Exosomal microRNA let-7-5p from Taenia pisiformis Cysticercus Prompted Macrophage to M2 Polarization through Inhibiting the Expression of C/EBP δ
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. T. pisiformis Larvae Cultures and Exosome-Like Vesicles Isolation
2.3. Transmission Electron Microscopy (TEM) and Immune TEM (ITEM)
2.4. Absolute Quantification of let-7-5p in Exosome-Like Vesicles
2.5. Cell Culture and Macrophage Polarization
2.6. Exosome-Like Vesicles Treatment and miRNA Mimics/Inhibitor Transfection
2.7. qPCR Analysis of the M1/M2 Marker Relative Expression
2.8. Sequence Conservation and Target Genes Analysis of let-7-5p
2.9. Luciferase Assay
2.10. Western Blot
2.11. Statistical Analysis
3. Results
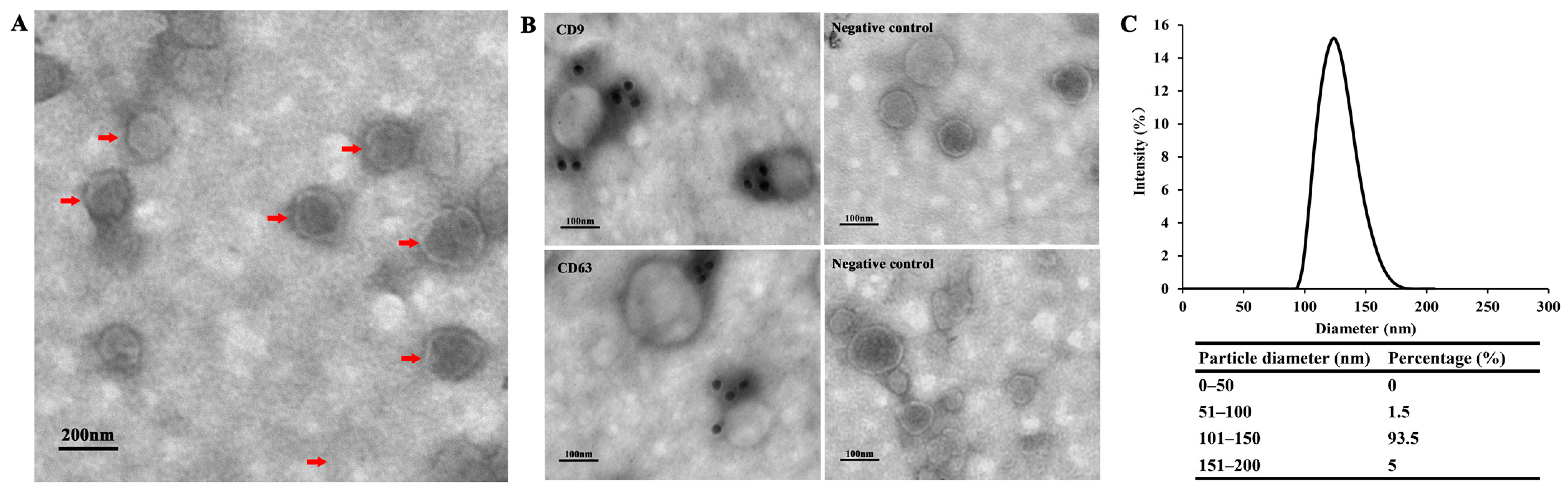
3.1. Exosome-Like Vesicles Secreted by T. pisiformis Metacestodes
3.2. Exosomal miRNA let-7-5p Derived from T. pisiformis Cysticercus Promoted M2 Macrophage Activation
3.3. C/EBP δ Was a Direct Target of miR-let-7-5p
3.4. C/EBP δ Was Involved in Macrophage Polarization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bansal, D.; Bhatti, H.S.; Sehgal, R. Role of Cholesterol in Parasitic Infections. Lipids. Health Dis. 2005, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Roldan, R.D.; Pérez-Martínez, M.; Rosetti, M.F.; Arias-Hernández, D.; Bernal-Fernández, G.; Flores-Pérez, F.I.; Hallal-Calleros, C. High Frequency of Taenia pisiformis Metacestodes and High Sex-Associated Susceptibility to Cysticercosis in Naturally Infected Wild Rabbits. Parasitol. Res. 2018, 117, 2201–2206. [Google Scholar] [CrossRef] [PubMed]
- Rajasekariah, G.R.; Rickard, M.D.; O’Donnell, I.J. Taenia pisiformis: Protective Immunization of Rabbits with Solubilized Oncospheral Antigens. Exp. Parasitol. 1985, 59, 321–327. [Google Scholar] [CrossRef]
- Sun, X.; Chen, H.; Cai, X. A Histopathologic Study on Cysticercus pisiformis Infected Rabbits. Acta Vet. Zootech. Sin. 2008, 39, 1100–1106. [Google Scholar]
- Hallal-Calleros, C.; Morales-Montor, J.; Orihuela-Trujillo, A.; Togno-Peirce, C.; Murcia-Mejía, C.; Bielli, A.; Hoffman, K.L.; Flores-Pérez, F.I. Taenia pisiformis Cysticercosis Induces Decreased Prolificacy and Increased Progesterone Levels in Rabbits. Vet. Parasitol. 2016, 229, 50–53. [Google Scholar] [CrossRef]
- McSorley, H.J.; Maizels, R.M. Helminth Infections and Host Immune Regulation. Clin. Microbiol. Rev. 2012, 25, 585–608. [Google Scholar] [CrossRef] [Green Version]
- Harnett, W. Secretory Products of Helminth Parasites as Immunomodulators. Mol. Biochem. Parasitol. 2014, 195, 130–136. [Google Scholar] [CrossRef]
- Hewitson, J.P.; Grainger, J.R.; Maizels, R.M. Helminth Immunoregulation: The Role of Parasite Secreted Proteins in Modulating Host Immunity. Mol. Biochem. Parasitol. 2009, 167, 1–11. [Google Scholar] [CrossRef]
- Coakley, G.; Buck, A.H.; Maizels, R.M. Host Parasite Communications-Messages from Helminths for the Immune System: Parasite Communication and Cell-Cell Interactions. Mol. Biochem. Parasitol. 2016, 208, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, L.; Wang, L.; Chen, Y.; Giri, B.R.; Li, J.; Cheng, G. Isolation and Characterization of Extracellular Vesicles from Adult Schistosoma Japonicum. J. Vis. Exp. 2018, 135, 57514. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, Z.; Shen, J.; Liu, Z.; Liang, J.; Wu, X.; Sun, X.; Wu, Z. Exosome-Like Vesicles Derived by Schistosoma Japonicum Adult Worms Mediates M1 Type Immune-Activity of Macrophage. Parasitol. Res. 2015, 114, 1865–1873. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, X.; Su, M.; Guo, A.; Ding, J.; Yang, J.; Xiang, H.; Cao, X.; Zhang, S.; Ayaz, M.; et al. Regulatory Effects of Echinococcus Multilocularis Extracellular Vesicles on RAW264.7 Macrophages. Vet. Parasitol. 2017, 235, 29–36. [Google Scholar] [CrossRef]
- Buck, A.H.; Coakley, G.; Simbari, F.; McSorley, H.J.; Quintana, J.F.; Bihan, T.L.; Kumar, S.; Abreu-Goodger, C.; Lear, M.; Harcus, Y.; et al. Exosomes secreted by Nematode Parasites Transfer Small RNAs to Mammalian Cells and Modulate Innate Immunity. Nat. Commun. 2014, 5, 5488. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, T.; Liang, P.; Zhang, S.; Li, T.; Li, Y.; Liu, G.; Mao, L.; Luo, X. Characterization of Exosome-Like Vesicles Derived from Cysticercus pisiformis and their Immunoregulatory Role on Macrophages. Parasit. Vectors. 2020, 13, 318. [Google Scholar] [CrossRef]
- Britton, C.; Winter, A.D.; Gillan, V.; Devaney, E. MicroRNAs of Parasitic Helminths-Identification, Characterization and Potential as Drug Targets. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Lai, E.C. Adult-specific Functions of Animal microRNAs. Nat. Rev. Genet. 2013, 14, 535–548. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Slack, F.J.; Basson, M.; Pasquinelli, A.E.; Bettinger, J.C.; Rougvie, A.E.; Horvitz, H.R.; Ruvkun, G. The 21-nucleotide Let-7 RNA Regulates Developmental Timing in Caenorhabditis Elegans. Nature 2000, 403, 901–906. [Google Scholar] [CrossRef]
- Baltimore, D.; Boldin, M.P.; O’Connell, R.M.; Rao, D.S.; Taganov, K.D. MicroRNAs: New Regulators of Immune Cell Development and Function. Nat. Immunol. 2008, 9, 839–845. [Google Scholar] [CrossRef]
- Banerjee, S.; Xie, N.; Cui, H.; Tan, Z.; Yang, S.; Icyuz, M.; Abraham, E.; Liu, G. MicroRNA Let-7c Regulates Macrophage Polarization. J. Immunol. 2013, 190, 6542–6549. [Google Scholar] [CrossRef]
- Liang, P.; Mao, L.; Zhang, S.; Guo, X.; Luo, X. Identification and Molecular Characterization of Exosome-Like Vesicles Derived from the Taenia asiatica Adult Worm. Acta Trop. 2019, 198, 105036. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wei, Z.; Ou, Q.; Liang, L.; Lin, X.; Wang, Y. Microrna-214 Suppresses Osteogenic Differentiation of Human Periodontal Ligament Stem Cells by Targeting Atf4. Stem Cells Int. 2017, 2017, 3028647. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Liu, X.; Zhou, Q.; Xie, J.; Ma, T.; Meng, J.; Li, J. Mir-146a Modulates Macrophage Polarization by Inhibiting Notch1 Pathway in RAW264.7 Macrophages. Int. Immunopharmacol. 2016, 32, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data using Real-time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lu, Y.; Kim, I.; Lye, E.; Shen, F.; Suzuki, N.; Suzuki, S.; Gerondakis, S.; Akira, S.; Gaffen, S.L.; Yeh, W.; et al. Differential Role for C-Rel and C/Ebpβ/Δ in Tlr-Mediated Induction of Proinflammatory Cytokines. J. Immunol. 2009, 182, 7212–7221. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cai, M.; Yang, J.; Li, Y.; Ding, J.; Kandil, O.M.; Kutyrev, I.; Ayaz, M.; Zheng, Y. Comparative Analysis of Different Extracellular Vesicles Secreted by Echinococcus Granulosus Protoscoleces. Acta Trop. 2021, 213, 105756. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Liu, X.; Zhang, Y.; Shi, H.; Jia, W.; Zhu, H.; Jia, H.; Liu, M.; Bai, X. Extracellular Vesicles Derived from Trichinella spiralis Muscle Larvae Ameliorate TNBS-Induced Colitis in Mice. Front. Immunol. 2020, 11, 1174. [Google Scholar] [CrossRef]
- Cruz, A.B.; Maia, G.M.; Pereira, I.S.; Taniwaki, N.N.; Namiyama, G.M.; Telles, J.P.M.; Mattos, C.C.B.; Mattos, L.C.; Meira-Strejevitch, C.S.; Pereira-Chioccola, V.L.P. Human Extracellular Vesicles and Correlation with Two Clinical Forms of Toxoplasmosis. PLoS ONE 2020, 15, e0229602. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Roush, S.; Slack, F.J. The Let-7 Family of MicroRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef]
- Tian, X.; Sun, M.; Wu, H.; Chen, C.; Li, H.; Qiu, S.; Wang, T.; Han, J.; Xiao, Q.; Chen, K. Exosome-derived miR-let-7c promotes angiogenesis in multiple myeloma by Polarizing M2 Macrophages in the Bone Marrow Microenvironment. Leuk. Res. 2021, 105, 106566. [Google Scholar] [CrossRef]
- Ancarola, M.E.; Marcilla, A.; Herz, M.; Macchiaroli, N.; Perez, M.; Asurmendi, S.; Brehm, K.; Poncini, C.; Rosenzvit, M.; Cucher, M. Cestode Parasites Release Extracellular Vesicles with MicroRNAs and Immunodiagnostic Protein Cargo. Int. J. Parasitol. 2017, 47, 675–686. [Google Scholar] [CrossRef]
- Hao, X.; Guo, Y.; Wang, R.; Yu, X.; He, L.; Shu, M. Exosomes from Adipose-Derived Mesenchymal Stem Cells Promote Survival of Fat Grafts by Regulating Macrophage Polarization via Let-7c. Acta Biochim. Biophys. Sin. 2021, 53, 501–510. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-Preconditioned Mesenchymal Stromal Cells Modify Macrophage Polarization for Resolution of Chronic Inflammation via Exosome-Shuttled Let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gottstein, B. Immunoregulation in Larval Echinococcus multilocularis Infection. Parasite Immunol. 2016, 38, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, R.M.; Sotillo, J.; Loukas, A. Immunobiology of Parasitic Worm Extracellular Vesicles. Immunol. Cell Biol. 2018, 96, 704–713. [Google Scholar] [CrossRef]





| Primer | Sequence (5′–3′) |
|---|---|
| iNOS forward primer | CGAAACGCTTCACTTCCAA |
| iNOS reverse primer | TGAGCCTATATTGCTGTGGCT |
| Arg-1 forward primer | TCATGGAAGTGAACCCAACTCTTG |
| Arg-1 reverse primer | TCAGTCCCTGGCTTATGGTTACC |
| IL-10 forward primer | GCTCTTACTGACTGGCATGAG |
| IL-10 reverse primer | CGCAGCTCTAGGAGCATGTG |
| IL-12 forward primer | TTGCTGGTGTCTCCACTCATG |
| IL-12 reverse primer | GTCACAGGTGAGGTTCACTGTTTC |
| GAPDH forward primer | GTCTTCACCACCATGGAG |
| GAPDH reverse primer | CCAAAGTTGTCATGGATGACC |
| RT primer | GCGAGCACAGAATTAATACGACTCACTATAGG(T)12VN a |
| Universal reverse primer | GCGAGCACAGAATTAATACGAC |
| miR-let-7-5p | TGAGGTAGTGTTTCGAATGTCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, T.; Chen, G.; Li, Y.; Zhang, S.; Mao, L.; Liang, P.; Fasihi Harandi, M.; Li, T.; Luo, X. Exosomal microRNA let-7-5p from Taenia pisiformis Cysticercus Prompted Macrophage to M2 Polarization through Inhibiting the Expression of C/EBP δ. Microorganisms 2021, 9, 1403. https://doi.org/10.3390/microorganisms9071403
Wang L, Liu T, Chen G, Li Y, Zhang S, Mao L, Liang P, Fasihi Harandi M, Li T, Luo X. Exosomal microRNA let-7-5p from Taenia pisiformis Cysticercus Prompted Macrophage to M2 Polarization through Inhibiting the Expression of C/EBP δ. Microorganisms. 2021; 9(7):1403. https://doi.org/10.3390/microorganisms9071403
Chicago/Turabian StyleWang, Liqun, Tingli Liu, Guoliang Chen, Yanping Li, Shaohua Zhang, Li Mao, Panhong Liang, Majid Fasihi Harandi, Taoshan Li, and Xuenong Luo. 2021. "Exosomal microRNA let-7-5p from Taenia pisiformis Cysticercus Prompted Macrophage to M2 Polarization through Inhibiting the Expression of C/EBP δ" Microorganisms 9, no. 7: 1403. https://doi.org/10.3390/microorganisms9071403
APA StyleWang, L., Liu, T., Chen, G., Li, Y., Zhang, S., Mao, L., Liang, P., Fasihi Harandi, M., Li, T., & Luo, X. (2021). Exosomal microRNA let-7-5p from Taenia pisiformis Cysticercus Prompted Macrophage to M2 Polarization through Inhibiting the Expression of C/EBP δ. Microorganisms, 9(7), 1403. https://doi.org/10.3390/microorganisms9071403







