Abstract
Sesquiterpenoids are one of the most diverse classes of isoprenoids which exhibit numerous potentials in industrial biotechnology. The methanotrophs-based methane bioconversion is a promising approach for sustainable production of chemicals and fuels from methane. With intrinsic high carbon flux though the ribulose monophosphate cycle in Methylotuvimicrobium alcaliphilum 20Z, we demonstrated here that employing a short-cut route from ribulose 5-phosphate to 1-deoxy-d-xylulose 5-phosphate (DXP) could enable a more efficient isoprenoid production via the methylerythritol 4-phosphate (MEP) pathway, using α-humulene as a model compound. An additional 2.8-fold increase in α-humulene production yield was achieved by the fusion of the nDXP enzyme and DXP reductase. Additionally, we utilized these engineering strategies for the production of another sesquiterpenoid, α-bisabolene. The synergy of the nDXP and MEP pathways improved the α-bisabolene titer up to 12.24 ± 0.43 mg/gDCW, twofold greater than that of the initial strain. This study expanded the suite of sesquiterpenoids that can be produced from methane and demonstrated the synergistic uses of the nDXP and MEP pathways for improving sesquiterpenoid production in methanotrophic bacteria.
1. Introduction
Isoprenoids are the largest family in natural products which are found as secondary metabolites in many organisms including plants, animals, and microbes and exhibit a wide range of applications in pharmaceuticals, nutraceuticals, flavors, cosmetics, food additives, and biofuels [1]. In the isoprenoid family, sesquiterpenoids are the largest and the most diverse subgroup with important medical and industrial properties [2]. However, there are several challenges for isoprenoid production via chemical synthesis or plant extraction [3]. While extraction from plants has several disadvantages such as low productivity, weather dependency, and waste production, chemical synthesis requires many steps with expensive procedures and environmental hazardous catalysts. Thus, the development of microbial cell factories for the production of diverse isoprenoids has been drawing a lot of attention in biotechnology for decades due to these challenges [2,3,4].
The microbial production described so far has used sugar as the major feedstock. However, due to increased sugar prices, inexpensive and non-food carbon feedstocks, therefore, are needed [5,6,7]. Methane is considered as an alternative carbon feedstock for industrial biomanufacturing because of its relatively high abundance and cheap price [7,8]. Additionally, methane has a degree of reduction per carbon of 8 which is much higher than that of 4 in glucose, indicating that methane has more electrons per carbon atom and those electrons can be used to enhance product yields [8]. Methanotrophs-based methane bioconversion does not compete with food production, which contrasts with other microbial hosts employing biomass-derived substrates, enabling its potential in industrial manufacturing for the sustainable bioproduction of isoprenoids as well as of other biochemicals [9,10]. Methylotuvimicrobium alcaliphilum 20Z is a model methanotrophic system because of its promising biotechnological potential with the availability of genetic tools, omics and physiological datasets, making it a potential methanotrophic biocatalyst for methane conversion [11,12,13]. However, the experience of generating isoprenoids from methane in methanotrophic bacteria as well as of this particular biocatalyst is very limited [14,15]. Some methanotrophic strains naturally produce carotenoids, an important class of isoprenoids, such as Methylomonas sp. DH-1 and Methylomonas sp. ZR1 [16,17]. In contrast, some are unable to synthesize isoprenoids without metabolic engineering, although they have full enzymes in the MEP pathway [14]. Previously, Methylomonas sp. 16a was demonstrated to produce a small amount of astaxanthin from methane by expressing an astaxanthin-synthesizing gene cluster [18]. Furthermore, M. alcaliphilum 20Z, a halophilic methanotroph, was engineered for α-humulene production via the methylerythritol 4-phosphate (MEP) pathway [19]. This demonstrates an important step forward in methanotrophic biocatalysts for the conversion of methane to isoprenoids. However, low titers of sesquiterpenoids highlight the need for more advanced engineering strategies to improve the production of isoprenoids using this promising biocatalyst.
DXP, 1-deoxy-D-xylulose 5-phosphate, has a similar structure to pentose phosphates. However, DXP is naturally produced by condensation of pyruvate (PYR) and glyceraldehyde 3-phosphate (G3P) using DXP synthase (Dxs) with the loss of one CO2 [16]. Dxs is a well-known gatekeeper for the MEP pathway which is regulated at both the transcriptional and the translational levels and the feedback is inhibited by prenyl phosphates [20]. To improve the carbon flux entering the MEP pathway, a short cut from C5 sugars to DXP (nDXP pathway) is a potential strategy since it possesses several advantages such as enhancement of the carbon conservation yield and avoidance of regulatory mechanisms [21]. With a high carbon flux through the ribulose monophosphate (RuMP) cycle in M. alcaliphilum 20Z for C5 regeneration, we demonstrated here the potential use of the nDXP pathway for improving the carbon flux through the MEP pathway, subsequently enhancing sesquiterpenoid production from methane in M. alcaliphilum 20Z. This study also represents the first demonstration of microbial production of α-bisabolene from methane.
2. Materials and Methods
2.1. Strains and Plasmids
The list of all strains used in this study is provided in Supplementary Table S1. Methylotuvimicrobium (formerly known as Methylomicrobium) alcaliphilum 20Z and the engineered strains were cultivated in 500-mL baffled flasks sealed with screw caps containing 50 mL nitrate mineral salt (NMS) medium with compositions described in detail by Ojala et al. at 30 °C and 230 rpm using methane as a carbon source [22]. Methane was supplied to a final concentration of 50% (v/v) by gas substitution using a gas-tight syringe, and the headspace was refreshed daily. Kanamycin with the final concentration of 50 µg/mL was used for selecting recombinant methanotrophs and Escherichia coli with recombinant plasmids.
The primers and procedures used for plasmids construction are provided in Supplementary Table S2, with the construction of platform plasmid pHM03 thoroughly described by Nguyen et al. [19]. The different recombinant plasmids were constructed with distinct genes but the constitutive promoter, Ptac, was kept consistently for driving the transcription. These recombinant plasmids were transformed into the host strain using electroporation described in detail by Nguyen et al. [11]. In brief, cells were harvested by centrifugation and washed twice using cold water. An amount of 100 µL of distilled sterile water was used to resuspend the cell pellets. Then, 50 µL cell suspension was mixed with the DNA plasmid and put into a cold 1-mm gap cuvette for electroporation at parameters of 1.3 kV, 25 µF, and 200 Ω. The electroporated cells were then recovered in 10 mL NMS overnight and spread on selective plates. The original ribB sequence from Methanococcus jannaschii was codon-optimized to maximize the codon adaptation index between the codon usage of ribB and the codon usage of all ORFs from M. alcaliphilum 20Z as described previously [19].
2.2. Production of α-Humulene and α-Bisabolene and the Analytical Method
Two-phase flask cultivation consisting of the NMS medium as the aqueous phase to cultivate methanotrophs and 20% (v/v) dodecane as the organic overlay to extract α-humulene and α-bisabolene produced from the engineered strains in situ was performed in all of the experiments as described in our previous report [19]. Briefly, precultures were grown in 10 mL NMS medium and then inoculated into 500-mL baffled flasks containing 40 mL fresh media to achieve OD600 of 0.1 and 10 mL (20% v/v) dodecane. Sampling was conducted after 96-h cultivation by centrifugation of 50 mL liquid culture at 3220 g for 10 min, and then the upper dodecane layer was collected for α-humulene and α-bisabolene quantification. An Agilent 5977B 5977E GC/MS system (Santa Clara, CA, USA) was used to analyze and quantify α-humulene and α-bisabolene according to previous protocols [23,24]. For quantification of α-humulene and α-bisabolene, standard curves were made from analytical standards dissolved in dodecane which was purchased from Sigma-Aldrich (St. Louis, MO, USA) and Alfa Aesar (Ward Hill, MA, USA). Due to the presence of other bisabolene isomers, the contribution of α-bisabolene to the molarity of 27.52 ± 0.27% in the commercial standard was calculated previously [24].
2.3. Calculation of the Maximum Theoretical Molar Yield Using a Genome-Scale Model
The iIA407 genome-scale model (GSM) of M. alcaliphilum 20Z was used for in silico calculation [12]. The iIA407-nDXP model was constructed by adding heterologous reactions for the nDXP reaction and an exchange reaction for converting isopentenyl pyrophosphate (IPP) into iIA407 by Cobrapy (Appendix A) [25]. The maximum theoretical molar yield of IPP (MTMYIPP) was calculated by means of the flux balance analysis using OptFlux [26].
3. Results and Discussion
3.1. Enhancing α-Humulene Production via the Synergy of the MEP and nDXP Pathways
In our previous study, metabolic flux analysis using a genome-scale model of M. alcaliphilum 20Z refined core metabolic pathways of M. alcaliphilum 20Z grown on C1 substrates and indicated that a large portion of carbon flux (~75%) enters the RuMP cycle for the regeneration of ribulose-5-phosphate (Ru5P) [13]. Consistent with the highest fluxes of the RuMP cycle, omics datasets also showed higher transcripts, protein abundances, and pool size of metabolites in the RuMP cycle compared to other pathways [12,13]. Therefore, M. alcaliphilum 20Z could be a suitable host for producing C5 sugar phosphate-derived products [27]. Based on the in silico analysis of the MTMYIPP and the thermodynamic properties, the MEP pathway is a suitable pathway for isoprenoid production in M. alcaliphilum 20Z [19]. However, as noted before, the MEP pathway starts with the condensation of G3P and PYR to produce DXP by DXP synthase (Dxs) which is known as an important control point for the MEP pathway. By using the nDXP route, DXP could be provided directly from C5 sugars. With the high carbon flux through the RuMP cycle, redirection of the carbon flux from the RuMP cycle to the DXP pathway is a promising strategy for improving the carbon flux through the MEP pathway and further enhancing the isoprenoid production (Figure 1). In order to confirm this hypothesis, we performed the flux balance analysis to calculate the MTMYIPP in the presence of the nDXP pathway. The nDXP reaction was manually added to the iIA407 model and the first enzyme (Dxs) that condenses G3P and PYR in the MEP pathway was inactivated before simulations (Appendix A). As a result, M. alcaliphilum 20Z employing the nDXP pathway resulted in the maximum theoretical molar IPP yield of 14.4% per mmol methane, which was slightly higher than that of the MEP pathway which was 13.6%, revealing that the nDXP route is a more efficient pathway for isoprenoid production in M. alcaliphilum 20Z as well as in other type I methanotrophs. Therefore, the synergy between the MEP and nDXP pathways is potentially more appealing to engineering isoprenoids in M. alcaliphilum 20Z.
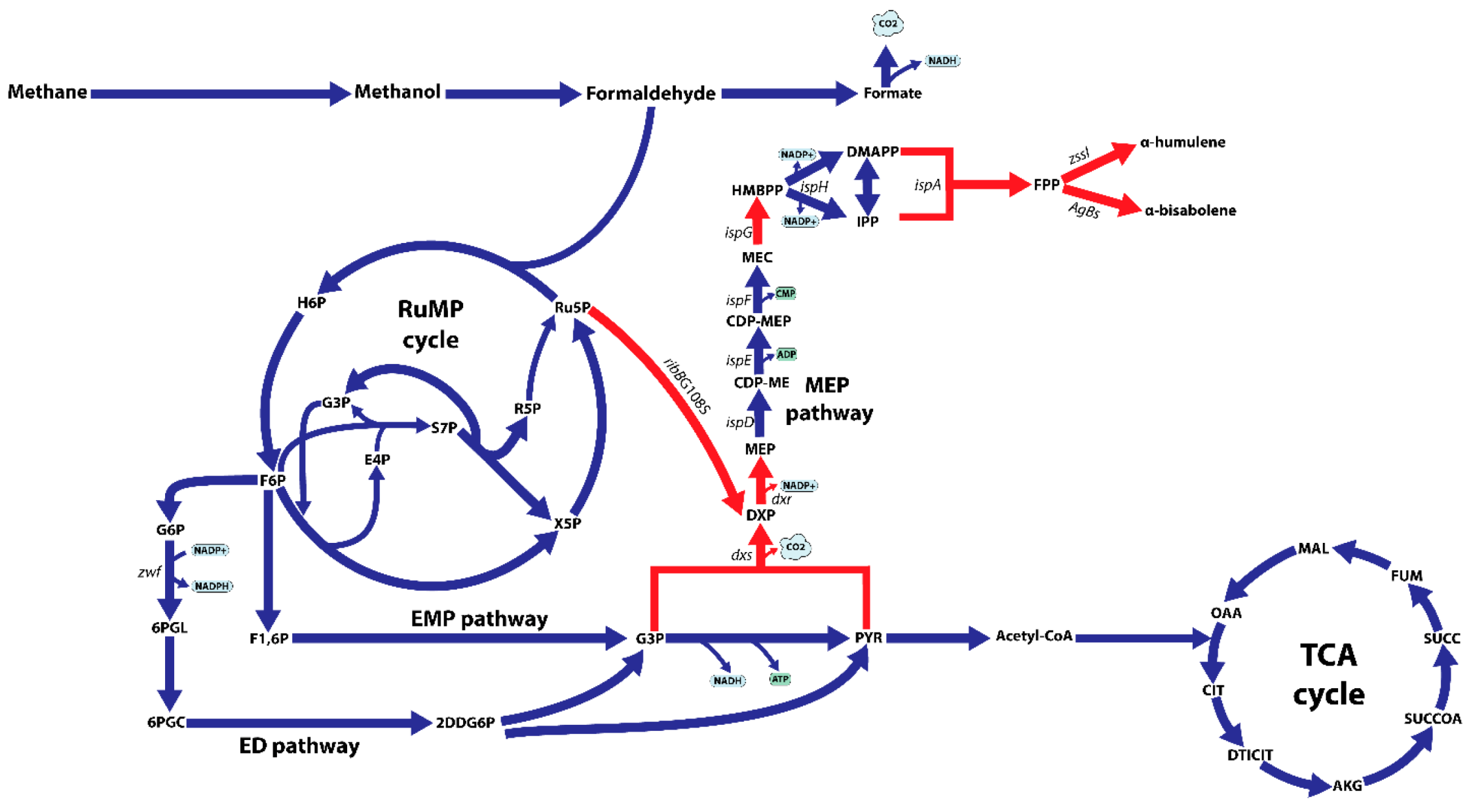
Figure 1.
Schematic overview of the central metabolism of M. alcaliphilum 20Z on methane, including the ribulose monophosphate (RuMP) cycle, the Embden–Meyerhof–Parnas (EMP) pathway, the Entner–Doudoroff (ED) pathway, the TCA cycle, endogenous isoprenoid synthesis via the methylerythritol 4-phosphate pathway (MEP). Abbreviations: H6P, hexulose 6-phosphate; F6P, fructose 6-phosphate; X5P, xylulose 5-phosphate; Ru5P, ribulose 5-phosphate; E4P, erythrose 4-phosphate; S7P, sedoheptulose 7-phosphate; R5P, ribose 5-phosphate; F1,6P, fructose 1,6-bisphosphate; G6P, glucose 6-phosphate; 6PGL, 6-phosphogluconolactonase; 6PGC, 6-phosphogluconate; 2DDG6P, 2-dehydro-3-deoxy-D-gluconate 6-phosphate; G3P, glyceraldehyde 3-phosphate; PYR, pyruvate; DXP, deoxyxylulose 5-phosphate; MEP, methylerythritol 4-phosphate; CDP-ME, diphosphocytidylyl methylerythritol; CDP-MEP, CDP-ME 2-phosphate; MEC, methylerythritol 2,4-cyclodiphosphate; HMBPP, hydroxymethylbutenyl diphosphate; IPP, isopentenyl diphosphate; DMAPP, dimethylallyl diphosphate; FPP, farnesyl pyrophosphate; OAA, oxaloacetate; CIT, citrate; DTICIT, D-threo-isocitrate; AKG, α-ketoglutarate; SUCCOA, succinyl-CoA; SUCC, succinate; FUM, fumarate; MAL, malate; dxs, DXP synthase; dxr, DXP reductoisomerase; ispD, CDP-ME synthase; ispE, CDP-ME kinase; ispF, MEC synthase; ispG, HMBPP synthase; ispH, HMBPP reductase; ispA, FPP synthase; zssI, α-humulene synthase; AgBs, Abies grandis α-bisabolene synthase; ribBG108S, nDXP enzyme catalyzing the reaction from ribulose 5-phosphate to DXP. Red arrows indicate the overexpression targets.
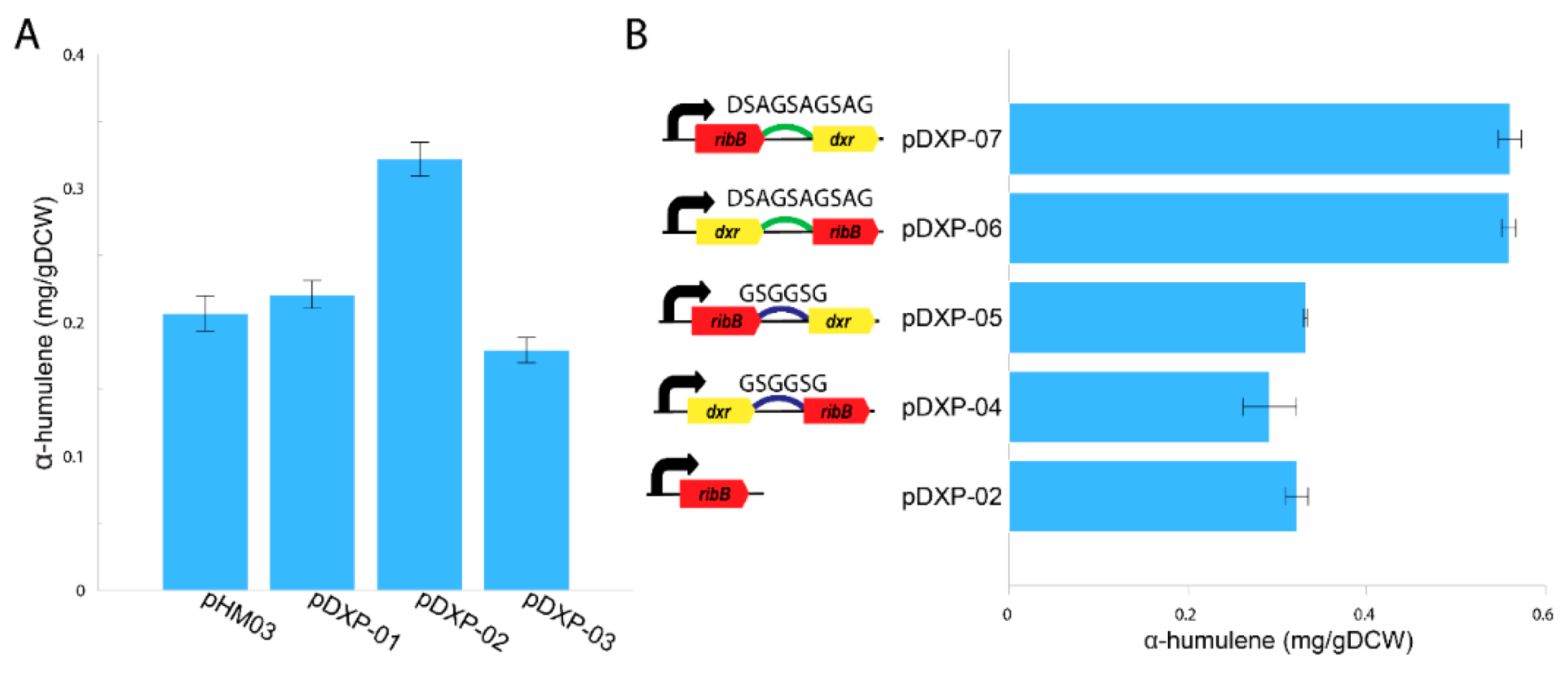
Recently, a novel route for synthesizing DXP from ribulose 5-phosphate was discovered using the directed evolution approach which uncovered two nDXP genes: yajO encoding putative xylose reductase and mutant of ribBG108S encoding 3,4-dihydroxy-2-butanone 4-phosphate synthase which involved riboflavin biosynthesis [21]. Therefore, to demonstrate the potential use of the nDXP pathway to enhance the production of isoprenoids, we chose to overexpress these nDXP enzymes in M. alcaliphilum 20Z (Figure 1). Besides, we selected α-humulene as the target sesquiterpenoid product. In our previous study, for the production of α-humulene, we constructed a pHM03 vector containing α-humulene synthase (zssI) along with some bottlenecks of the MEP pathway in M. alcaliphilum 20Z (Dxs, IspG, and IspA) that had previously shown to improve the flux pathway [19]. Then, yajO and the ribBG108S variant from E. coli which was generated by Gibson assembly-based site-directed mutagenesis were cloned into pHM03 under control of a strong constitutive Ptac promoter, resulting in the pDXP-01 and pDXP-02 vectors (Supplementary Tables S1 and S2). Additionally, we also found other nDXP candidates for screening. The RibB encoding 3,4-dihydroxy-2-butanone 4-phosphate synthase in the archaebacterium Methanococcus jannaschii was well-investigated with the availability of the crystal structure [28]. Multiple alignment was performed using RibB from E. coli and other well-studied RibB including RibB from M. jannaschii, which showed that the amino acid G at position 108 in the RibB from E. coli is conserved among these species (Supplementary Figure S1). Therefore, we hypothesized that the replacement of amino acid G to S in other RibB variants might work as nDXP enzymes. Thus, we employed the ribBG113S variant from M. jannaschii as an nDXP enzyme, resulting in vector pDXP-03 (Appendix B). The resultant strain (pDXP-01-03) was employed using a two-phase culture wherein 20% dodecane was used as the organic phase and methane was used as the carbon source, and α-humulene production in these engineered strains was assayed. Interestingly, as expected, the overexpression of ribBG108S and yajO from E. coli boosted higher α-humulene production compared to the parent strain pHM03, while ribBG113S from M. jannaschii did not (Figure 2A). In particular, pDXP-02 harboring ribBG108S from E. coli showed the highest improvement, which was ~1.6-fold higher than the pHM03 strain with the productivity of 0.32 mg/gDCW. In agreement with this result, the previous study had shown that ribBG108S has a more efficient enzyme activity for the conversion of Ru5P to DXP as compared to yajO. Therefore, the pDXP-02 strain was used for further experiments.
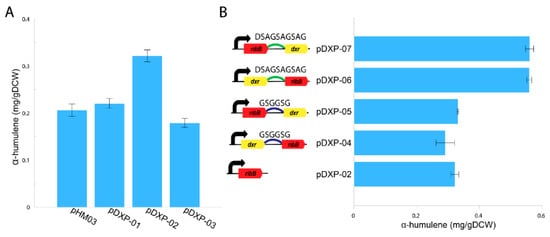
Figure 2.
Production of α-humulene in the M. alcaliphilum 20Z pHM03, pDXP-01, pDXP-02, and pDXP-03 strains using methane (50% v/v) as the sole carbon substrate (A). Schematic overview of the protein fusions between ribBG108S and dxr and the α-humulene production of the M. alcaliphilum 20Z pDXP-02, pDXP-04, pDXP-05, pDXP-06 and pDXP-07 strains (B). Analysis of α-humulene in the dodecane layer was performed, and the concentrations were compared after 96-h cultivation. The data represent the means from three replicates ± standard deviations.
3.2. Fusion of nDXP/Dxr Enhanced the Carbon Flux through the nDXP Pathway
There are several protein engineering strategies employed to improve isoprenoid production [29]. Protein fusions or scaffolds were reported to enhance the carbon flux in several cases such as isoprenoid pathway engineering [30]. Due to poor kinetics of RibBG108S enzymes, we hypothesized that protein fusion of RibBG108S and DXP reductase (Dxr) might improve the kinetic property in the delivery of Ru5P to the MEP pathway [21]. Therefore, the fusions of ribBG108S and native dxr were constructed with different orders and linkers. We used two peptide linkers: G2 corresponds to a GSGGSG linker and DSAG corresponds to a DSAGSAGSAG linker. As a result, the four fused proteins were constructed including dxr-G2-ribB, ribB-G2-dxr, dxr-DSAG-ribB, and ribB-DSAG-dxr (Figure 2B). These fused proteins were co-overexpressed with the pHM03 vector, resulting in pDXP04–pDXP07 vectors (Supplementary Tables S1 and S2). These vectors also harbored a strong constitutive Ptac promoter, which was applied to heterologous expression in M. alcaliphilum 20Z. Subsequently, the production of α-humulene from methane in these engineering strains was assayed using aqueous–organic two-phase flask cultivation where 20% dodecane was added as the organic phase. As shown in Figure 2B, the fusions of ribBG108S and dxr using the DSAG linker (ribB-DSAG-dxr, dxr-DSAG-ribB) both yielded higher productivity, which was ~2.8-fold higher than that of the pHM03 strain with the productivity of ~0.56 mg/gDCW. In contrast, the fusions by the G2 linker did not improve the production of α-humulene, suggesting that the G2 linker might not work in M. alcaliphilum 20Z. Likewise, Kirby et al. also reported that the expression of the Dxr-RibB (G108S) fusion improved α-bisabolene titers more than fourfold [21]. In summary, these results suggest that the fusion of dxr to ribBG108S could enhance the carbon flux through the isoprenoid biosynthesis pathway in M. alcaliphilum 20Z.
3.3. Engineering of M. alcaliphilum 20Z for α-Bisabolene Production via Synergy of the MEP and nDXP Pathways
To expand the suite of products that can be generated from methane using methanotrophic biocatalysts, we further demonstrated the bioconversion of methane to another sesquiterpenoid, α-bisabolene. It is a monocyclic sesquiterpene and a candidate biodiesel fuel that is produced by several microbial hosts via the heterologous expression of α-bisabolene synthase from various plant sources [31]. Recently, to improve the economic feasibility of the biological production of α-bisabolene, various microbial hosts using different alternative carbon sources were used to produce α-bisabolene via the MEP pathway [24,32,33]. However, α-bisabolene production from the next-generation feedstock, such as C1 compounds, is still in its infancy.
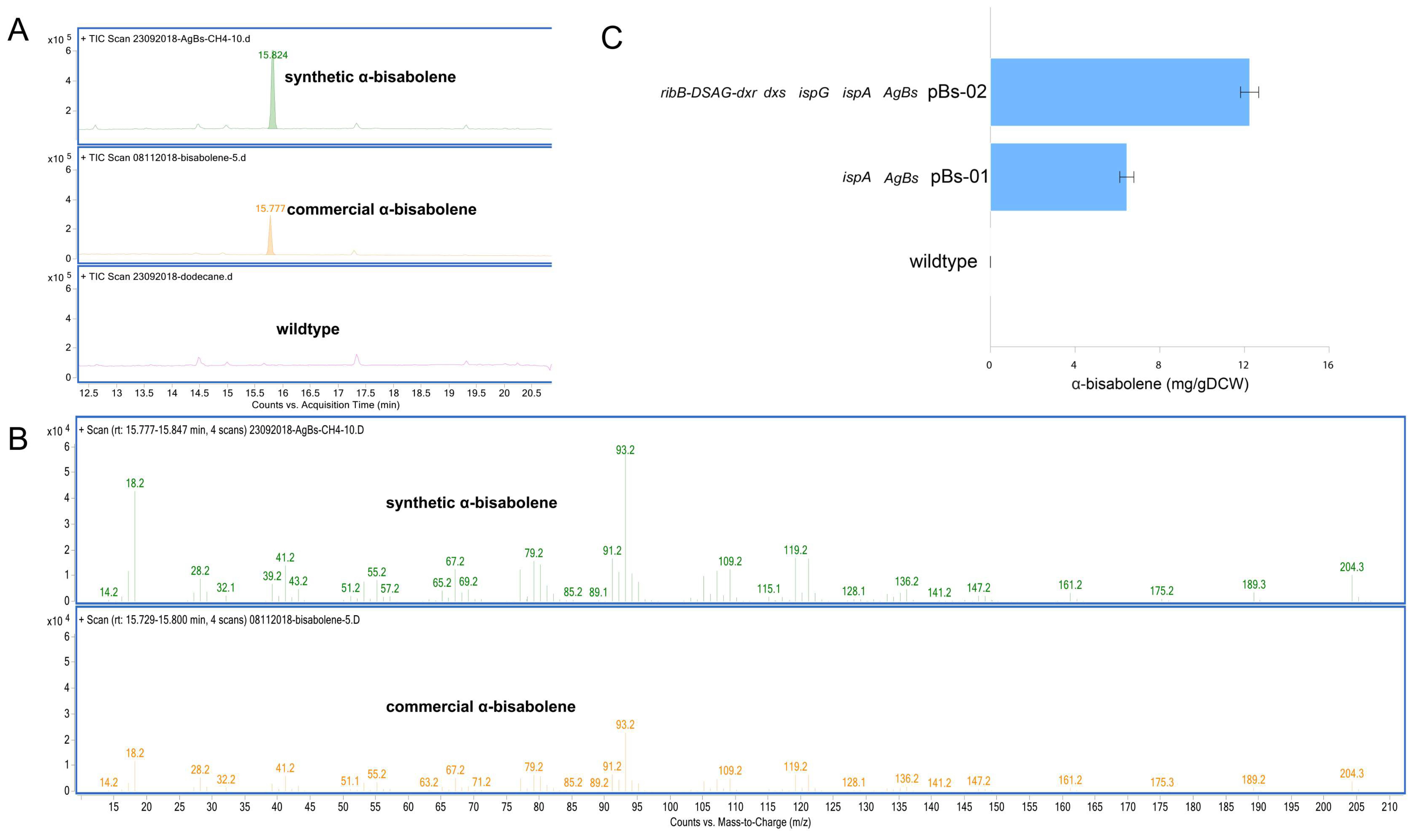
To further increase the supply of the farnesyl pyrophosphate (FPP) pool, the native FPP synthase (ispA) from M. alcaliphilum 20Z was expressed along with α-bisabolene synthase from Abies grandis (AgBs). The pBs01 vector was constructed for expression of a codon-optimized AgBs and native ispA, driven by the Ptac promoter (Supplementary Tables S1 and S2). Interestingly, the pBs-01 strain could produce α-bisabolene as characterized by GC-MS analysis. As shown in Figure 3A, B, α-bisabolene produced from pBs-01 has a similar retention time and mass spectrum compared to the α-bisabolene standard. In contrast, α-bisabolene was not produced in wildtype. In particular, the pBs-01 strain was able to produce 6.44 ± 0.33 mg/gDCW (12.8 ± 0.66 mg/L) of α-bisabolene (Figure 3C).
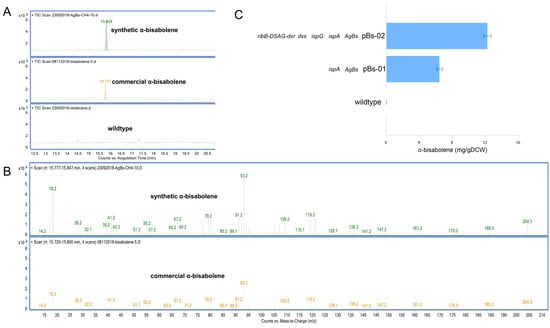
Figure 3.
GC-MS chromatogram (A), extracted ion GC-MS chromatograms (B) of the α-bisabolene standard and synthetic α-bisabolene produced by the pBs-01 strain and α-bisabolene production of the M. alcaliphilum 20Z pBs-01 and pBs-02 strains using methane (50% v/v) as the sole carbon substrate (C). Analysis of α-bisabolene in the dodecane layer was performed, and the concentrations were compared after 96-h cultivation. The data represent the means from three replicates ± standard deviations.
As noted in the previous section, the utility of nDXP along with the MEP pathway could improve isoprenoid production using a methanotrophic biocatalyst. Therefore, we expected that the synergy of the DXP route along with the optimized MEP pathway might also enhance α-bisabolene production in M. alcaliphilum 20Z. Thus, we constructed a vector for the expression of AgBs along with MEP pathway enzymes (ispA, dxs, and ispG) and the optimized nDXP route (dxr-DSAG-ribB), resulting in the pBs-02 vector (Supplementary Tables S1 and S2). As a result, α-bisabolene was produced in the pBs-02 strain with the titer of 12.24 ± 0.43 mg/gDCW (24.55 ± 0.86 mg/L), which was twofold higher than that in the pBs-01 strain (Figure 3C). These results suggested that the engineering strategies proposed in this study could become a powerful approach for enhancing the production of isoprenoid-related products.
However, in comparison with other conventional bacteria, the resulting titer of isoprenoids from methanotrophic bacteria was still far below. Methanotrophic bacteria utilize methane as the sole carbon source through the oxidation process, which requires a large amount of energy [34]. Besides, the generation of multi-carbon products such as isoprenoids from a C1 compound such as methane or methanol is very difficult. Therefore, the heterologous expression of the assimilation pathway for utilizing an additional carbon source such as xylose beside methane and the addition of a micromineral such as tungsten into the media could be fantastic options to significantly enhance the production of isoprenoids in methanotrophic bacteria. Indeed, the addition of xylose, as well as of tungsten, led to the increase in the growth of methanotrophic bacteria resulting in the improvement of some valuable products such as shinorine, acetoin, 2,3-butanediol, or 3-hydroxybutyric acid [27]. Moreover, for further improvement of the isoprenoid titer, a fed-batch bioreactor with a continuous supply of methane could also be applied [35].
4. Conclusions
In summary, we demonstrated a novel engineering strategy for enhancing sesquiterpenoid production from methane in metabolically engineered methanotrophic bacteria by the synergy of the MEP pathway and a novel short-cut nDXP pathway. This study also expanded the suite of sesquiterpenoids converted from methane using methanotrophic biocatalysts. The approaches developed in this study enabled enhanced sesquiterpenoid production and can be applied to the production of diverse isoprenoids from methane.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9061236/s1, Figure S1: Multiple alignment of some RibB variants, Table S1: Strains and plasmids used in this study, Table S2: Primers and procedures used for plasmid construction in this study.
Author Contributions
The manuscript was written through the contributions of all the authors. A.D.N. performed the experiments and wrote the manuscript. D.N.P. and T.H.T.C. revised the experimental data and the manuscript. E.Y.L. performed conceptualization, supervision of the study, and secured the funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the C1 Gas Refinery Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2015M3D3A1A01064882).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are included in the manuscript, supplementary data, Appendix A and Appendix B.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Genome-scale model of iIA407-nDXP.
Appendix B
Codon-optimized ribB from Methanococcus jannaschii.
References
- Withers, S.T.; Keasling, J.D. Biosynthesis and engineering of isoprenoid small molecules. Appl. Microbiol. Biotechnol. 2007, 73, 980–990. [Google Scholar] [CrossRef]
- Wang, C.; Zada, B.; Wei, G.; Kim, S.-W. Metabolic engineering and synthetic biology approaches driving isoprenoid production in Escherichia coli. Bioresour. Technol. 2017, 241, 430–438. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Keasling, J.D. Advanced biofuel production in microbes. Biotechnol. J. 2010, 5, 147–162. [Google Scholar] [CrossRef]
- Mewalal, R.; Rai, D.K.; Kainer, D.; Chen, F.; Külheim, C.; Peter, G.F.; Tuskan, G.A. Plant-derived terpenes: A feedstock for specialty biofuels. Trends Biotechnol. 2017, 35, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.A.; Gonzalez, R. Rethinking biological activation of methane and conversion to liquid fuels. Nat. Chem. Biol. 2014, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhnaya, M.G.; Puri, A.W.; Lidstrom, M.E. Metabolic engineering in methanotrophic bacteria. Metab. Eng. 2015, 29, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Clomburg, J.M.; Crumbley, A.M.; Gonzalez, R. Industrial biomanufacturing: The future of chemical production. Science 2017, 355. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.Y.; Nguyen, A.D.; Nguyen, T.T.; Nguyen, L.T.; Lee, O.K.; Lee, E.Y. Biological conversion of methane to chemicals and fuels: Technical challenges and issues. Appl. Microbiol. Biotechnol. 2018, 102, 3071–3080. [Google Scholar] [CrossRef]
- Kabimoldayev, I.; Nguyen, A.D.; Yang, L.; Park, S.; Lee, E.Y.; Kim, D. Basics of genome-scale metabolic modeling and applications on C1-utilization. FEMS Microbiol. Lett. 2018, 365, fny241. [Google Scholar] [CrossRef]
- Lee, E.Y. Methanotrophs: Microbiology Fundamentals and Biotechnological Applications; Springer Nature: Basingstoke, UK, 2019; Volume 32. [Google Scholar]
- Nguyen, A.D.; Hwang, I.Y.; Lee, O.K.; Kim, D.; Kalyuzhnaya, M.G.; Mariyana, R.; Hadiyati, S.; Kim, M.S.; Lee, E.Y. Systematic metabolic engineering of Methylomicrobium alcaliphilum 20Z for 2, 3-butanediol production from methane. Metab. Eng. 2018, 47, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Akberdin, I.R.; Thompson, M.; Hamilton, R.; Desai, N.; Alexander, D.; Henard, C.A.; Guarnieri, M.T.; Kalyuzhnaya, M.G. Methane utilization in Methylomicrobium alcaliphilum 20ZR: A systems approach. Sci. Rep. 2018, 8, 2512. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Park, J.Y.; Hwang, I.Y.; Hamilton, R.; Kalyuzhnaya, M.G.; Kim, D.; Lee, E.Y. Genome-scale evaluation of core one-carbon metabolism in gammaproteobacterial methanotrophs grown on methane and methanol. Metab. Eng. 2020, 57, 1–12. [Google Scholar] [CrossRef]
- Jeon, Y.C.; Nguyen, A.D.; Lee, E.Y. Bioproduction of isoprenoids and other secondary metabolites using methanotrophic bacteria as an alternative microbial cell factory option: Current stage and future aspects. Catalysts 2019, 9, 883. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Lee, E.Y. Engineered methanotrophy: A sustainable solution for methane-based industrial biomanufacturing. Trends Biotechnol. 2020, 39, 381–396. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Kim, D.; Lee, E.Y. A comparative transcriptome analysis of the novel obligate methanotroph Methylomonas sp. DH-1 reveals key differences in transcriptional responses in C1 and secondary metabolite pathways during growth on methane and methanol. BMC Genom. 2019, 20, 130. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, D.; He, R.; Wu, M.; Chen, W.; Gao, F.; Zhang, Z.; Yao, Y.; Yu, L.; Chen, S. Synthesizing value-added products from methane by a new Methylomonas. J. Appl. Microbiol. 2017, 123, 1214–1227. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.W.; Yao, H.; Stead, K.; Wang, T.; Tao, L.; Cheng, Q.; Sharpe, P.L.; Suh, W.; Nagel, E.; Arcilla, D. Construction of the astaxanthin biosynthetic pathway in a methanotrophic bacterium Methylomonas sp. strain 16a. J. Ind. Microbiol. Biotechnol. 2007, 34, 289. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.D.; Kim, D.; Lee, E.Y. Unlocking the biosynthesis of sesquiterpenoids from methane via the methylerythritol phosphate pathway in methanotrophic bacteria, using α-humulene as a model compound. Metab. Eng. 2020, 61, 69–78. [Google Scholar] [CrossRef]
- Banerjee, A.; Wu, Y.; Banerjee, R.; Li, Y.; Yan, H.; Sharkey, T.D. Feedback inhibition of deoxy-D-xylulose-5-phosphate synthase regulates the methylerythritol 4-phosphate pathway. J. Biol. Chem. 2013, 288, 16926–16936. [Google Scholar] [CrossRef]
- Kirby, J.; Nishimoto, M.; Chow, R.W.; Baidoo, E.E.; Wang, G.; Martin, J.; Schackwitz, W.; Chan, R.; Fortman, J.L.; Keasling, J.D. Enhancing terpene yield from sugars via novel routes to 1-deoxy-d-xylulose 5-phosphate. Appl. Environ. Microbiol. 2015, 81, 130–138. [Google Scholar] [CrossRef]
- Ojala, D.S.; Beck, D.A.; Kalyuzhnaya, M.G. Genetic systems for moderately halo (alkali) philic bacteria of the genus Methylomicrobium. In Methods Enzymol; Elsevier: Amsterdam, The Netherlands, 2011; Volume 495, pp. 99–118. [Google Scholar]
- Sonntag, F.; Kroner, C.; Lubuta, P.; Peyraud, R.; Horst, A.; Buchhaupt, M.; Schrader, J. Engineering Methylobacterium extorquens for de novo synthesis of the sesquiterpenoid α-humulene from methanol. Metab. Eng. 2015, 32, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, J.; Baier, T.; Wentnagel, E.; Lauersen, K.J.; Kruse, O. Tailored carbon partitioning for phototrophic production of (E)-α-bisabolene from the green microalga Chlamydomonas reinhardtii. Metab. Eng. 2018, 45, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, A.; Lerman, J.A.; Palsson, B.O.; Hyduke, D.R. COBRApy: Constraints-based reconstruction and analysis for python. BMC Syst. Biol. 2013, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Maia, P.; Evangelista, P.; Vilaça, P.; Soares, S.; Pinto, J.P.; Nielsen, J.; Patil, K.R.; Ferreira, E.C.; Rocha, M. OptFlux: An open-source software platform for in silico metabolic engineering. BMC Syst. Biol. 2010, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.D.; Chau, T.H.T.; Lee, E.Y. Methanotrophic microbial cell factory platform for simultaneous conversion of methane and xylose to value-added chemicals. Chem. Eng. J. 2020, 127632. [Google Scholar] [CrossRef]
- Fischer, M.; Römisch, W.; Schiffmann, S.; Kelly, M.; Oschkinat, H.; Steinbacher, S.; Huber, R.; Eisenreich, W.; Richter, G.; Bacher, A. Biosynthesis of riboflavin in archaea studies on the mechanism of 3, 4-dihydroxy-2-butanone-4-phosphate synthase of Methanococcus jannaschii. J. Biol. Chem. 2002, 277, 41410–41416. [Google Scholar] [CrossRef]
- Dueber, J.E.; Wu, G.C.; Malmirchegini, G.R.; Moon, T.S.; Petzold, C.J.; Ullal, A.V.; Prather, K.L.; Keasling, J.D. Synthetic protein scaffolds provide modular control over metabolic flux. Nat. Biotechnol. 2009, 27, 753–759. [Google Scholar] [CrossRef]
- Brodelius, M.; Lundgren, A.; Mercke, P.; Brodelius, P.E. Fusion of farnesyldiphosphate synthase and epi-aristolochene synthase, a sesquiterpene cyclase involved in capsidiol biosynthesis in Nicotiana tabacum. Eur. J. Biochem. 2002, 269, 3570–3577. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T.S. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2011, 2, 483. [Google Scholar] [CrossRef]
- Davies, F.K.; Work, V.H.; Beliaev, A.S.; Posewitz, M.C. Engineering limonene and bisabolene production in wild type and a glycogen-deficient mutant of Synechococcus sp. PCC 7002. Front. Bioeng. Biotechnol. 2014, 2, 21. [Google Scholar] [CrossRef]
- Phelan, R.M.; Sekurova, O.N.; Keasling, J.D.; Zotchev, S.B. Engineering terpene biosynthesis in Streptomyces for production of the advanced biofuel precursor bisabolene. ACS Synth. Biol. 2015, 4, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhnaya, M.; Yang, S.; Rozova, O.; Smalley, N.; Clubb, J.; Lamb, A.; Gowda, G.N.; Raftery, D.; Fu, Y.; Bringel, F. Highly efficient methane biocatalysis revealed in a methanotrophic bacterium. Nat. Commun. 2013, 4, 2785. [Google Scholar] [CrossRef] [PubMed]
- Wehrs, M.; Tanjore, D.; Eng, T.; Lievense, J.; Pray, T.R.; Mukhopadhyay, A. Engineering robust production microbes for large-scale cultivation. Trends Microbiol. 2019, 27, 524–537. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).