Malnutrition Aggravates Alterations Observed in the Gut Structure and Immune Response of Mice Infected with Leishmania infantum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Parasite Culture
2.3. Mice, Feeding Protocol and Experimental Infection
2.4. Hormone and Cytokine Levels
2.5. Positivity of Infection and Parasite Load
2.6. Histopathological Analysis
2.7. Immunohistochemistry for Detection of Leishmania
2.8. Gene Expression Analysis
2.9. Quantification of Local and Systemic Soluble Immunoglobulin A (IgA) Levels
2.10. Statistical Analysis
3. Results
3.1. Whereas Systemic Levels of IGF-1 and Leptin Are Significantly Decreased and Corticosterone and IgA Are Significantly Increased in Malnourished BALB/c Mice
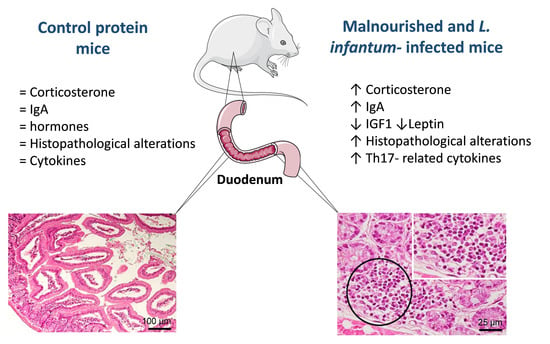
3.2. Infection with L. infantum Induced Structural and Inflammatory Alterations in the Duodenum of BALB/c Mice and Such Changes Were Aggravated by Preceding Malnutrition
3.3. Malnutrition Upregulated mRNA Levels of il-17a, tgfβ, and il-10 in the Duodenum of BALB/c Mice
3.4. Infection with L. infantum Induced a Significant Decrease in TNFα, IL-12 and TGFβ Protein Levels in the Duodenum of BALB/c Mice, Whereas Malnutrition Increased the Concentration of Th17-Related Cytokines
3.5. Whereas CCL5 Protein Levels Were Significantly Increased in the Duodenum of Malnourished-Infected Mice, CXCL10 Was Decreased in These Animals
3.6. L. infantum Infection Induced a Significant Increase in sIgA Levels in the Duodenum of Well-Nourished Mice, but Malnourished-Infected Animals Exhibited Impaired Secretion of IgA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourke, C.D.; Berkley, J.A.; Prendergast, A.J. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol. 2016, 37, 386–398. [Google Scholar] [CrossRef] [Green Version]
- Badaro, R.; Carvalho, E.M.; Rocha, H.; Queiroz, A.C.; Jones, T.C. Leishmania donovani: An opportunistic microbe associated with progressive disease in three immunocompromised patients. Lancet 1986, 1, 647–649. [Google Scholar] [CrossRef]
- Rosenthal, P.J.; Chaisson, R.E.; Hadley, W.K.; Leech, J.H. Rectal leishmaniasis in a patient with acquired immunodeficiency syndrome. Am. J. Med. 1988, 84, 307–309. [Google Scholar] [CrossRef]
- Anstead, G.M.; Chandrasekar, B.; Zhang, Q.; Melby, P.C. Multinutrient undernutrition dysregulates the resident macrophage proinflammatory cytokine network, nuclear factor-kappaB activation, and nitric oxide production. J. Leukoc. Biol. 2003, 74, 982–991. [Google Scholar] [CrossRef] [Green Version]
- Anstead, G.M.; Chandrasekar, B.; Zhao, W.; Yang, J.; Perez, L.E.; Melby, P.C. Malnutrition alters the innate immune response and increases early visceralization following Leishmania donovani infection. Infect. Immun. 2001, 69, 4709–4718. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.K.; Barnes, J.L.; Anstead, G.M.; Jimenez, F.; Travi, B.L.; Peniche, A.G.; Osorio, E.Y.; Ahuja, S.S.; Melby, P.C. The malnutrition-related increase in early visceralization of Leishmania donovani is associated with a reduced number of lymph node phagocytes and altered conduit system flow. PLoS Negl. Trop. Dis. 2013, 7, e2329. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.K.; Zambruni, M.; Melby, C.L.; Melby, P.C. Impact of Childhood Malnutrition on Host Defense and Infection. Clin. Microbiol. Rev. 2017, 30, 919–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuervo-Escobar, S.; Losada-Barragan, M.; Umana-Perez, A.; Porrozzi, R.; Saboia-Vahia, L.; Miranda, L.H.; Morgado, F.N.; Menezes, R.C.; Sanchez-Gomez, M.; Cuervo, P. T-cell populations and cytokine expression are impaired in thymus and spleen of protein malnourished BALB/c mice infected with Leishmania infantum. PLoS ONE 2014, 9, e114584. [Google Scholar] [CrossRef]
- Losada-Barragan, M.; Umana-Perez, A.; Cuervo-Escobar, S.; Berbert, L.R.; Porrozzi, R.; Morgado, F.N.; Mendes-da-Cruz, D.A.; Savino, W.; Sanchez-Gomez, M.; Cuervo, P. Protein malnutrition promotes dysregulation of molecules involved in T cell migration in the thymus of mice infected with Leishmania infantum. Sci. Rep. 2017, 7, 45991. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barragan, M.; Umana-Perez, A.; Rodriguez-Vega, A.; Cuervo-Escobar, S.; Azevedo, R.; Morgado, F.N.; de Carvalho, V.F.; Aquino, P.; Carvalho, P.C.; Porrozzi, R.; et al. Proteomic profiling of splenic interstitial fluid of malnourished mice infected with Leishmania infantum reveals defects on cell proliferation and pro-inflammatory response. J. Proteom. 2019, 208, 103492. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barragan, M.; Umana-Perez, A.; Duraes, J.; Cuervo-Escobar, S.; Rodriguez-Vega, A.; Ribeiro-Gomes, F.L.; Berbert, L.R.; Morgado, F.; Porrozzi, R.; Mendes-da-Cruz, D.A.; et al. Thymic Microenvironment Is Modified by Malnutrition and Leishmania infantum Infection. Front. Cell. Infect. Microbiol. 2019, 9, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, M.M.; Deoti, B.; Amorim, I.F.; Pinto, A.J.; Moraes, A.; Carvalho, C.S.; da Silva, S.M.; de Assis, A.C.; de Faria, A.M.; Tafuri, W.L. Expression of regulatory T cells in jejunum, colon, and cervical and mesenteric lymph nodes of dogs naturally infected with Leishmania infantum. Infect. Immun. 2014, 82, 3704–3712. [Google Scholar] [CrossRef] [Green Version]
- Barati, M.; Sharifi, I.; Daie Parizi, M.; Fasihi Harandi, M. Bacterial infections in children with visceral leishmaniasis: Observations made in Kerman province, southern Iran, between 1997 and 2007. Ann. Trop. Med. Parasitol. 2008, 102, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Adamama-Moraitou, K.K.; Rallis, T.S.; Koytinas, A.F.; Tontis, D.; Plevraki, K.; Kritsepi, M. Asymptomatic colitis in naturally infected dogs with Leishmania infantum: A prospective study. Am. J. Trop. Med. Hyg. 2007, 76, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Luz, K.G.; Tuon, F.F.; Duarte, M.I.; Maia, G.M.; Matos, P.; Ramos, A.M.; Nicodemo, A.C. Cytokine expression in the duodenal mucosa of patients with visceral leishmaniasis. Rev. Soc. Bras. Med. Trop. 2010, 43, 393–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losada-Barragan, M.; Cavalcanti, A.; Umana-Perez, A.; Porrozzi, R.; Cuervo-Escobar, S.; Vallejo, A.F.; Sanchez-Gomez, M.; Cuervo, P. Detection and quantification of Leishmania infantum in naturally and experimentally infected animal samples. Vet. Parasitol. 2016, 226, 57–64. [Google Scholar] [CrossRef]
- Carson, F.L.; Cappellano, C.H. Histotechnology: A self-Instructional Text, 4th ed.; American Society for Clinical Pathology, ASCP Press: Chicago, IL, USA, 2015. [Google Scholar]
- Boechat, V.C.; Mendes, A.A., Jr.; Mde, F.M.; Ferreira, L.C.; Figueiredo, F.B.; Rodrigues, F.; Vda, C.O.; de Rde, V.O.; Menezes, R.C. Occurrence of Leishmania infantum and associated histological alterations in the genital tract and mammary glands of naturally infected dogs. Parasitol. Res. 2016, 115, 2371–2379. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Bankoti, R.; Stager, S. Differential Regulation of the Immune Response in the Spleen and Liver of Mice Infected with Leishmania donovani. J. Trop. Med. 2012, 2012, 639304. [Google Scholar] [CrossRef] [Green Version]
- Nylen, S.; Maurya, R.; Eidsmo, L.; Manandhar, K.D.; Sundar, S.; Sacks, D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J. Exp. Med. 2007, 204, 805–817. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, D.; Demartino, S.; Anjuere, F.; Ferrua, B.; Fragaki, K.; Le Fichoux, Y.; Kubar, J. Sustained parasite burden in the spleen of Leishmania infantum-infected BALB/c mice is accompanied by expression of MCP-1 transcripts and lack of protection against challenge. Eur. Cytokine Netw. 2001, 12, 340–347. [Google Scholar]
- Alves, G.B.; Pinho, F.A.; Silva, S.M.; Cruz, M.S.; Costa, F.A. Cardiac and pulmonary alterations in symptomatic and asymptomatic dogs infected naturally with Leishmania (Leishmania) chagasi. Braz. J. Med. Biol. Res. 2010, 43, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Diro, E.; van Griensven, J.; Mohammed, R.; Colebunders, R.; Asefa, M.; Hailu, A.; Lynen, L. Atypical manifestations of visceral leishmaniasis in patients with HIV in north Ethiopia: A gap in guidelines for the management of opportunistic infections in resource poor settings. Lancet Infect. Dis. 2015, 15, 122–129. [Google Scholar] [CrossRef]
- Oliveira, V.D.C.; Boechat, V.C.; Mendes, A.A.V., Jr.; Madeira, M.F.; Ferreira, L.C.; Figueiredo, F.B.; Campos, M.P.; de Rodrigues, F.D.C.C.; de Oliveira, R.V.C.; Amendoeira, M.R.R.; et al. Occurrence of Leishmania infantum in the central nervous system of naturally infected dogs: Parasite load, viability, co-infections and histological alterations. PLoS ONE 2017, 12, e0175588. [Google Scholar] [CrossRef]
- Hu, G.; Gong, A.Y.; Roth, A.L.; Huang, B.Q.; Ward, H.D.; Zhu, G.; Larusso, N.F.; Hanson, N.D.; Chen, X.M. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog. 2013, 9, e1003261. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shen, Y.; Liu, H.; Yin, J.; Zhang, X.T.; Gong, A.Y.; Chen, X.; Chen, S.; Mathy, N.W.; Cao, J.; et al. Induction of Inflammatory Responses in Splenocytes by Exosomes Released from Intestinal Epithelial Cells following Cryptosporidium parvum Infection. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilic, M.; Taskin, E.; Ustundag, B.; Aygun, A.D. The evaluation of serum leptin level and other hormonal parameters in children with severe malnutrition. Clin. Biochem. 2004, 37, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.D.; Dickinson, L.; Lee, J.; Kilgore, B.; Branham, B.; Elders, M.J. Effects of dietary composition on somatomedin activity in growing rats. J. Nutr. 1979, 109, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.O.; Oliveira, L.M.; Marchini, J.S.; Antunes-Rodrigues, J.; Elias, L.L.; Almeida, S.S. Effects of early protein malnutrition and environmental stimulation on behavioral and biochemical parameters in rats submitted to the elevated plus-maze test. Nutr. Neurosci. 2013, 16, 104–112. [Google Scholar] [CrossRef] [PubMed]
- McMurray, D.N.; Rey, H.; Casazza, L.J.; Watson, R.R. Effect of moderate malnutrition on concentrations of immunoglobulins and enzymes in tears and saliva of young Colombian children. Am. J. Clin. Nutr. 1977, 30, 1944–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rho, S.; Kim, H.; Shim, S.H.; Lee, S.Y.; Kim, M.J.; Yang, B.G.; Jang, M.H.; Han, B.W.; Song, M.K.; Czerkinsky, C.; et al. Protein energy malnutrition alters mucosal IgA responses and reduces mucosal vaccine efficacy in mice. Immunol. Lett. 2017, 190, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Sirisinha, S.; Suskind, R.; Edelman, R.; Asvapaka, C.; Olson, R.E. Secretory and serum IgA in children with protein-calorie malnutrition. Pediatrics 1975, 55, 166–170. [Google Scholar] [PubMed]
- Olas, K.; Butterweck, H.; Teschner, W.; Schwarz, H.P.; Reipert, B. Immunomodulatory properties of human serum immunoglobulin A: Anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin. Exp. Immunol. 2005, 140, 478–490. [Google Scholar] [CrossRef]
- Wolf, H.M.; Fischer, M.B.; Puhringer, H.; Samstag, A.; Vogel, E.; Eibl, M.M. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood 1994, 83, 1278–1288. [Google Scholar] [CrossRef] [Green Version]
- Nikolova, E.B.; Russell, M.W. Dual function of human IgA antibodies: Inhibition of phagocytosis in circulating neutrophils and enhancement of responses in IL-8-stimulated cells. J. Leukoc. Biol. 1995, 57, 875–882. [Google Scholar] [CrossRef]
- van Epps, D.E.; Williams, R.C., Jr. Suppression of leukocyte chemotaxis by human IgA myeloma components. J. Exp. Med. 1976, 144, 1227–1242. [Google Scholar] [CrossRef] [PubMed]
- Wilton, J.M. Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin. Exp. Immunol. 1978, 34, 423–428. [Google Scholar]
- Monteiro, R.C. Role of IgA and IgA fc receptors in inflammation. J. Clin. Immunol. 2010, 30. [Google Scholar] [CrossRef]
- Fagarasan, S.; Honjo, T. Intestinal IgA synthesis: Regulation of front-line body defences. Nat. Rev. Immunol. 2003, 3, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.W.; Ding, J.L. The unexplored roles of human serum IgA. DNA Cell Biol. 2014, 33, 823–829. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Yilmaz, B.; Limenitakis, J.P.; Ganal-Vonarburg, S.C. IgA Function in Relation to the Intestinal Microbiota. Annu. Rev. Immunol. 2018, 36, 359–381. [Google Scholar] [CrossRef] [PubMed]
- Yel, L. Selective IgA deficiency. J. Clin. Immunol. 2010, 30, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiberg, D.; Basic, M.; Smoczek, M.; Bode, U.; Bornemann, M.; Buettner, M. Participation of the spleen in the IgA immune response in the gut. PLoS ONE 2018, 13, e0205247. [Google Scholar] [CrossRef] [Green Version]
- Cao, A.T.; Yao, S.; Gong, B.; Elson, C.O.; Cong, Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J. Immunol. 2012, 189, 4666–4673. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Jaime, H.; de Oliveira, R.P.; Kang, B.; Spolski, R.; Vaziri, T.; Myers, T.G.; Thovarai, V.; Shen, Z.; Fox, J.G.; et al. Defective IgA response to atypical intestinal commensals in IL-21 receptor deficiency reshapes immune cell homeostasis and mucosal immunity. Mucosal Immunol. 2019, 12, 85–96. [Google Scholar] [CrossRef]
- Carmody, R.N.; Gerber, G.K.; Luevano, J.M., Jr.; Gatti, D.M.; Somes, L.; Svenson, K.L.; Turnbaugh, P.J. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015, 17, 72–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef] [Green Version]
- Villarino, N.F.; LeCleir, G.R.; Denny, J.E.; Dearth, S.P.; Harding, C.L.; Sloan, S.S.; Gribble, J.L.; Campagna, S.R.; Wilhelm, S.W.; Schmidt, N.W. Composition of the gut microbiota modulates the severity of malaria. Proc. Natl. Acad. Sci. USA 2016, 113, 2235–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abusleme, L.; Moutsopoulos, N.M. IL-17: Overview and role in oral immunity and microbiome. Oral Dis. 2017, 23, 854–865. [Google Scholar] [CrossRef]
- Eyerich, K.; Dimartino, V.; Cavani, A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur. J. Immunol. 2017, 47, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Sarra, M.; Pallone, F.; Macdonald, T.T.; Monteleone, G. IL-23/IL-17 axis in IBD. Inflamm. Bowel Dis. 2010, 16, 1808–1813. [Google Scholar] [CrossRef]
- Maxwell, J.R.; Zhang, Y.; Brown, W.A.; Smith, C.L.; Byrne, F.R.; Fiorino, M.; Stevens, E.; Bigler, J.; Davis, J.A.; Rottman, J.B.; et al. Differential Roles for Interleukin-23 and Interleukin-17 in Intestinal Immunoregulation. Immunity 2015, 43, 739–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, W., Jr.; Kamanaka, M.; Booth, C.J.; Town, T.; Nakae, S.; Iwakura, Y.; Kolls, J.K.; Flavell, R.A. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 2009, 10, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Lindemans, C.A.; Calafiore, M.; Mertelsmann, A.M.; O’Connor, M.H.; Dudakov, J.A.; Jenq, R.R.; Velardi, E.; Young, L.F.; Smith, O.M.; Lawrence, G.; et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015, 528, 560–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aujla, S.J.; Kolls, J.K. IL-22: A critical mediator in mucosal host defense. J. Mol. Med. 2009, 87, 451–454. [Google Scholar] [CrossRef] [PubMed]









| Type of Alteration | CP | LP | CPi | LPi | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | J | I | C | D | J | I | C | D | J | I | C | D | J | I | C | ||
| Lymphocytic inflammatory infiltrate in lamina propria | Mild | 17% (1/6) | 17% (1/6) | 17% (1/6) | 0/6 | 50% (3/6) | 50% (3/6) | 33% (2/6) | 0/6 | 50% (3/6) | 33% (2/6) | 17% (1/6) | 17% (1/6) | 50% (3/6) | 50% (3/6) | 33% (2/6) | 33% (2/6) |
| Moderate | 17% (1/6) | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 17% (1/6) | 17% (1/6) | 0/6 | 0/6 | 33% (2/6) | 33% (2/6) | 17% (1/6) | 17% (1/6) | |
| Transmural | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 17% (1/6) | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | |
| Lymphocytic inflammatory infiltrate in submucose (SM) | Mild | 0/6 | 0/6 | 17% (1/6) | 0/6 | 0/6 | 0/6 | 0/6 | 17% (1/6) | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 |
| Moderate | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 17% (1/6) | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | |
| Neutrophils, macrophages and plasma cells infiltrate | 0/6 | 0/6 | 17% (1/6) | 0/6 | 17% (1/6) | 17% (1/6) | 0/6 | 0/6 | 33% (2/6) | 50% (3/6) | 17% (1/6) | 0/6 | 83% (5/6) | 83% (5/6) | 50% (3/6) | 50% (3/6) | |
| Lymphoid hyperplasia | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 17% (1/6) | 0/6 | 33% (2/6) | 0/6 | 0/6 | 17% (1/6) | 0/6 | 17% (1/6) | 17% (1/6) | |
| Villus atrophy | 0/6 | 33% (2/6) | 17% (1/6) | 0/6 | 17% (1/6) | 0/6 | 17% (1/6) | 0/6 | 0/6 | 17% (1/6) | 17% (1/6) | 0/6 | 0/6 | 0/6 | 17% (1/6) | 0/6 | |
| Ulcers | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 33% (2/6) | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaitán-Albarracín, F.; Losada-Barragán, M.; Pinho, N.; Azevedo, R.; Durães, J.; Arcila-Barrera, J.S.; Menezes, R.C.; Morgado, F.N.; Carvalho, V.d.F.; Umaña-Pérez, A.; et al. Malnutrition Aggravates Alterations Observed in the Gut Structure and Immune Response of Mice Infected with Leishmania infantum. Microorganisms 2021, 9, 1270. https://doi.org/10.3390/microorganisms9061270
Gaitán-Albarracín F, Losada-Barragán M, Pinho N, Azevedo R, Durães J, Arcila-Barrera JS, Menezes RC, Morgado FN, Carvalho VdF, Umaña-Pérez A, et al. Malnutrition Aggravates Alterations Observed in the Gut Structure and Immune Response of Mice Infected with Leishmania infantum. Microorganisms. 2021; 9(6):1270. https://doi.org/10.3390/microorganisms9061270
Chicago/Turabian StyleGaitán-Albarracín, Felipe, Monica Losada-Barragán, Nathalia Pinho, Renata Azevedo, Jonathan Durães, Juan Sebastián Arcila-Barrera, Rodrigo C. Menezes, Fernanda N. Morgado, Vinicius de Frias Carvalho, Adriana Umaña-Pérez, and et al. 2021. "Malnutrition Aggravates Alterations Observed in the Gut Structure and Immune Response of Mice Infected with Leishmania infantum" Microorganisms 9, no. 6: 1270. https://doi.org/10.3390/microorganisms9061270
APA StyleGaitán-Albarracín, F., Losada-Barragán, M., Pinho, N., Azevedo, R., Durães, J., Arcila-Barrera, J. S., Menezes, R. C., Morgado, F. N., Carvalho, V. d. F., Umaña-Pérez, A., & Cuervo, P. (2021). Malnutrition Aggravates Alterations Observed in the Gut Structure and Immune Response of Mice Infected with Leishmania infantum. Microorganisms, 9(6), 1270. https://doi.org/10.3390/microorganisms9061270