The First Report on the Transovarial Transmission of Microsporidian Nosema bombycis in Lepidopteran Crop Pests Spodoptera litura and Helicoverpa armigera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of N. bombycis Spores
2.2. Rearing and Inoculating S. litura and H. armigera
2.3. Microscopic Observation of the Infections
2.4. Paraffin Section and Indirect Immunofluorescent Assay (IFA)
2.5. PCR Amplification
3. Results
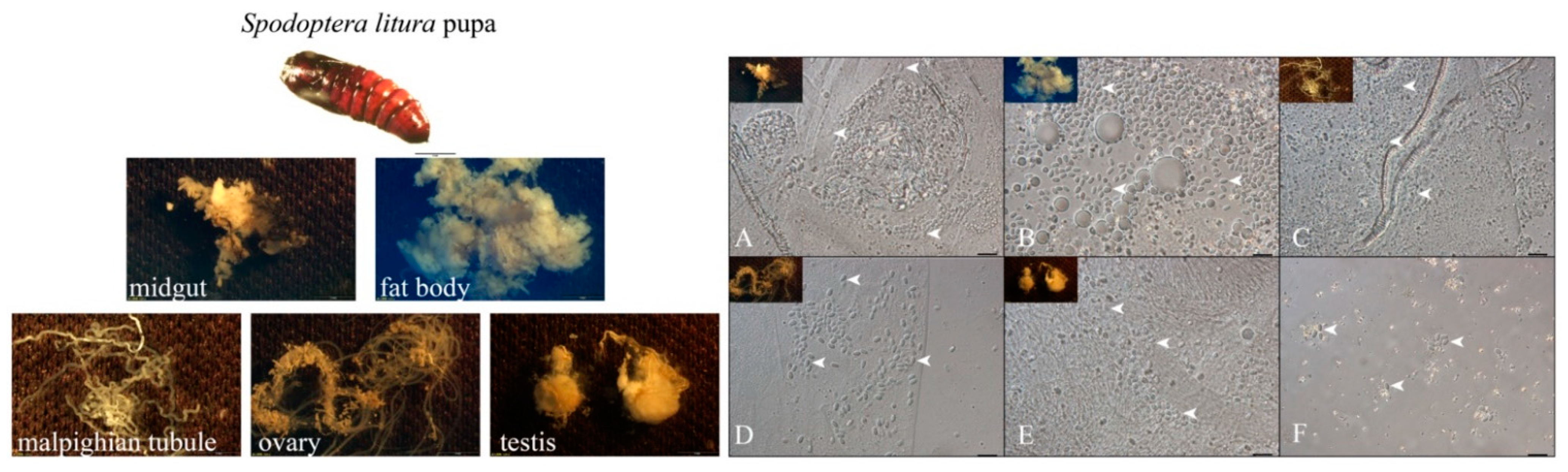
3.1. NbCQ1 Infection in the Pupa of S. litura and H. armigera
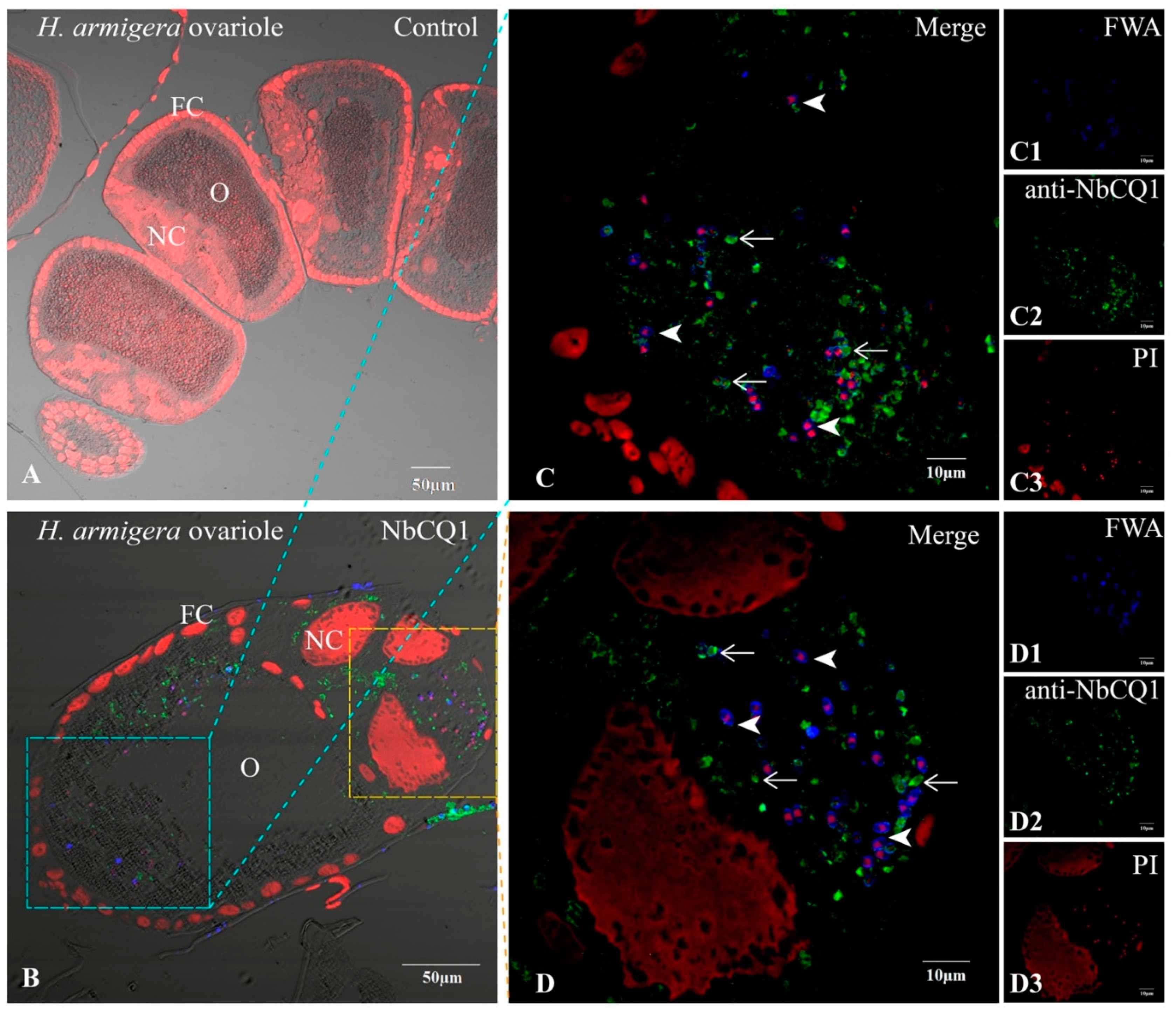
3.2. NbCQ1 Infection in the Ovariole of S. litura and H. armigera
3.3. NbCQ1 Infection in the Egg of S. litura and H. armigera
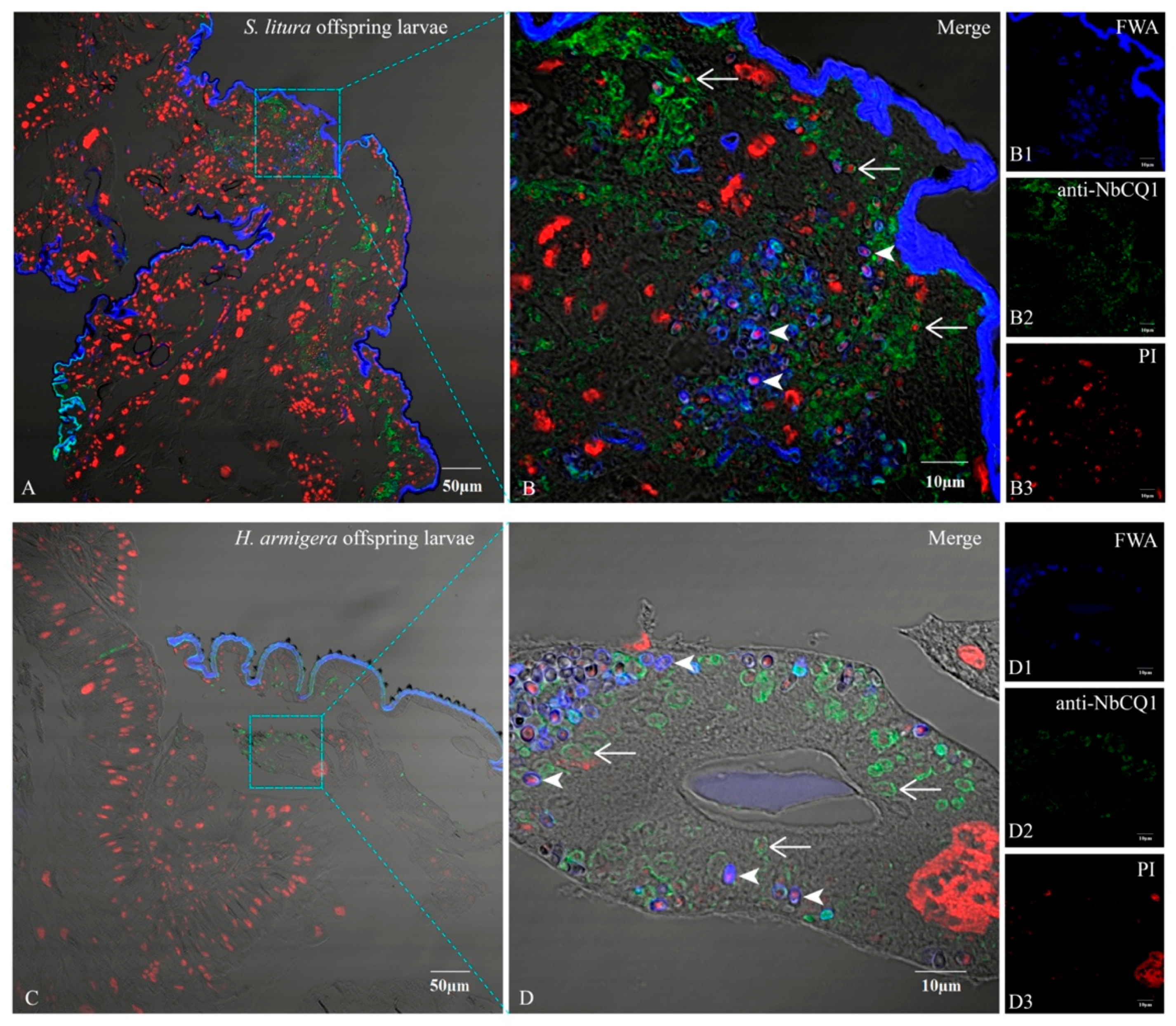
3.4. NbCQ1 Infection in the Offspring Larva of S. litura and H. armigera
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fayer, R.; Santin-Duran, M. Epidemiology of Microsporidia in Human Infections. In Microsporidia Pathogens of Opportunity; Weiss, L.M., Becnel, J.J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 135–164. [Google Scholar]
- Ruan, Y.; Xu, X.; He, Q.; Li, L.; Guo, J.; Bao, J.; Pan, G.; Li, T.; Zhou, Z. The largest meta-analysis on the global prevalence of microsporidia in mammals, avian and water provides insights into the epidemic features of these ubiquitous pathogens. Parasites Vectors 2021, 14, 186. [Google Scholar] [CrossRef]
- Han, B.; Weiss, L.M. Microsporidia: Obligate Intracellular Pathogens Within the Fungal Kingdom. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Oguz Kaya, I.; Dogruman Al, F.; Mumcuoglu, I. Investigation of Microsporidia prevalence with calcofluor white and uvitex 2B chemiluminescence staining methods and molecular analysis of species in diarrheal patients. Mikrobiyol. Bul. 2018, 52, 401–412. [Google Scholar] [CrossRef]
- Joseph, J.; Sharma, S.; Murthy, S.I.; Krishna, P.V.; Garg, P.; Nutheti, R.; Kenneth, J.; Balasubramanian, D. Microsporidial keratitis in India: 16S rRNA gene-based PCR assay for diagnosis and species identification of microsporidia in clinical samples. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzen, C.; Muller, A. Molecular techniques for detection, species differentiation, and phylogenetic analysis of microsporidia. Clin. Microbiol. Rev. 1999, 12, 243–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, M.-S.; Watanabe, H. Transovarian transmission of two microsporidia in the silkworm Bombyx mori and disease occurrence in the progeny popultion. J. Invertebr. Pathol. 1988, 51, 41–45. [Google Scholar] [CrossRef]
- Pan, G.; Xu, J.; Li, T.; Xia, Q.; Liu, S.L.; Zhang, G.; Li, S.; Li, C.; Liu, H.; Yang, L.; et al. Comparative genomics of parasitic silkworm microsporidia reveal an association between genome expansion and host adaptation. BMC Genom. 2013, 14, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashkarova, L.F.; Khakhanov, A.I. Range of the hosts of the causative agent of pebrine (Nosema bombycis) in the mulberry silkworm. Parazitologiia 1980, 14, 164–167. [Google Scholar]
- Mereghetti, V.; Chouaia, B.; Montagna, M. New Insights into the Microbiota of Moth Pests. Int. J. Mol. Sci. 2017, 18, 2450. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, E.; Goncalves, C.; Duarte, S.; Godinho, M.C.; Mexia, A.; Torres, L. Risk Assessment for Tomato Fruitworm in Processing Tomato Crop-Egg Location and Sequential Sampling. Insects 2020, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.-A.; Jaques Miret, J.A.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; et al. Pest categorisation of Spodoptera litura. EFSA J. 2019, 17, e05765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, S.J.; Lo, C.F.; Soichi, Y.; Wang, C.H. The characterization of microsporidian isolates (Nosematidae: Nosema) from five important lepidopteran pests in Taiwan. J. Invertebr. Pathol. 2003, 83, 51–59. [Google Scholar] [CrossRef]
- Dunn, A.M.; Terry, R.S.; Smith, J.E. Transovarial transmission in the microsporidia. Adv. Parasitol. 2001, 48, 57–100. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.S.; Dunn, A.M.; Smith, J.E. Cellular distribution of a feminizing microsporidian parasite: A strategy for transovarial transmission. Parasitology 1997, 115, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Ni, X.; Backus, E.A.; Maddox, J.V. Transmission Mechanisms of Nosema empoascae (Microspora: Nosematidae) in Empoasca fabae (Homoptera: Cicadellidae). J. Invertebr. Pathol. 1997, 69, 269–275. [Google Scholar] [CrossRef]
- Kotkova, M.; Sak, B.; Hlaskova, L.; Kvetonova, D.; Kvac, M. Evidence of transplacental transmission of Encephalitozoon cuniculi genotype II in murine model. Exp. Parasitol. 2018, 193, 51–57. [Google Scholar] [CrossRef]
- Kellen, W.R.; Lindegren, J.E. Transovarian transmission of Nosema plodiae in the Indian-meal moth, Plodia interpunctella. J. Invertebr. Pathol. 1973, 21, 248–254. [Google Scholar] [CrossRef]
- Vavra, J.; Undeen, A.H. Nosema algerae n. sp. (Cnidospora, Microsporida) a pathogen in a laboratory colony of Anopheles stephensi Liston (Diptera, Culicidae). J. Protozool. 1970, 17, 240–249. [Google Scholar] [CrossRef]
- Canning, E.U. An evaluation of protozoal characteristics in relation to biological control of pests. Parasitology 1982, 84, 119–149. [Google Scholar] [CrossRef]
- Chen, J.; Geng, L.; Long, M.; Li, T.; Li, Z.; Yang, D.; Ma, C.; Wu, H.; Ma, Z.; Li, C.; et al. Identification of a novel chitin-binding spore wall protein (NbSWP12) with a BAR-2 domain from Nosema bombycis (microsporidia). Parasitology 2013, 140, 1394–1402. [Google Scholar] [CrossRef]
- De Araujo-Coutinho, C.J.; Cunha Ade, B.; Serra-Freire, N.M.; de Mello, R.P. Evaluation of the impact of Bacillus thuringiensis serovar israelensis and Temephos, used for the control of Simulium (Chirostilbia) pertinax Kollar, 1832 (Diptera, Simuliidae) on the associated entomofauna, Paraty, state of Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 2003, 98, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Jurc, M.; Hauptman, T.; Pavlin, R.; Borkovič, D. Target and non-target beetles in semiochemical-baited cross vane funnel traps used in monitoring Bursaphelenchus xylophilus (PWN) vectors in pine stands. Phytoparasitica 2016, 44, 151–164. [Google Scholar] [CrossRef]
- Miles, M.; Kemmitt, G.; Bakker, F.; Aldershof, S. The impact of mancozeb on entomofauna communities in apple orchards. Commun. Agric. Appl. Biol. Sci. 2008, 73, 409–417. [Google Scholar]
- Shapiro-Ilan, D.I.; Bruck, D.J.; Lacey, L.A. Chapter 3—Principles of Epizootiology and Microbial Control. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Elsevier: San Diego, CA, USA, 2012; pp. 29–72. [Google Scholar] [CrossRef]
- Kong, F.; Song, Y.; Zhang, Q.; Wang, Z.; Liu, Y. Sublethal Effects of Chlorantraniliprole on Spodoptera litura (Lepidoptera: Noctuidae) Moth: Implication for Attract-And-Kill Strategy. Toxics 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.A.; Ahmad, M.; Ahmad, M.; Aslam, M.; Sayyed, A.H. Resistance to selected organochlorin, organophosphate, carbamate and pyrethroid, in Spodoptera litura (Lepidoptera: Noctuidae) from Pakistan. J. Econ. Entomol. 2008, 101, 1667–1675. [Google Scholar] [CrossRef]
- Tian, L.; Gao, X.; Zhang, S.; Zhang, Y.; Ma, D.; Cui, J. Dynamic changes of transcriptome of fifth-instar spodoptera litura larvae in response to insecticide. 3 Biotech 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Laborde, A. Impact of pesticide exposure in childhood. Rev. Environ. Health 2020, 35, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Slamovits, C.H.; Williams, B.A.; Keeling, P.J. Transfer of Nosema locustae (Microsporidia) to Antonospora locustae n. comb. based on molecular and ultrastructural data. J. Eukaryot. Microbiol. 2004, 51, 207–213. [Google Scholar] [CrossRef]
- Lewis, L.C.; Bruck, D.J.; Prasifka, J.R.; Raun, E.S. Nosema pyrausta: Its biology, history, and potential role in a landscape of transgenic insecticidal crops. Biol. Control 2009, 48, 223–231. [Google Scholar] [CrossRef]
- Solter, L.F.; Hajek, A.E. Control of Gypsy Moth, Lymantria dispar, in North America since 1878. In Use of Microbes for Control and Eradication of Invasive Arthropods; Hajek, A.E., Glare, T.R., O’Callaghan, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 181–212. [Google Scholar] [CrossRef]
- Andreadis, T.G. Host specificity of Amblyospora connecticus (Microsporida: Amblyosporidae), a polymorphic microsporidian parasite of Aedes cantator (Diptera: Culicidae). J. Med. Entomol. 1989, 26, 140–145. [Google Scholar] [CrossRef]
- Becnel, J.J.; Johnson, M.A. Mosquito host range and specificity of Edhazardia aedis (Microspora: Culicosporidae). J. Am. Mosq. Control Assoc. 1993, 9, 269–274. [Google Scholar] [PubMed]
- Nordin, G.L. Transovarial transmission of a Nosema sp. infecting Malacosoma americanum. J. Invertebr. Pathol. 1975, 25, 221–228. [Google Scholar] [CrossRef]
- Raina, S.K.; Das, S.; Rai, M.M.; Khurad, A.M. Transovarial transmission of Nosema locustae (Microsporida: Nosematidae) in the migratory locust Locusta migratoria migratorioides. Parasitol. Res. 1995, 81, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Liu, W.; Zhang, F.; Chen, X.; Li, L.; Liu, Q.; Zhou, Y.; Wei, T.; Fang, R.; Wang, X. Transovarial transmission of a plant virus is mediated by vitellogenin of its insect vector. PLoS Pathog. 2014, 10, e1003949. [Google Scholar] [CrossRef]
- Guo, Y.; Hoffmann, A.A.; Xu, X.Q.; Mo, P.W.; Huang, H.J.; Gong, J.T.; Ju, J.F.; Hong, X.Y. Vertical Transmission of Wolbachia Is Associated With Host Vitellogenin in Laodelphax striatellus. Front. Microbiol. 2018, 9, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; He, Y.Z.; Guo, Q.; Guo, T.; Liu, Y.Q.; Zhou, X.P.; Liu, S.S.; Wang, X.W. Vector development and vitellogenin determine the transovarial transmission of begomoviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 6746–6751. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, B.; Wang, C.; Yu, B.; Xia, D.; Li, T.; Zhou, Z. The First Report on the Transovarial Transmission of Microsporidian Nosema bombycis in Lepidopteran Crop Pests Spodoptera litura and Helicoverpa armigera. Microorganisms 2021, 9, 1442. https://doi.org/10.3390/microorganisms9071442
Pei B, Wang C, Yu B, Xia D, Li T, Zhou Z. The First Report on the Transovarial Transmission of Microsporidian Nosema bombycis in Lepidopteran Crop Pests Spodoptera litura and Helicoverpa armigera. Microorganisms. 2021; 9(7):1442. https://doi.org/10.3390/microorganisms9071442
Chicago/Turabian StylePei, Boyan, Chunxia Wang, Bin Yu, Dan Xia, Tian Li, and Zeyang Zhou. 2021. "The First Report on the Transovarial Transmission of Microsporidian Nosema bombycis in Lepidopteran Crop Pests Spodoptera litura and Helicoverpa armigera" Microorganisms 9, no. 7: 1442. https://doi.org/10.3390/microorganisms9071442





