The Respiratory Commensal Bacterium Dolosigranulum pigrum 040417 Improves the Innate Immune Response to Streptococcus pneumoniae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Animals and Experimental Infection
2.3. Broncho-Alveolar Lavage (BAL) Sampling
2.4. Lung Tissue Injury Studies
2.5. Determination of Cell Populations
2.6. Primary Cultures of Alveolar Macrophages
2.7. Cytokine Concentrations in BAL and Culture Supernatants
2.8. Analysis of Alveolar Macrophages by Flow Cytometry
2.9. In Vivo Depletion of Alveolar Macrophages
2.10. Statistical Analysis
3. Results
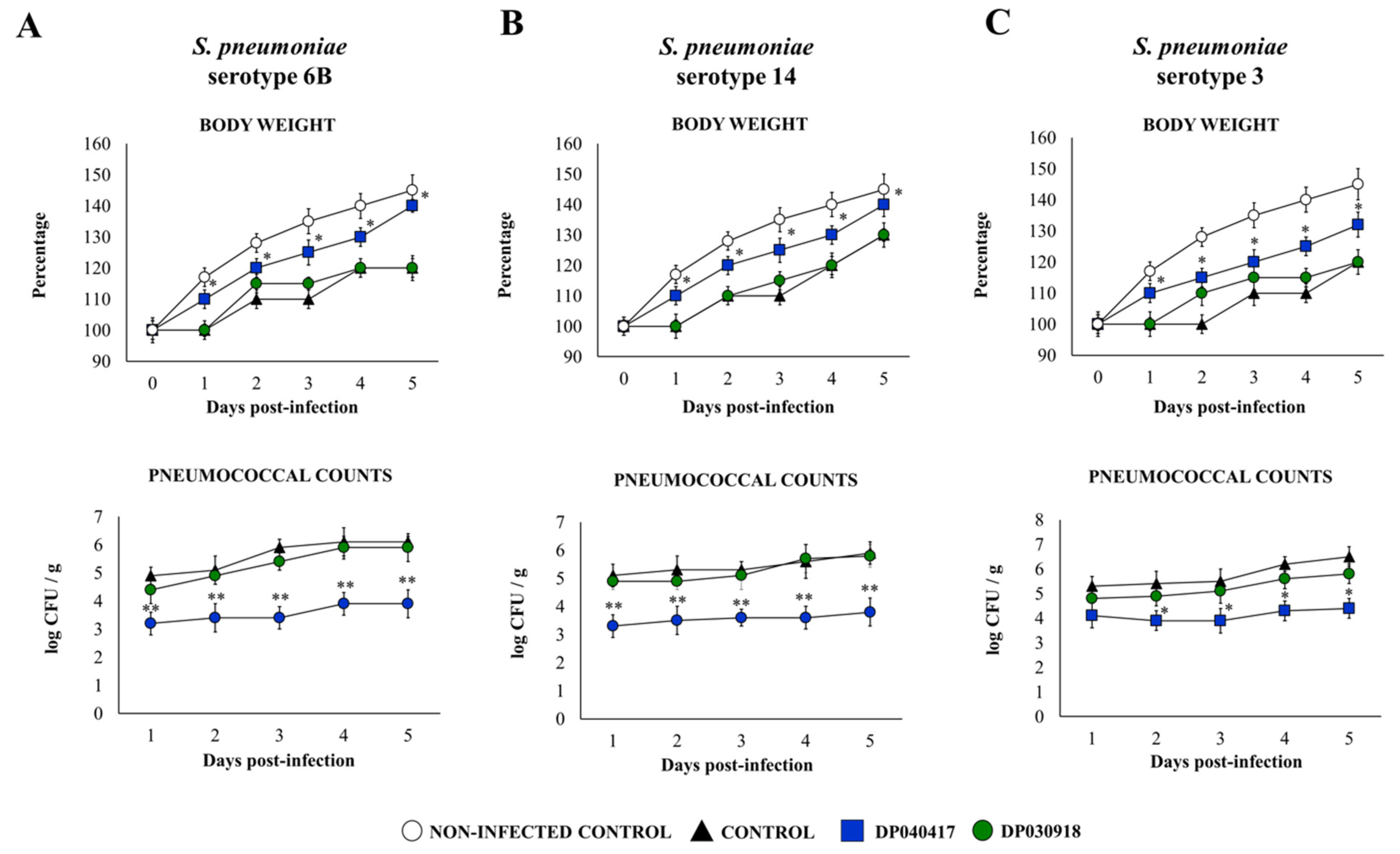
3.1. The Respiratory Commensal Bacteria D. pigrum Improve Resistance to Pneumococcal Infection in a Strain-Dependent Manner
3.2. D. pigrum 040417 Differentially Modulates the Respiratory Innate Immunity against S. pneumoniae
3.3. D. pigrum 040417 Differentially Modulates the Activation of Alveolar Macrophages
3.4. D. pigrum 040417 Modulates Alveolar Macrophages Cytokine Production
3.5. The Depletion of Alveolar Macrophages Significantly Affects the Ability of D. pigrum 040417 to Protect against Pneumococcal Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumpitsch, C.; Koskinen, K.; Schöpf, V.; Moissl-Eichinger, C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019, 17, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Hardy, B.L.; Merrell, D.S. Friend or Foe: Interbacterial Competition in the Nasal Cavity. J. Bacteriol. 2020, 203. [Google Scholar] [CrossRef]
- Man, W.H.; De Steenhuijsen Piters, W.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Hoggard, M.; Waldvogel-Thurlow, S.; Zoing, M.; Chang, K.; Radcliff, F.J.; MacKenzie, B.W.; Biswas, K.; Douglas, R.G.; Taylor, M.W. Inflammatory Endotypes and Microbial Associations in Chronic Rhinosinusitis. Front. Immunol. 2018, 9, 2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellussi, L.M.; Passali, F.M.; Ralli, M.; De Vincentiis, M.; Greco, A.; Passali, D. An overview on upper respiratory tract infections and bacteriotherapy as innovative therapeutic strategy. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 27–38. [Google Scholar]
- De Boeck, I.; Wittouck, S.; Wuyts, S.; Oerlemans, E.F.M.; van den Broek, M.F.L.; Vandenheuvel, D.; Vanderveken, O.; Lebeer, S. Comparing the Healthy Nose and Nasopharynx Microbiota Reveals Continuity As Well As Niche-Specificity. Front. Microbiol. 2017, 8, 2372. [Google Scholar] [CrossRef] [Green Version]
- Camelo- Castillo, A.; Henares, D.; Brotons, P.; Galiana, A.; Rodríguez, J.C.; Mira, A.; Muñoz-Almagro, C. on behalf of the Catalan Study Group of Host- Pathogen Interaction in Patients With IPD. Nasopharyngeal Microbiota in Children with Invasive Pneumococcal Disease: Identification of Bacteria with Potential Disease-Promoting and Protective Effects. Front. Microbiol. 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Laufer, A.S.; Metlay, J.P.; Gent, J.F.; Fennie, K.P.; Kong, Y.; Pettigrew, M.M. Microbial Communities of the Upper Respiratory Tract and Otitis Media in Children. mBio 2011, 2, e00245-10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettigrew, M.M.; Laufer, A.S.; Gent, J.F.; Kong, Y.; Fennie, K.P.; Metlay, J.P. Upper Respiratory Tract Microbial Communities, Acute Otitis Media Pathogens, and Antibiotic Use in Healthy and Sick Children. Appl. Environ. Microbiol. 2012, 78, 6262–6270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugger, S.D.; Eslami, S.M.; Pettigrew, M.M.; Escapa, I.F.; Henke, M.T.; Kong, Y.; Lemon, K.P. Dolosigranulum pigrum Cooperation and Competition in Human Nasal Microbiota. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Clua, P.; Vizoso-Pinto, M.G.; Rodriguez, C.; Alvarez, S.; Melnikov, V.; Takahashi, H.; Kitazawa, H.; Villena, J. Respiratory Commensal Bacteria Corynebacterium pseudodiphtheriticum Improves Resistance of Infant Mice to Respiratory Syncytial Virus and Streptococcus pneumoniae Superinfection. Front. Microbiol. 2017, 8, 1613. [Google Scholar] [CrossRef] [Green Version]
- Moyano, R.O.; Tonetti, F.R.; Tomokiyo, M.; Kanmani, P.; Vizoso-Pinto, M.G.; Kim, H.; Quilodrán-Vega, S.; Melnikov, V.; Alvarez, S.; Takahashi, H.; et al. The Ability of Respiratory Commensal Bacteria to Beneficially Modulate the Lung Innate Immune Response Is a Strain Dependent Characteristic. Microorganisms 2020, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Molimard, P.; Courau, S.; Crociani, J.; Dufour, C.; Le Vacon, F.; Carton, T. Beneficial effects of probiotics in upper respiratory tract infections and their mechanical actions to antagonize pathogens. J. Appl. Microbiol. 2012, 113, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- Spacova, I.; De Boeck, I.; Bron, P.A.; Delputte, P.; Lebeer, S. Topical Microbial Therapeutics against Respiratory Viral Infections. Trends Mol. Med. 2021, 27, 538–553. [Google Scholar] [CrossRef]
- Gan, W.; Yang, F.; Meng, J.; Liu, F.; Liu, S.; Xian, J. Comparing the nasal bacterial microbiome diversity of allergic rhinitis, chronic rhinosinusitis and control subjects. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.A.; Levin, E.; van Houten, M.A.; Hasrat, R.; Kalkman, G.; Biesbroek, G.; Piters, W.D.S.; de Groot, P.-K.C.; Pernet, P.; Keijser, B.J.; et al. Development of Upper Respiratory Tract Microbiota in Infancy is Affected by Mode of Delivery. EBioMedicine 2016, 9, 336–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, K.; Linnemann, R.W.; Mansbach, J.M.; Ajami, N.J.; Espinola, J.A.; Petrosino, J.F.; Piedra, P.A.; Stevenson, M.D.; Sullivan, A.F.; Thompson, A.D.; et al. Nasal Airway Microbiota Profile and Severe Bronchiolitis in Infants. Pediatr. Infect. Dis. J. 2017, 36, 1044–1051. [Google Scholar] [CrossRef]
- Wen, Z.; Xie, G.; Zhou, Q.; Qiu, C.; Li, J.; Hu, Q.; Dai, W.; Li, D.; Zheng, Y.; Wen, F. Distinct Nasopharyngeal and Oropharyngeal Microbiota of Children with Influenza A Virus Compared with Healthy Children. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laiño, J.; Villena, J.; Suvorov, A.; Zelaya, H.; Moyano, R.O.; Salva, S.; Alvarez, S. Nasal immunization with recombinant chimeric pneumococcal protein and cell wall from immunobiotic bacteria improve resistance of infant mice to Streptococcus pneumoniae infection. PLoS ONE 2018, 13, e0206661. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Racedo, S.; Aguero, G.; Bru, E.; Medina, M.; Alvarez, S. Lactobacillus casei Improves Resistance to Pneumococcal Respiratory Infection in Malnourished Mice. J. Nutr. 2005, 135, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Clua, P.; Tomokiyo, M.; Tonetti, F.R.; Islam, A.; Castillo, V.G.; Marcial, G.; Salva, S.; Alvarez, S.; Takahashi, H.; Kurata, S.; et al. The Role of Alveolar Macrophages in the Improved Protection against Respiratory Syncytial Virus and Pneumococcal Superinfection Induced by the Peptidoglycan of Lactobacillus rhamnosus CRL 1505. Cells 2020, 9, 1653. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castillo, V.; Tomokiyo, M.; Tonetti, F.R.; Islam, A.; Takahashi, H.; Kitazawa, H.; Villena, J. Alveolar Macrophages Are Key Players in the Modulation of the Respiratory Antiviral Immunity Induced by Orally Administered Lacticaseibacillus rhamnosus CRL 1505. Front. Immunol. 2020, 11, 568636. [Google Scholar] [CrossRef] [PubMed]
- Piters, W.A.A.D.S.; Jochems, S.P.; Mitsi, E.; Rylance, J.; Pojar, S.; Nikolaou, E.; German, E.L.; Holloway, M.; Carniel, B.F.; Chu, M.L.J.N.; et al. Interaction between the nasal microbiota and S. pneumoniae in the context of live-attenuated influenza vaccine. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Biesbroek, G.; Bosch, A.A.; Wang, X.; Keijser, B.J.; Veenhoven, R.H.; Sanders, E.A.; Bogaert, D. The Impact of Breastfeeding on Nasopharyngeal Microbial Communities in Infants. Am. J. Respir. Crit. Care Med. 2014, 190, 298–308. [Google Scholar] [CrossRef]
- Lappan, R.; Imbrogno, K.; Sikazwe, C.; Anderson, D.; Mok, D.; Coates, H.; Vijayasekaran, S.; Bumbak, P.; Blyth, C.C.; Jamieson, S.E.; et al. A microbiome case-control study of recurrent acute otitis media identified potentially protective bacterial genera. BMC Microbiol. 2018, 18, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Piters, W.D.S.; Bogaert, D. Unraveling the Molecular Mechanisms Underlying the Nasopharyngeal Bacterial Community Structure. mBio 2016, 7, e00009-16. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.; Walter, J.M.; Misharin, A.V. Alveolar Macrophages. Cell. Immunol. 2018, 330, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.C.; Yamada, M.; Martin, J.R.; Dang, H.; Brickey, W.J.; Bergmeier, W.; Dinauer, M.C.; Doerschuk, C.M. Mechanisms of Interferon-γ Production by Neutrophils and Its Function duringStreptococcus pneumoniaePneumonia. Am. J. Respir. Cell Mol. Biol. 2015, 52, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Marqués, J.M.; Rial, A.; Muñoz-Wolf, N.; Pellay, F.-X.; Van Maele, L.; Léger, H.; Camou, T.; Sirard, J.-C.; Benecke, A.; Chabalgoity, J.A. Protection against Streptococcus pneumoniae serotype 1 acute infection shows a signature of Th17- and IFN-γ-mediated immunity. Immunobiol. 2012, 217, 420–429. [Google Scholar] [CrossRef]
- Maier, B.B.; Hladik, A.; Lakovits, K.; Korosec, A.; Martins, R.; Kral, J.B.; Mesteri, I.; Strobl, B.; Müller, M.; Kalinke, U.; et al. Type I interferon promotes alveolar epithelial type II cell survival during pulmonary Streptococcus pneumoniae infection and sterile lung injury in mice. Eur. J. Immunol. 2016, 46, 2175–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeMessurier, K.S.; Häcker, H.; Chi, L.; Tuomanen, E.; Redecke, V. Type I Interferon Protects against Pneumococcal Invasive Disease by Inhibiting Bacterial Transmigration across the Lung. PLoS Pathog. 2013, 9, e1003727. [Google Scholar] [CrossRef] [Green Version]
- Peñaloza, H.F.; Nieto, P.A.; Muñoz-Durango, N.; Salazar-Echegarai, F.J.; Torres, J.; Parga, M.J.; Alvarez-Lobos, M.; Riedel, C.A.; Kalergis, A.; Bueno, S.M. Interleukin-10 plays a key role in the modulation of neutrophils recruitment and lung inflammation during infection byStreptococcus pneumoniae. Immunology 2015, 146, 100–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyle, C.J.; Uwadiae, F.I.; Swieboda, D.P.; Harker, J.A. Early IL-6 signalling promotes IL-27 dependent maturation of regulatory T cells in the lungs and resolution of viral immunopathology. PLoS Pathog. 2017, 13, e1006640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soroosh, P.; Doherty, T.; Duan, W.; Mehta, A.K.; Choi, H.; Adams, Y.F.; Mikulski, Z.; Khorram, N.; Rosenthal, P.; Broide, D.H.; et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J. Exp. Med. 2013, 210, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Kamada, R.; Yang, W.; Zhang, Y.; Patel, M.C.; Yang, Y.; Ouda, R.; Dey, A.; Wakabayashi, Y.; Sakaguchi, K.; Fujita, T.; et al. Interferon stimulation creates chromatin marks and establishes transcriptional memory. Proc. Natl. Acad. Sci. USA 2018, 115, E9162–E9171. [Google Scholar] [CrossRef] [Green Version]
- Wager, C.M.L.; Hole, C.; Campuzano, A.; Castro-Lopez, N.; Cai, H.; Van Dyke, M.C.C.; Wozniak, K.L.; Wang, Y. IFN-γ immune priming of macrophages in vivo induces prolonged STAT1 binding and protection against Cryptococcus neoformans. PLoS Pathog. 2018, 14, e1007358. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Jeyanathan, M.; Haddadi, S.; Barra, N.G.; Vaseghi-Shanjani, M.; Damjanovic, D.; Lai, R.; Afkhami, S.; Chen, Y.; Dvorkin-Gheva, A.; et al. Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell 2018, 175, 1634–1650.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albarracin, L.; Garcia-Castillo, V.; Masumizu, Y.; Indo, Y.; Islam, A.; Suda, Y.; Garcia-Cancino, A.; Aso, H.; Takahashi, H.; Kitazawa, H.; et al. Efficient Selection of New Immunobiotic Strains With Antiviral Effects in Local and Distal Mucosal Sites by Using Porcine Intestinal Epitheliocytes. Front. Immunol. 2020, 11, 543. [Google Scholar] [CrossRef]
- Tonetti, F.R.; Arce, L.; Salva, S.; Alvarez, S.; Takahashi, H.; Kitazawa, H.; Vizoso-Pinto, M.G.; Villena, J. Immunomodulatory Properties of Bacterium-Like Particles Obtained From Immunobiotic Lactobacilli: Prospects for Their Use as Mucosal Adjuvants. Front. Immunol. 2020, 11, 15. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raya Tonetti, F.; Tomokiyo, M.; Ortiz Moyano, R.; Quilodrán-Vega, S.; Yamamuro, H.; Kanmani, P.; Melnikov, V.; Kurata, S.; Kitazawa, H.; Villena, J. The Respiratory Commensal Bacterium Dolosigranulum pigrum 040417 Improves the Innate Immune Response to Streptococcus pneumoniae. Microorganisms 2021, 9, 1324. https://doi.org/10.3390/microorganisms9061324
Raya Tonetti F, Tomokiyo M, Ortiz Moyano R, Quilodrán-Vega S, Yamamuro H, Kanmani P, Melnikov V, Kurata S, Kitazawa H, Villena J. The Respiratory Commensal Bacterium Dolosigranulum pigrum 040417 Improves the Innate Immune Response to Streptococcus pneumoniae. Microorganisms. 2021; 9(6):1324. https://doi.org/10.3390/microorganisms9061324
Chicago/Turabian StyleRaya Tonetti, Fernanda, Mikado Tomokiyo, Ramiro Ortiz Moyano, Sandra Quilodrán-Vega, Hikari Yamamuro, Paulraj Kanmani, Vyacheslav Melnikov, Shoichiro Kurata, Haruki Kitazawa, and Julio Villena. 2021. "The Respiratory Commensal Bacterium Dolosigranulum pigrum 040417 Improves the Innate Immune Response to Streptococcus pneumoniae" Microorganisms 9, no. 6: 1324. https://doi.org/10.3390/microorganisms9061324
APA StyleRaya Tonetti, F., Tomokiyo, M., Ortiz Moyano, R., Quilodrán-Vega, S., Yamamuro, H., Kanmani, P., Melnikov, V., Kurata, S., Kitazawa, H., & Villena, J. (2021). The Respiratory Commensal Bacterium Dolosigranulum pigrum 040417 Improves the Innate Immune Response to Streptococcus pneumoniae. Microorganisms, 9(6), 1324. https://doi.org/10.3390/microorganisms9061324







