- Hosseinkhani, B.; Hosseinkhani, G. Analysis of phytase producing bacteria (Pseudomonas sp.) from poultry faeces and optimization of this enzyme production. Afr. J. Biotechnol 2009, 8, 4229–4232. [Google Scholar]
- Yu, S.; Cowieson, A.; Gilbert, C.; Plumstead, P.; Dalsgaard, S. Interactions of phytate and myo-inositol phosphate esters (IP1-5) including IP5 isomers with dietary protein and iron and inhibition of pepsin. J. Anim. Sci. 2012, 90, 1824–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shastak, Y.; Zeller, E.; Witzig, M.; Schollenberger, M.; Rodehutscord, M. Effects of the composition of the basal diet on the evaluation of mineral phosphorus sources and interactions with phytate hydrolysis in broilers. Poult. Sci. 2014, 93, 2548–2559. [Google Scholar] [CrossRef] [PubMed]
- Blaabjerg, K.; Jørgensen, H.; Tauson, A.-H.; Poulsen, H.D. Heat-treatment, phytase and fermented liquid feeding affect the presence of inositol phosphates in ileal digesta and phosphorus digestibility in pigs fed a wheat and barley diet. Animal 2010, 4, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Cowieson, A.J.; Wilson, J.W.; Ajuwon, K.M.; Adeola, O. Extra-phosphoric effects of super dosing phytase on growth performance of pigs is not solely due to release of myo-inositol. J. Anim. Sci. 2019, 97, 3898–3906. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, C.; Oster, M.; Reyer, H.; Polley, C.; Vollmar, B.; Muráni, E.; Wimmers, K.; Wolf, P. Effects of excessive or restricted phosphorus and calcium intake during early life on markers of bone architecture and composition in pigs. J. Anim. Physiol. Anim. Nutr. 2020. [Google Scholar] [CrossRef] [Green Version]
- Gesellschaft Für Ernährungsphysiologie (GFE). Empfehlungen zur Energie-und Nährstoffversorgung beim Schwein. In Ausschuss für Bedarfsnormen der Gesellschaft für Ernährungsphysiologie; DLG Verlag: Frankfurt, Germany, 2006. [Google Scholar]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugerth, L.W.; Wefer, H.A.; Lundin, S.; Jakobsson, H.E.; Lindberg, M.; Rodin, S.; Engstrand, L.; Andersson, A.F. Degeprime, a program for degenerate primer design for broad-taxonomic-range pcr in microbial ecology studies. Appl. Environ. Microbiol. 2014, 80, 5116–5123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paraskova, J.V.; Jørgensen, C.; Reitzel, K.; Pettersson, J.; Rydin, E.; Sjöberg, P.J. Speciation of inositol phosphates in lake sediments by ion-exchange chromatography coupled with mass spectrometry, inductively coupled plasma atomic emission spectroscopy, and 31P NMR spectroscopy. Anal. Chem. 2015, 87, 2672–2677. [Google Scholar] [CrossRef]
- Sjöberg, P.J.; Thelin, P.; Rydin, E. Separation of inositol phosphate isomers in environmental samples by ion-exchange chromatography coupled with electrospray ionization tandem mass spectrometry. Talanta 2016, 161, 392–397. [Google Scholar] [CrossRef]
- Seynaeve, M.; Janssens, G.; Hesta, M.; Van Nevel, C.; De Wilde, R. Effects of dietary Ca/P ratio, P level and microbial phytase supplementation on nutrient digestibilities in growing pigs: Breakdown of phytic acid, partition of P and phytase activity along the intestinal tract. J. Anim. Physiol. Anim. Nutr. 2000, 83, 193–204. [Google Scholar] [CrossRef]
- De Rodas, B.; Youmans, B.P.; Danzeisen, J.L.; Tran, H.; Johnson, T.J. Microbiome profiling of commercial pigs from farrow to finish. J. Anim. Sci. 2018, 96, 1778–1794. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Mann, E.; Schmitz-Esser, S.; Wagner, M.; Ritzmann, M.; Zebeli, Q. Changing dietary calcium-phosphorus level and cereal source selectively alters abundance of bacteria and metabolites in the upper gastrointestinal tracts of weaned pigs. Appl. Environ. Microbiol. 2013, 79, 7264–7272. [Google Scholar] [CrossRef] [Green Version]
- Heyer, C.M.; Weiss, E.; Schmucker, S.; Rodehutscord, M.; Hoelzle, L.E.; Mosenthin, R.; Stefanski, V. The impact of phosphorus on the immune system and the intestinal microbiota with special focus on the pig. Nutr. Res. Rev. 2015, 28, 67–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyer, C.M.E.; Schmucker, S.; Burbach, K.; Weiss, E.; Eklund, M.; Aumiller, T.; Capezzone, F.; Steuber, J.; Rodehutscord, M.; Hoelzle, L.E. Phytate degradation, intestinal microbiota, microbial metabolites and immune values are changed in growing pigs fed diets with varying calcium–phosphorus concentration and fermentable substrates. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Boutard, M.; Cerisy, T.; Nogue, P.-Y.; Alberti, A.; Weissenbach, J.; Salanoubat, M.; Tolonen, A.C. Functional diversity of carbohydrate-active enzymes enabling a bacterium to ferment plant biomass. PLoS Genet. 2014, 10, e1004773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedderich, R.; Whitman, W.B. Physiology and Biochemistry of the Methane-Producing Archaea. In The Prokaryotes: Volume 2: Ecophysiology and Biochemistry; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 1050–1079. [Google Scholar]
- Klinsoda, J.; Vötterl, J.; Koger, S.; Metzler-Zebeli, B.U. Dietary phytase and lactic acid treated cereals caused greater taxonomic than functional adaptations in the cecal metagenome of growing pigs. Appl. Environ. Microbiol. 2020, 87, e02240-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shah, A.M.; Liu, Y.; Jin, L.; Wang, Z.; Xue, B.; Peng, Q. Relationship between true digestibility of dietary phosphorus and gastrointestinal bacteria of goats. PLoS ONE 2020, 15, e0225018. [Google Scholar] [CrossRef]
- Pieper, R.; Tudela, C.V.; Taciak, M.; Bindelle, J.; Pérez, J.F.; Zentek, J. Health relevance of intestinal protein fermentation in young pigs. Anim. Health Res. Rev. 2016, 17, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.; Gálvez, E.J.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yanke, L.; Bae, H.; Selinger, L.; Cheng, K. Phytase activity of anaerobic ruminal bacteria. Microbiology 1998, 144, 1565–1573. [Google Scholar] [CrossRef] [Green Version]
- Leytem, A.B.; Turner, B.L.; Thacker, P. Phosphorus composition of manure from swine fed low-phytate grains: Evidence for hydrolysis in the animal. J. Environ. Qual. 2004, 33, 2380–2383. [Google Scholar] [CrossRef] [Green Version]
- Wubuli, A.; Reyer, H.; Muráni, E.; Ponsuksili, S.; Wolf, P.; Oster, M.; Wimmers, K. Tissue-wide gene expression analysis of sodium/phosphate co-transporters in pigs. Int. J. Mol. Sci. 2019, 20, 5576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seynaeve, M.; Janssens, G.; Hesta, M.; Van Nevel, C.; De Wilde, R. Effects of dietary Ca/P ratio, P level and microbial phytase supplementation on nutrient digestibilities in growing pigs: Precaecal, post-ileal and total tract disappearances of OM. J. Anim. Physiol. Anim. Nutr. 2000, 83, 36–48. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyer, H.; Sjöberg, P.J.R.; Oster, M.; Wubuli, A.; Murani, E.; Ponsuksili, S.; Wolf, P.; Wimmers, K. Mineral Phosphorus Supply in Piglets Impacts the Microbial Composition and Phytate Utilization in the Large Intestine. Microorganisms 2021, 9, 1197. https://doi.org/10.3390/microorganisms9061197
Reyer H, Sjöberg PJR, Oster M, Wubuli A, Murani E, Ponsuksili S, Wolf P, Wimmers K. Mineral Phosphorus Supply in Piglets Impacts the Microbial Composition and Phytate Utilization in the Large Intestine. Microorganisms. 2021; 9(6):1197. https://doi.org/10.3390/microorganisms9061197
Chicago/Turabian StyleReyer, Henry, Per J. R. Sjöberg, Michael Oster, Aisanjiang Wubuli, Eduard Murani, Siriluck Ponsuksili, Petra Wolf, and Klaus Wimmers. 2021. "Mineral Phosphorus Supply in Piglets Impacts the Microbial Composition and Phytate Utilization in the Large Intestine" Microorganisms 9, no. 6: 1197. https://doi.org/10.3390/microorganisms9061197
APA StyleReyer, H., Sjöberg, P. J. R., Oster, M., Wubuli, A., Murani, E., Ponsuksili, S., Wolf, P., & Wimmers, K. (2021). Mineral Phosphorus Supply in Piglets Impacts the Microbial Composition and Phytate Utilization in the Large Intestine. Microorganisms, 9(6), 1197. https://doi.org/10.3390/microorganisms9061197






