Dynamics of Fermentation Parameters and Bacterial Community in High-Moisture Alfalfa Silage with or without Lactic Acid Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Forage Harvest and Silage Preparation
2.2. Analysis of Fermentation Parameters and Chemical Composition
2.3. Bacterial Community Analyses
2.4. Statistical Analyses
3. Results
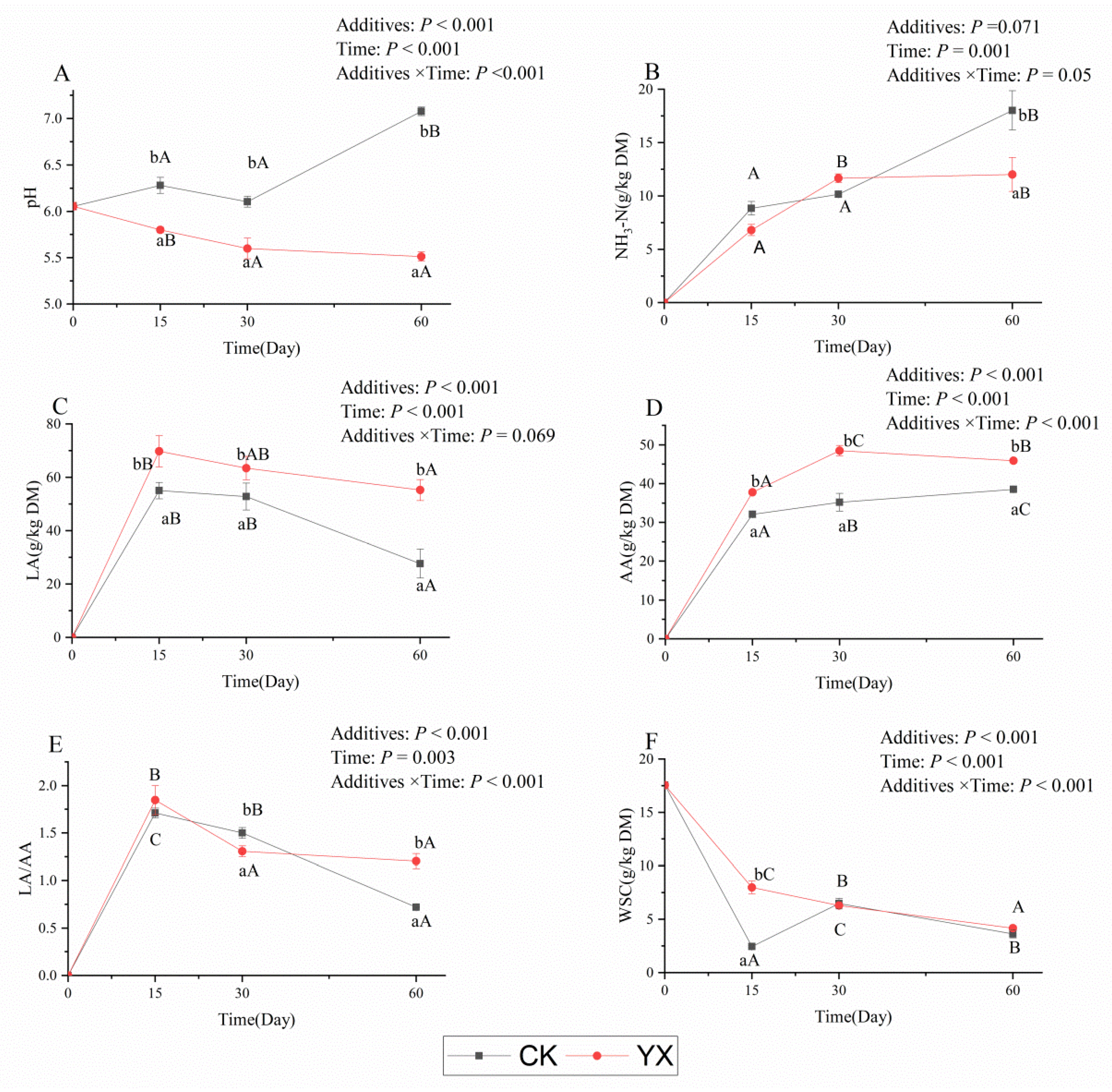
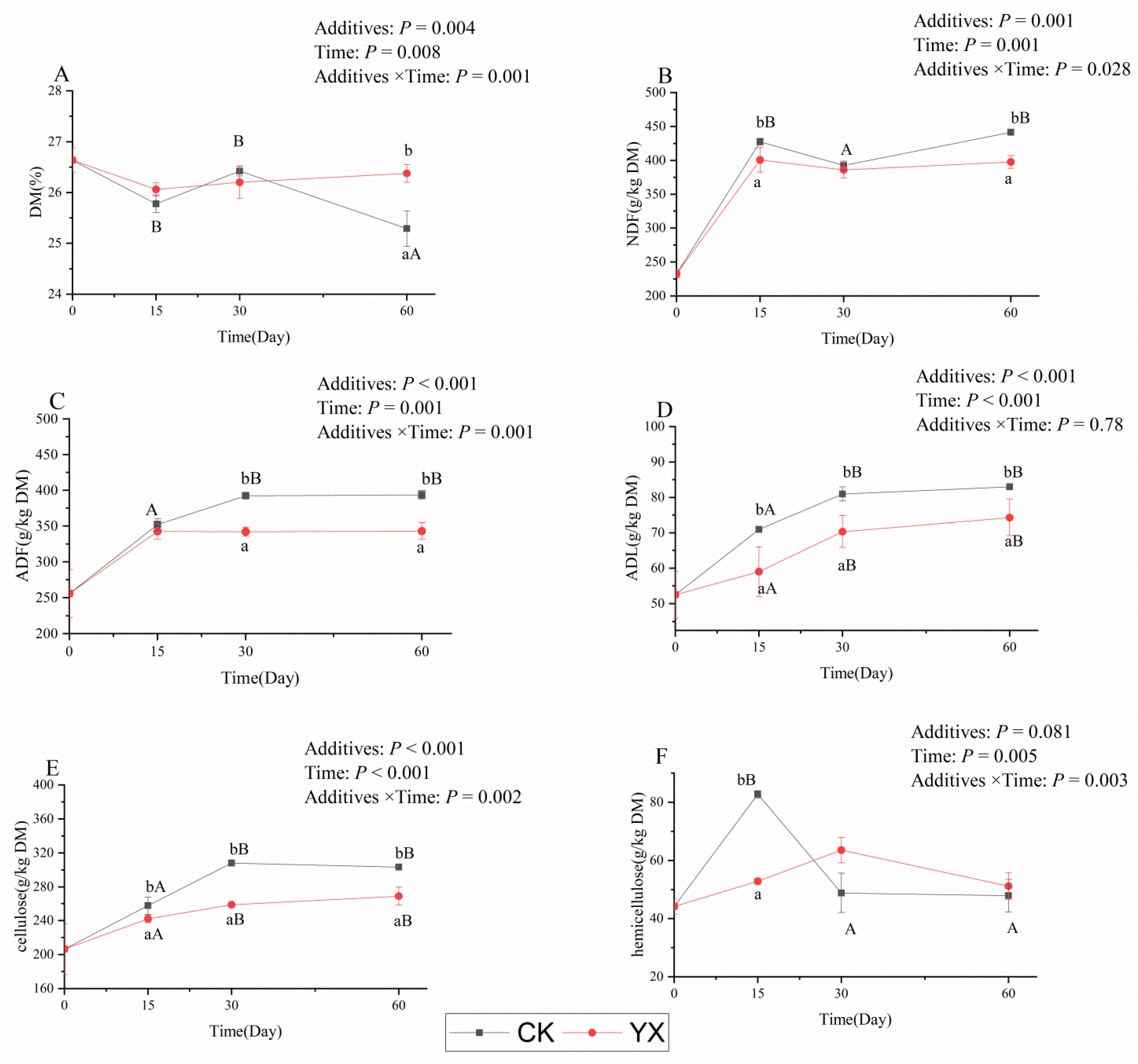
3.1. Changes in Fermentation Parameters in Silages during Ensiling with or without LAB
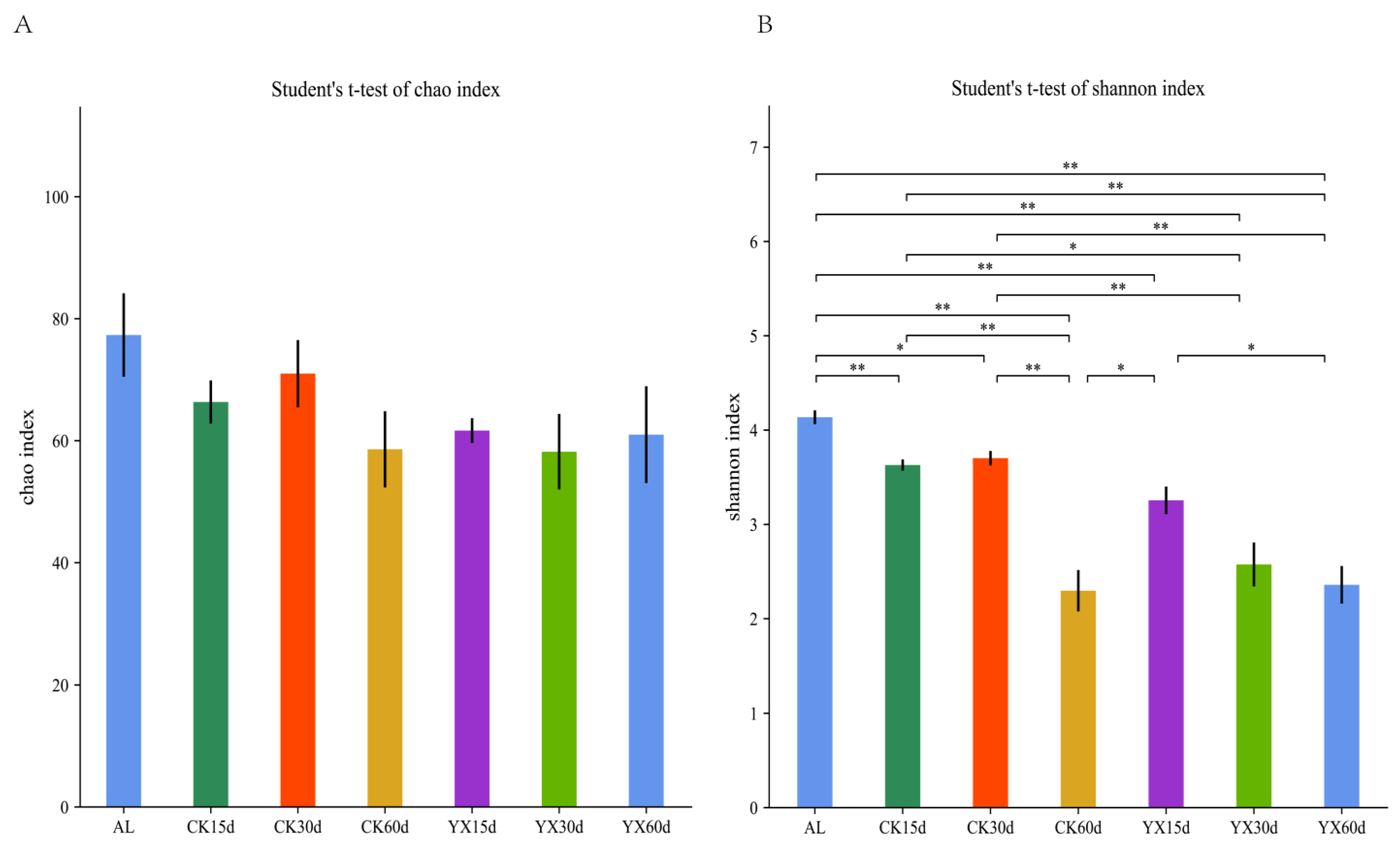
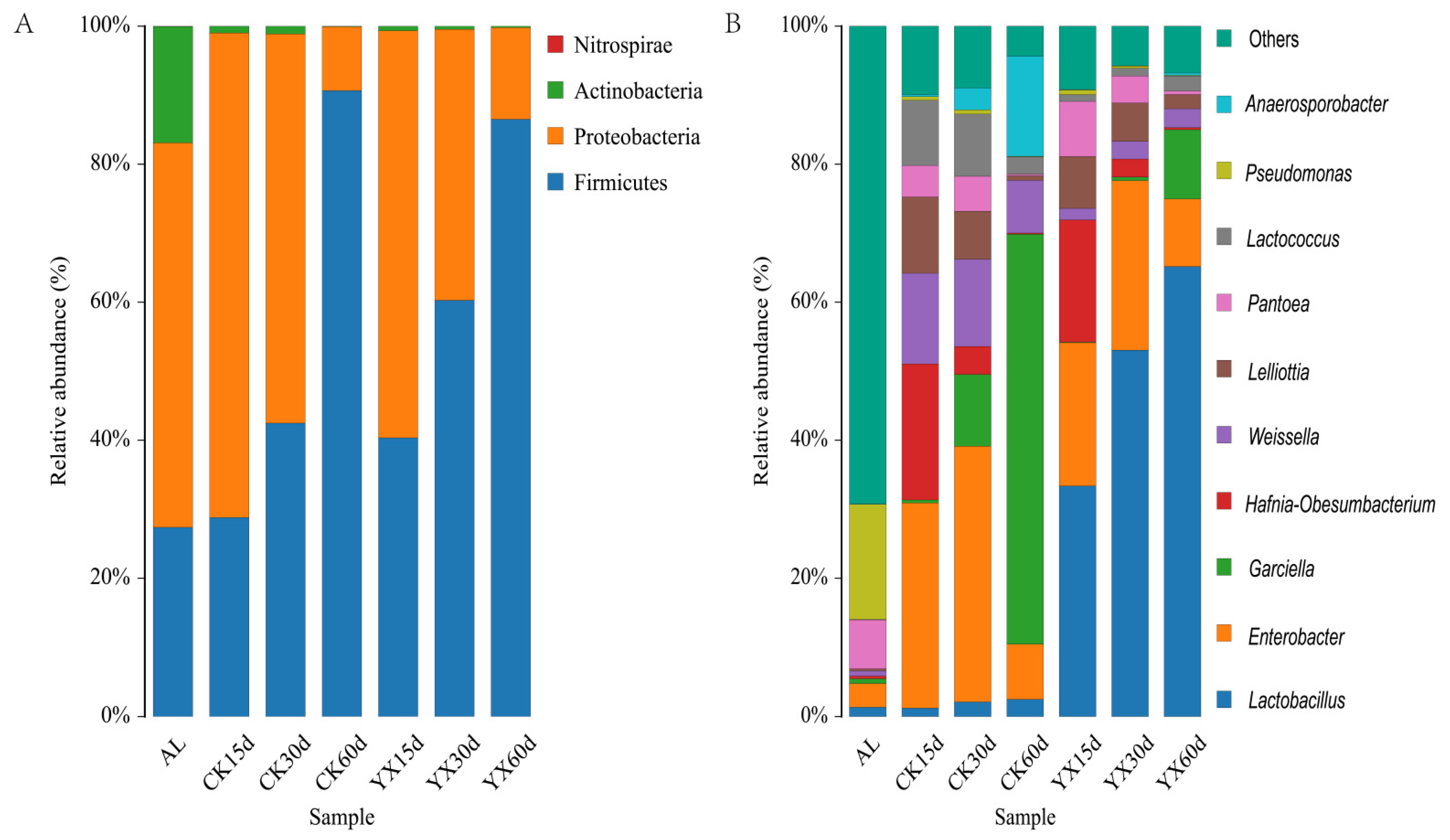
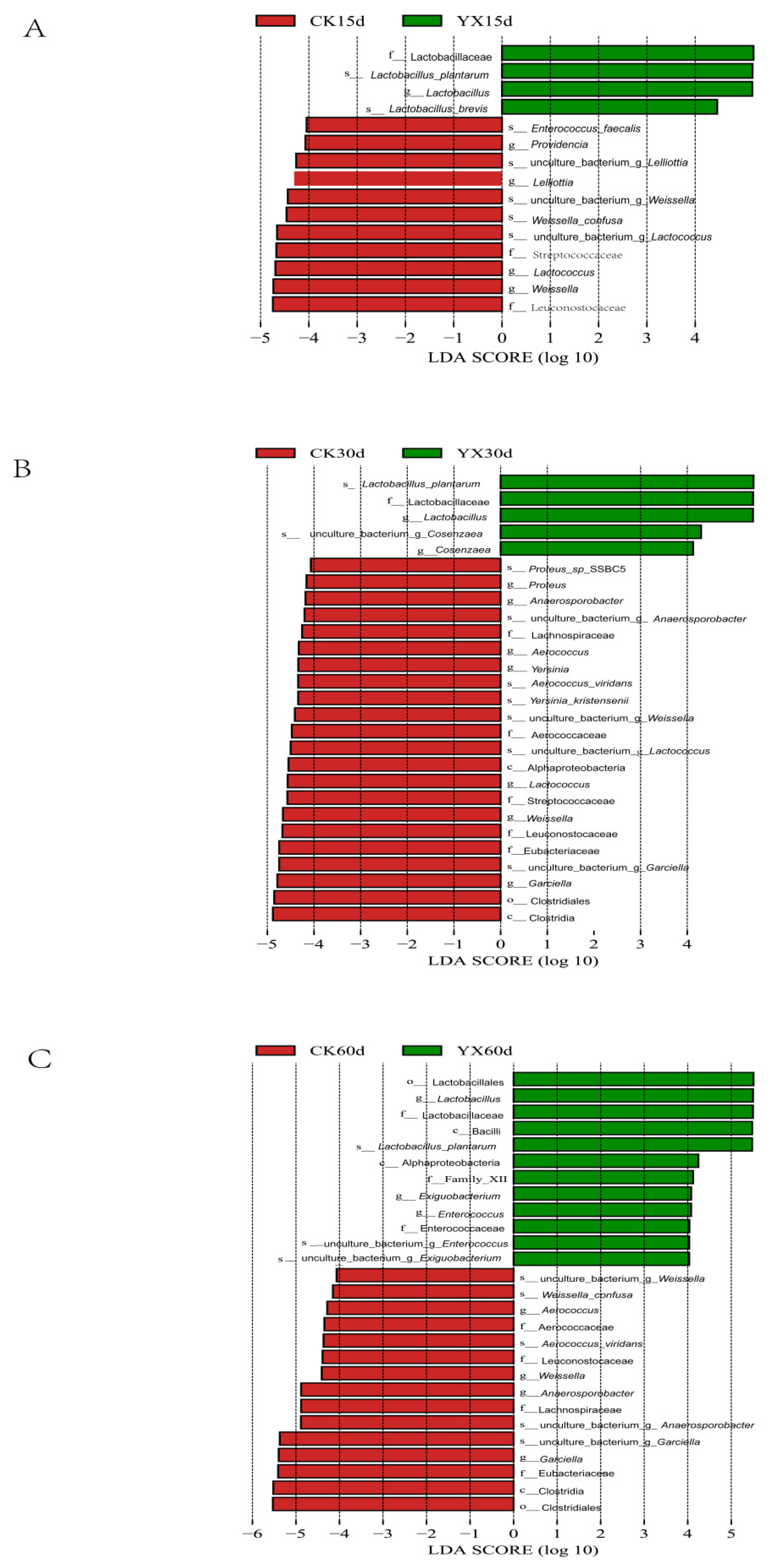
3.2. Changes in Bacterial Community in Silages during Ensiling with or without LAB
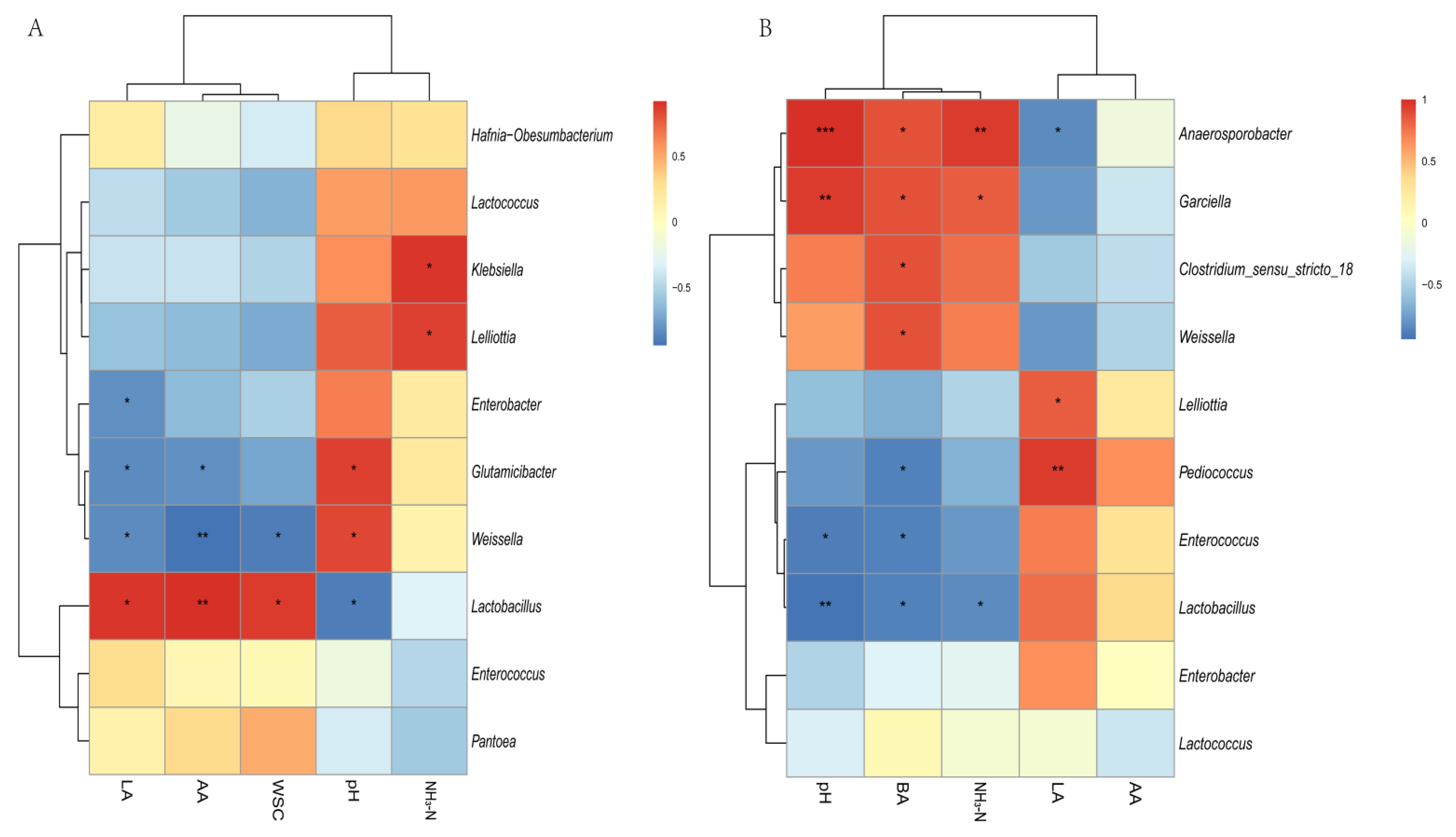
3.3. Relationships between Fermentation Parameters and Bacterial Community
4. Discussion
4.1. Changes in Fermentation Parameters, DM, and Structural Carbohydrates in Silages during Ensiling with or without LAB
4.2. Changes in Bacterial Community in Silages during Ensiling with or without LAB
4.3. Relationships between Fermentation Parameters and Bacterial Community
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duniere, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Thevenot-Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Tech. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Radovic, J.; Sokolović, D.; Marković, J. Alfalfa-most important perennial forage legume in animal husbandry. Biotechnol. Anim. Husb. 2009, 25, 465–475. [Google Scholar] [CrossRef]
- Eikmeyer, F.G.; Kofinger, P.; Poschenel, A.; Junemann, S.; Zakrzewski, M.; Heinl, S. Metagenome analyses reveal the influence of the inoculant Lactobacillus buchneri CD034 on the microbial community involved in grass ensiling. J. Biotechnol. 2013, 167, 334–343. [Google Scholar] [CrossRef]
- Nkosi, B.D.; Meeske, R.; Langa, T.; Motiang, M.D.; Modiba, S.; Mkhize, N.R. Effects of ensiling forage soybean (Glycine max (L.) Merr.) with or without bacterial inoculants on the fermentation characteristics, aerobic stability and nutrient digestion of the silage by Damara rams. Small Rumin. Res. 2016, 134, 90–96. [Google Scholar] [CrossRef]
- Kung, L.; Stough, E.C.; McDonell, E.E.; Schmidt, R.J.; Hofherr, M.W.; Reich, L.J.; Klingerman, C.M. The effect of wide swathing on wilting times and nutritive value of alfalfa haylage. J. Dairy Sci. 2010, 93, 1770–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coblentz, W.K.; Muck, R.E. Effects of natural and simulated rainfall on indicators of ensilability and nutritive value for wilting alfalfa forages sampled before preservation as silage. J. Dairy Sci. 2012, 95, 6635–6653. [Google Scholar] [CrossRef] [Green Version]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; He, L.W.; Xing, Y.Q.; Zhou, W.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Effects of mixing Neolamarckia cadamba leaves on fermentation quality, microbial community of high moisture alfalfa and stylo silage. Microb. Biotechnol. 2019, 12, 869–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombari, G.; Borreani, G.; Crovetto, G.M. Effect of ensiling alfalfa at low and high dry matter on production of milk used to make Grana cheese. J. Dairy Sci. 2001, 84, 2494–2502. [Google Scholar] [CrossRef]
- Franco, R.T.; Buffiere, P.; Bayard, R. Optimizing storage of a catch crop before biogas production: Impact of ensiling and wilting under unsuitable weather conditions. Biomass. Bioenerg. 2017, 100, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Muck, R.E. Factors Influencing Silage Quality and Their Implications for Management. J. Dairy Sci. 1988, 71, 2992–3002. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, M.C.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Dong, Z.H.; Shao, T. Effect of additives on fatty acid profile of high moisture alfalfa silage during ensiling and after exposure to air. Anim. Feed Sci. Tech. 2018, 236, 29–38. [Google Scholar] [CrossRef]
- Yuan, X.J.; Wen, A.Y.; Wang, J.; Desta, S.T.; Dong, Z.H.; Shao, T. Effects of four short–chain fatty acids or salts on fermentation characteristics and aerobic stability of alfalfa (Medicago sativa L.) silage. J. Sci. Food Agric. 2018, 98, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Fabiszewska, A.U.; Zielinska, K.J.; Wrobel, B. Trends in designing microbial silage quality by biotechnological methods using lactic acid bacteria inoculants: A minireview. World J. Microb. Biot. 2019, 35, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.P.; Li, Z.Z.; Yu, Z.; Zhang, Q.; Li, X.J. Interactive effect of inoculant and dried jujube powder on the fermentation quality and nitrogen fraction of alfalfa silage. Anim. Sci. J. 2017, 88, 633–642. [Google Scholar] [CrossRef]
- Zheng, M.L.; Niu, D.Z.; Jiang, D.; Zuo, S.S.; Xu, C.C. Dynamics of microbial community during ensiling direct-cut alfalfa with and without LAB inoculant and sugar. J. Appl. Microbiol. 2017, 122, 1456–1470. [Google Scholar] [CrossRef]
- Yang, L.L.; Yuan, X.J.; Li, J.F.; Dong, Z.H.; Shao, T. Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 2019, 275, 280–287. [Google Scholar] [CrossRef]
- Ercolini, D. PCR-DGGE fingerprinting: Novel strategies for detection of microbes in food. J. Microbiol. Meth. 2004, 56, 297–314. [Google Scholar] [CrossRef]
- Stevenson, D.M.; Muck, R.E.; Shinners, K.J.; Weimer, P.J. Use of real time PCR to determine population profiles of individual species of lactic acid bacteria in alfalfa silage and stored corn stover. Appl. Microbiol. Biot. 2006, 71, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.E.; Merry, R.J.; Davies, D.R.; Kell, D.B.; Theodorou, M.K.; Griffith, G.W. Vacuum packing: A model system for laboratory–scale silage fermentations. J. Appl. Microbiol. 2005, 98, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hoedtke, S.; Zeyner, A. Comparative evaluation of laboratory-scale silages using standard glass jar silages or vacuum-packed model silages. J. Sci. Food Agric. 2011, 91, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.C.; Ding, W.R.; Xu, D.M.; Ding, L.M.; Zhang, P.; Li, F.D.; Guo, X.S. Effects of addition of malic or citric acids on fermentation quality and chemical characteristics of alfalfa silage. J. Dairy Sci. 2017, 100, 8958–8966. [Google Scholar] [CrossRef] [Green Version]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Zhao, S.S.; Wang, Y.P.; Yang, F.Y.; Wang, Y.; Zhang, H. Screening a Lactobacillus plantarum strain for good adaption in alfalfa ensiling and demonstrating its improvement of alfalfa silage quality. J. Appl. Microbiol. 2020, 129, 233–242. [Google Scholar] [CrossRef]
- Arthur Thomas, T. An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Playne, M.J.; McDonald, P. The buffering constituents of herbage and of silage. J. Sci. Food Agric. 1966, 17, 264–268. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Yang, F.Y.; Wang, Y.P.; Zhao, S.S.; Wang, Y. Lactobacillus plantarum Inoculants Delay Spoilage of High Moisture Alfalfa Silages by Regulating Bacterial Community Composition. Front. Microbiol. 2020, 11, 1989. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microb. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.H.; Li, J.F.; Zhao, J.; Wu, J.X.; Shao, T. Enhancement of lignocellulosic degradation in high-moisture alfalfa via anaerobic bioprocess of engineered Lactococcus lactis with the function of secreting cellulase. Biotechnol. Biofuels 2019, 12, 88. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.R.; Yuan, X.J.; Dong, Z.H.; Li, J.F.; Shao, T. Effect of ensiling corn stover with legume herbages in different proportions on fermentation characteristics, nutritive quality and in vitro digestibility on the Tibetan Plateau. Grassl Sci. 2017, 63, 236–244. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Su, R.N.; Ni, K.K.; Wang, T.W.; Yang, X.P.; Zhang, J.; Liu, Y.Y.; Shi, W.X.; Yan, L.; Jie, C.; Zhong, J. Effects of ferulic acid esterase-producing Lactobacillus fermentum and cellulase additives on the fermentation quality and microbial community of alfalfa silage. PeerJ 2019, 7, e7712. [Google Scholar] [CrossRef] [Green Version]
- Kung, L.; Shaver, R. Interpretation and use of silage fermentation analysis reports. Focus Forage 2001, 13, 20–28. [Google Scholar]
- Tao, L.; Guo, X.S.; Zhou, H.; Undersander, D.J.; Nandety, A. Short communication: Characteristics of proteolytic activities of endo- and exopeptidases in alfalfa herbage and their implications for proteolysis in silage. J. Dairy Sci. 2012, 95, 4591–4595. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.K.; Wang, F.F.; Zhu, B.G.; Yang, J.X.; Zhou, G.A.; Pan, Y.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.K.; Zhao, J.Y.; Zhu, B.G.; Su, R.N.; Pan, Y.; Ma, J.K.; Zhou, G.A.; Tao, Y.; Liu, X.R.; Zhong, J. Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 2018, 265, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Ennahar, S.; Cai, Y.M.; Fujita, Y. Phylogenetic diversity of lactic acid bacteria associated with paddy rice silage as determined by 16S ribosomal DNA analysis. Appl. Environ. Microb. 2003, 69, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Namihira, T.; Shinzato, N.; Akamine, H.; Maekawa, H.; Matsui, T. Influence of nitrogen fertilization on tropical-grass silage assessed by ensiling process monitoring using chemical and microbial community analyses. J. Appl. Microbiol. 2010, 108, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.S.; Fang, C.; Sun, X.X.; Han, L.J.; He, X.Q.; Huang, G.Q. Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane under slight positive pressure. Bioresource Technol. 2018, 259, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Mi, Z.; Xu, H.; Zheng, Y.; Kwok, L.Y.; Zhang, H.; Zhang, W. Assessing quality of Medicago sativa silage by monitoring bacterial composition with single molecule, real-time sequencing technology and various physiological parameters. Sci. Rep. 2016, 6, 28358. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.R.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela, S. Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biot. 2018, 102, 4025–4037. [Google Scholar] [CrossRef]
- Yang, J.; Cao, Y.; Cai, Y.; Terada, F. Natural populations of lactic acid bacteria isolated from vegetable residues and silage fermentation. J. Dairy Sci. 2010, 93, 3136–3145. [Google Scholar] [CrossRef]
- Filya, I.; Muck, R.E.; Contreras-Govea, F.E. Inoculant effects on alfalfa silage: Fermentation products and nutritive value. J. Dairy Sci. 2007, 90, 5108–5114. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Zhao, J.; Dong, Z.; Li, J.; Nazar, M.; Shao, T. Assessment of inoculating various epiphytic microbiota on fermentative profile and microbial community dynamics in sterile Italian ryegrass. J. Appl. Microbiol. 2020, 129, 509–520. [Google Scholar] [CrossRef]
- Amir, A.; Zeisel, A.; Zuk, O.; Elgart, M.; Stern, S.; Shamir, O.; Turnbaugh, P.J.; Soen, Y.; Shental, N. High–resolution microbial community reconstruction by integrating short reads from multiple 16S rRNA regions. Nucleic Acids. Res. 2013, 41, e205. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.J.; Dong, Z.H.; Li, J.F.; Shao, T. Microbial community dynamics and their contributions to organic acid production during the early stage of the ensiling of Napier grass (Pennisetum purpureum). Grass Forage Sci. 2020, 75, 37–44. [Google Scholar] [CrossRef]
- Pereira, O.G.; Rocha, K.D.; de Luces Fortes Ferreira, C.L. Chemical composition, characterization, and population of microorganisms on elephantgrass “Cameroon” (Pennisetum purpureum, Schum) and its silages. Rev. Bras. Zootec. 2007, 36, 1742–1750. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.N.; Yao, D.D.; Li, D.X.; Lin, Y.L.; Bureenok, S.; Ni, K.K.; Yang, F.Y. Effects of Lactic Acid Bacteria Isolated From Rumen Fluid and Feces of Dairy Cows on Fermentation Quality, Microbial Community, and in vitro Digestibility of Alfalfa Silage. Front. Microbiol. 2020, 10, 2998. [Google Scholar] [CrossRef] [PubMed]
- Kung, L. Silage additives: Where are we going? In Proceedings of the XVII International Silage Conference, Piracicaba, São Paulo, Brazil, 1–3 July 2015; pp. 1–3. [Google Scholar]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Elferink, S.J.W.H.O.; Spoelstra, S.F. Microbiology of Ensiling. Agronomy 2003, 42, 31–94. [Google Scholar] [CrossRef]
- Miranda-Tello, E.; Fardeau, M.L.; Sepúlveda, J.; Fernández, L.; Cayol, J.L.; Thomas, P.; Ollivier, B. Garciella nitratireducens gen. nov., sp. nov., ananaerobic, thermophilic, nitrate- and thiosulfate-reducing bacterium isolated from an oilfield separator in the Gulf of Mexico. Int. J. Syst. Evol. Microbiol. 2003, 53, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Lim, Y.W.; Yi, H.; Sekiguchi, Y.; Kamagata, Y.; Chun, J. Anaerosporobacter mobilis gen. nov., sp nov., isolated from forest soil. Int. J. Syst. Evol. Microbiol. 2007, 57, 1784–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heron, S.J.E.; Wilkinson, J.F.; Duffus, C.M. Enterobacteria associated with grass and silages. J. Appl. Bacteriol. 1993, 75, 13–17. [Google Scholar] [CrossRef]
- Heinritz, S.N.; Martens, S.D.; Avila, P.; Hoedtke, S. The effect of inoculant and sucrose addition on the silage quality of tropical forage legumes with varying ensilability. Anim. Feed Sci. Technol. 2012, 174, 201–210. [Google Scholar] [CrossRef]
- Östling, C.; Lindgren, S. Influences of enterobacteria on the fermentation and aerobic stability of grass silages. Grass Forage Sci. 2006, 50, 41–47. [Google Scholar] [CrossRef]
- Yang, F.Y.; Zhao, S.S.; Wang, Y.; Fan, X.M.; Wang, Y.P.; Feng, C.S. Assessment of Bacterial Community Composition and Dynamics in Alfalfa Silages with and without Lactobacillus plantarum Inoculation Using Absolute Quantification 16S rRNA Sequencing. Front. Microbiol. 2021, 11, 629894. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Yang, F.; Wang, Y.; Fan, X.; Feng, C.; Wang, Y. Dynamics of Fermentation Parameters and Bacterial Community in High-Moisture Alfalfa Silage with or without Lactic Acid Bacteria. Microorganisms 2021, 9, 1225. https://doi.org/10.3390/microorganisms9061225
Zhao S, Yang F, Wang Y, Fan X, Feng C, Wang Y. Dynamics of Fermentation Parameters and Bacterial Community in High-Moisture Alfalfa Silage with or without Lactic Acid Bacteria. Microorganisms. 2021; 9(6):1225. https://doi.org/10.3390/microorganisms9061225
Chicago/Turabian StyleZhao, Shanshan, Fengyuan Yang, Yuan Wang, Xiaomiao Fan, Changsong Feng, and Yanping Wang. 2021. "Dynamics of Fermentation Parameters and Bacterial Community in High-Moisture Alfalfa Silage with or without Lactic Acid Bacteria" Microorganisms 9, no. 6: 1225. https://doi.org/10.3390/microorganisms9061225
APA StyleZhao, S., Yang, F., Wang, Y., Fan, X., Feng, C., & Wang, Y. (2021). Dynamics of Fermentation Parameters and Bacterial Community in High-Moisture Alfalfa Silage with or without Lactic Acid Bacteria. Microorganisms, 9(6), 1225. https://doi.org/10.3390/microorganisms9061225




