Abstract
This systematic review aims to identify probiotics and prebiotics for modulating oral bacterial species associated with mental disorders. Using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guideline, we search the electronic MEDLINE database published till January 2021 to identify the studies on probiotics and/or prebiotics for preventing and treating major oral dysbiosis that provokes mental disorders. The outcome of the search produces 374 records. After excluding non-relevant studies, 38 papers were included in the present review. While many studies suggest the potential effects of the oral microbiota on the biochemical signalling events between the oral microbiota and central nervous system, our review highlights the limited development concerning the use of prebiotics and/or probiotics in modulating oral dysbiosis potentially involved in the development of mental disorders. However, the collected studies confirm prebiotics and/or probiotics interest for a global or targeted modulation of the oral microbiome in preventing or treating mental disorders. These outcomes also offer exciting prospects for improving the oral health of people with mental disorders in the future.
1. Introduction
The oral cavity is an important bacterial gateway that plays a crucial role in the first-step digestion. Food entering the mouth is chewed and mixed with saliva before being swallowed. Microorganisms from the air pass through the nose and mouth into the trachea and lungs, and colonize the oral cavity. They spread to the epithelial surfaces and the body via the bloodstream. Thus, oral cavity is colonized by 50 to 100 billion bacteria [1], and they are responsible for many infectious diseases in the mouth, which include caries (tooth decay) and periodontitis (gum disease) [2,3]. Evidence confirms that oral bacteria are linked to many systemic diseases [4], such as cardiovascular disease [5], stroke [6], premature birth [7], diabetes [8], pneumonia [9], cancer development [10], kidney diseases [11], and mental disorders [12].
In reverse, Bifidobacterium dentium in the oral cavity may offer health benefits to the host [13]. Benefits include the barrier against pathogens or immunomodulating properties [14]. Thus, many bacterial strains are identified as probiotics—microorganisms whose ingestion in adequate quantities is beneficial to the health of the host. According to the International Scientific Association for Probiotics and Prebiotics, Bifidobacterium (adolescentis, animalis, bifidum, breve and longum) and Lactobacillus (acidophilus, casei, fermentum, gasseri, johnsonii, paracasei, plantarum, rhamnosus and salivarius) represents a core group of well-studied species likely to impart some general benefits [15]. Similarly, prebiotics are substrates, such as fructans and galactans, selectively used by host microorganisms and offered a health benefit by modulating the microbiome of individuals [16].
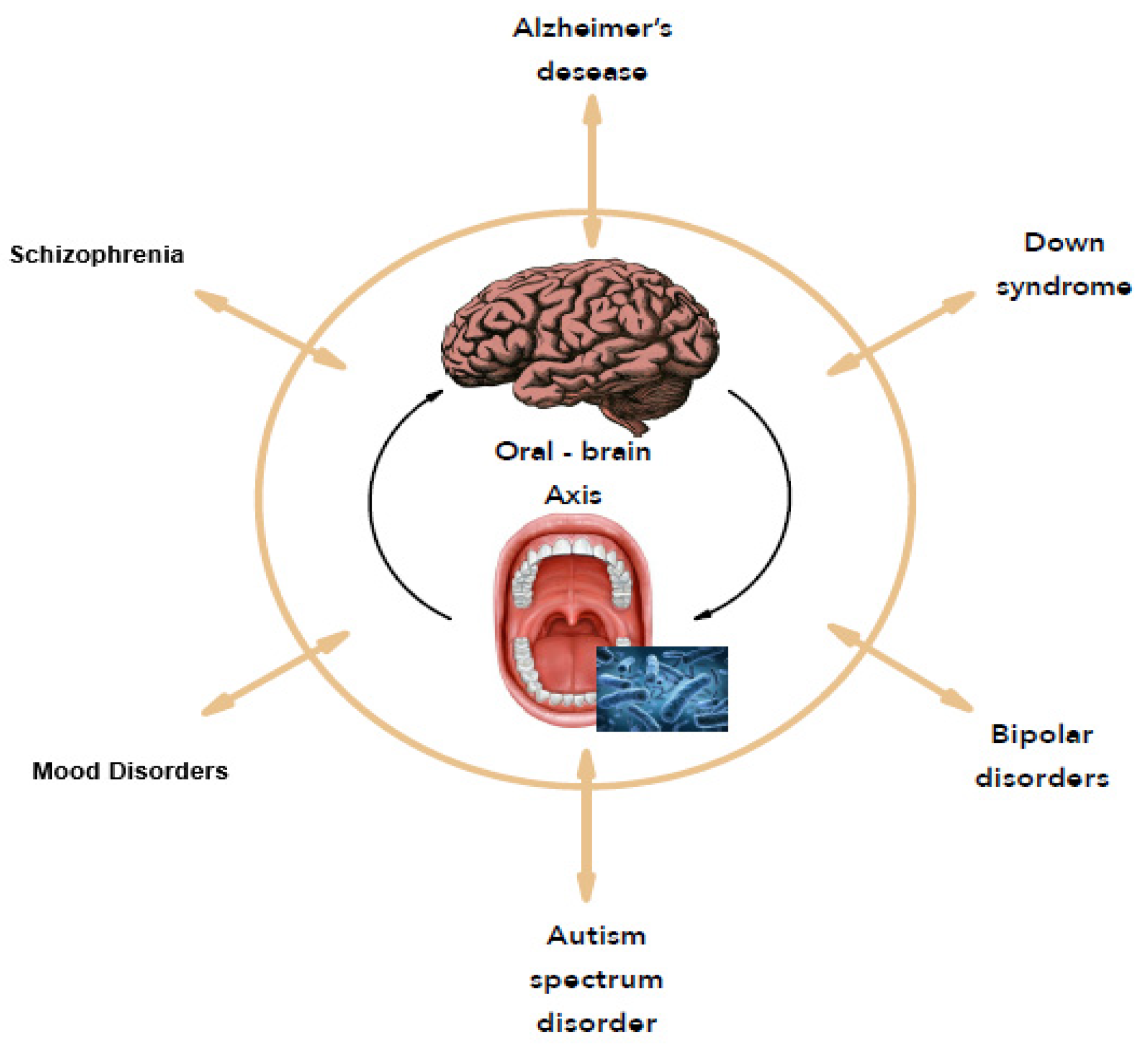
In 2001, Joshua Lederberg introduced the term “microbiome” [17] referred to as dynamic communities of microbes that colonized the body and provided many metabolic functions and molecular signals to maintain good health. With the concept of the microbiome, microbiologists have refocused on microbial communities rather than on individual organisms in pure culture, as in Koch’s postulates. In this case, the consortia of organisms in a biofilm rather than a single pathogen are responsible for many infections like caries, periodontitis, and others [18]. As many people never develop dental caries, some authors suggest that certain bacterial species have a potential antagonistic effect against cariogenic bacteria [19]. Thus, replacing pathogens with harmless isolates obtained from healthy strains prevent health disorders. A metagenomic approach confirms that the bacteria with a protective effect against cariogenic species offers probiotic benefits for the oral health of the host [20]. From an ever-growing understanding of how the microbiome affects health and disease, the human microbiome offers new therapeutic pathways like reversing or rebalancing the microbiome towards health. In this context, this study focuses on the oral microbiome and its correlation to mental disorders, named the “Oral-Brain Axis” [12] to highlight new therapeutic perspectives for mental disorders (Figure 1).
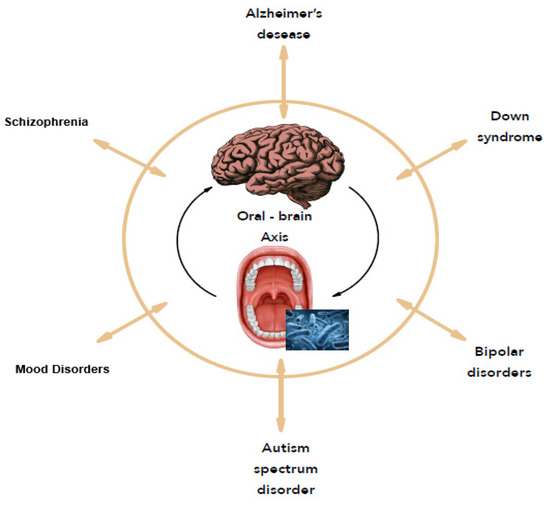
Figure 1.
The oral-brain axis.
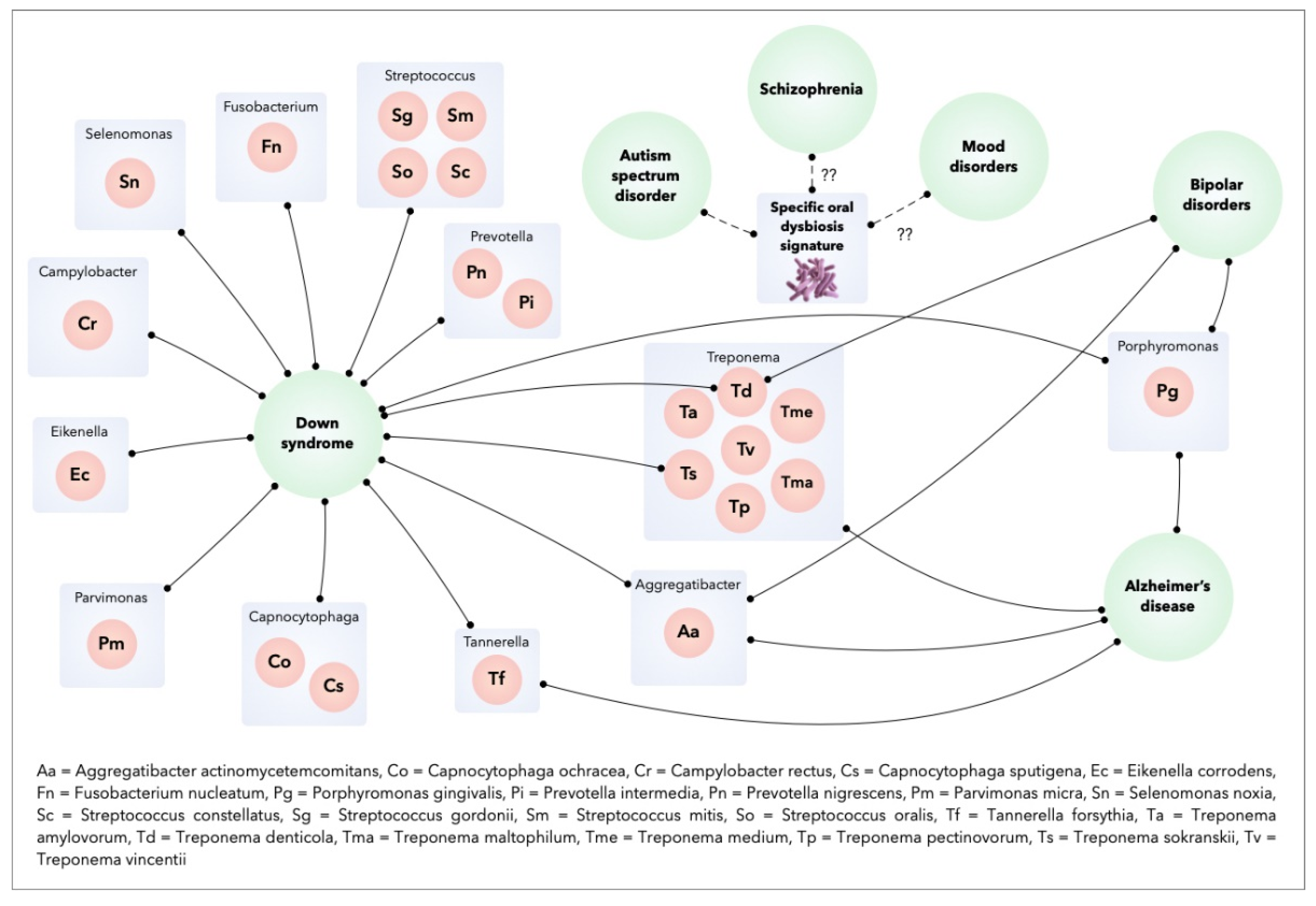
The identification of a specific microflora in patients with mental disorders suggests that these disorders could be influenced by the oral microbiome. For example, the inflammatory process induced from the Gram-negative periodontal pathogen Porphyromonas gingivalis is evoked in individuals with dementia or Alzheimer’s Disease [21,22]. In Autism Spectrum Disorder, the specific oral dysbiosis observed in the oral microbial community of these patients suggests a potential role for microorganisms in the progression of this disease [23]. The microflora of individuals with Down’s Syndrome (DS) with periodontitis show similarities with those of patients without DS with periodontitis, including the main periodontal pathogens (Aggregatibacter actinomycetemcomitans, P. gingivalis, Tannarella forsythia, Prevotella intermedia, Parvimonas micra, Fusobacterium nucleatum, Campylobacter rectus, etc.). In addition, patents with DS have increased colonization of some bacterial species in the subgingival flora (Selenomonas noxia, Propionibacterium acnes, Streptococcus gordonii, Streptococcus mitis, Streptococcus oralis, Treponema socranskii and Streptococcus constellatus). S. gordonii, S. mitis, and S. oralis initiate early microbial colonization and contribute to the development of microbial plaque. The presence of P. acnes (normally found on the skin) at high levels in the subgingival microbiota of subjects with DS may be related to the habit of putting fingers to the mouth [24]. S. gordonii contains genes that facilitate the attachment of free-floating P. gingivalis to the adherent plaque biofilm [25]. S. noxia is associated with periodontal disease activity at interproximal sites [26] and T. socranskii has been associated with the severity of periodontal tissue destruction [27]. Finally, S. constellatus is associated with refractory forms of periodontitis [28]. Although this overall periodontal dysbiosis may be influenced by oral hygiene habits and deficient immune status of DS patients, it could contribute to an increased susceptibility to periodontitis in patients with DS [29]. Bipolar disorder was correlated with an increased risk of periodontitis, a higher frequency of periodontopathogens, and a higher total bacterial load like A. actinomycetemcomitans and P. gingivalis [30]. Finally, the oropharynx microbiome in schizophrenics is significantly different from that of healthy controls, with some bacterial species more abundant in schizophrenic patients than in controls (Lactobacillus gasseri, Lactobacillus salivarius, Bifidobacterium pseudocatenulatum) [31].
Therefore, the concept of the “oral-brain axis” refers to the role of the oral microbiota in the biochemical signaling events between the oral microbiota and central nervous system [10]. The bacterial species associated with mental disorders through the “oral-brain axis” are mainly periodontopathogen bacteria (A. actinomycetemcomitans, P. gingivalis, T. forsythia, Treponema denticola, C. rectus, P. intermedia, etc.). Figure 2 presents the main bacterial species that may be potentially associated with mental disorders through the “oral-brain axis” [10,22,29,30,32,33].
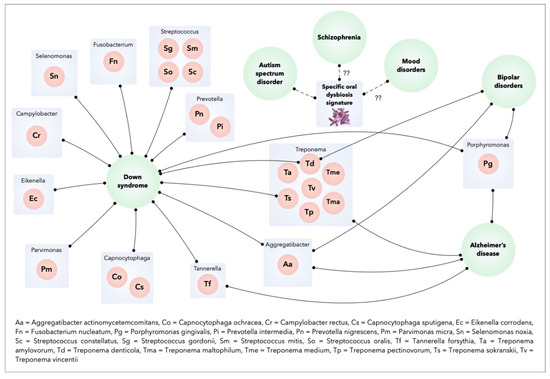
Figure 2.
Oral bacteria and mental disorders.
Similar to the “gut–brain axis” that emphasizes the bidirectional communication between the central and enteric nervous systems [34], the possible relationship between the oral microbiome and mental disorders suggests that probiotics or prebiotics use could prevent and manage mental disorders by modulating oral dysbiosis and its inflammatory and immune consequences. Prebiotics are a type of fiber that the human body cannot digest. They serve as food for probiotics, which are microorganisms. Both prebiotics and probiotics may support helpful bacteria and other organisms in the gut, and we hypothesized in the oral cavity.
The objective of this systematic review is to identify probiotics and prebiotics involved in modulating oral bacterial species associated with mental disorders.
2. Materials and Methods
The present systematic review was conducted according to the PRISMA guidelines for Systematic Reviews [35].
2.1. Search Strategy
To identify studies that met the inclusion criteria, an electronic search was conducted through the MEDLINE (PubMed) database up to January 2021 without restriction. While modulation of the microbiome represents an innovative therapeutic axis to treat or prevent human pathologies [36], the impact of probiotics on human health has long been documented [37]. Consequently, the search was performed without time restriction to identify the studies that explore the application of probiotics and/or prebiotics in preventing and treating major oral dysbiosis potentially involved in mental disorders. The search terms were used in combination with Boolean operator “AND”/”OR” according to the following equations:
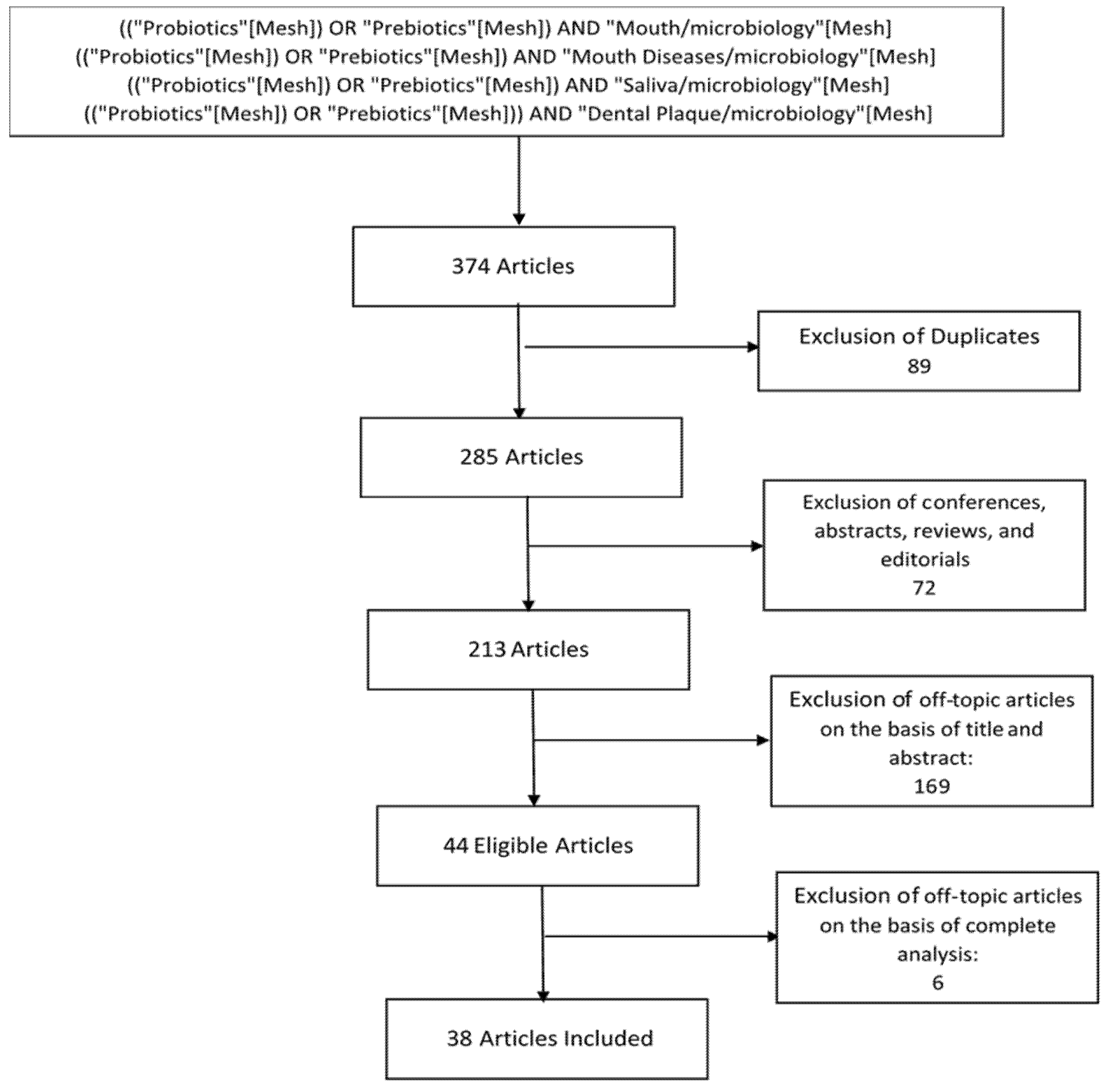
((“Probiotics”[Mesh]) OR “Prebiotics”[Mesh]) AND “Mouth/microbiology”[Mesh]
((“Probiotics”[Mesh]) OR “Prebiotics”[Mesh]) AND “Mouth Diseases/microbiology”[Mesh]
((“Probiotics”[Mesh]) OR “Prebiotics”[Mesh]) AND “Saliva/microbiology”[Mesh]
((“Probiotics”[Mesh]) OR “Prebiotics”[Mesh])) AND “Dental Plaque/microbiology”[Mesh]
2.2. Study Detection
A manual reference check of eligible studies on the subject was performed by two operators who reviewed the studies according to the inclusion/exclusion criteria.
2.3. Inclusion and Exclusion Criteria
The bacterial species associated with mental disorders (Alzheimer diseases, Autism Spectrum Disorders, Down Syndrome, and Bipolar Disorders) through the oral-brain axis are mainly periodontopathogen bacteria (A. actinomycetemcomitans, P. gingivalis, T. forsythia, T. denticola, C. rectus, P. intermedia, etc.). We included experimental or clinical studies (longitudinal, cross-sectional, or randomized) that identified the effects of probiotics strains or prebiotics compounds on these bacterial species or the host response associated. Conferences, abstracts, reviews, and editorials were excluded.
2.4. Data Collection
Two independent reviewers (M.G. and Y.M.) screened titles and abstracts to identify the relevant papers based on the inclusion criteria. The full studies were reviewed to decide whether they should be included when the abstract information was judged to be insufficient. The reviewers reached a consensus on the eligibility criteria for selecting the studies. In case of discrepancy, a third reviewer (F.D.) resolved the conflicts regarding the eligibility.
3. Results
3.1. Study Selection
The initial studies retrieved from the databases were first selected. We selected 38 studies from 285 studies (Figure 3), and we reviewed and analyzed studies that met the eligibility criteria.
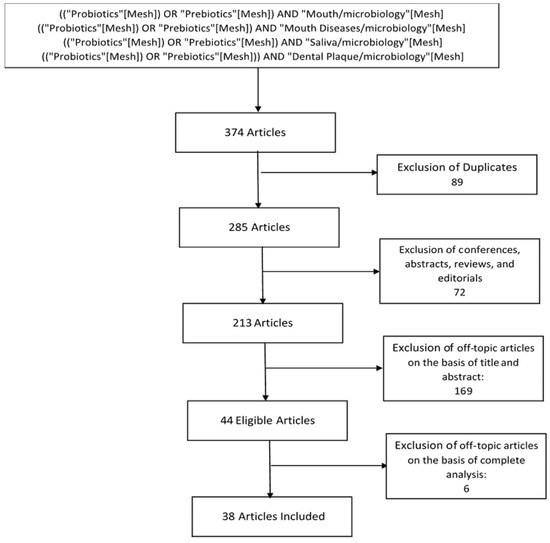
Figure 3.
Flow chart of study selection.
Each study that met the inclusion criteria was analyzed to identify prebiotic compounds or probiotic strains and their effects on the oral microbial flora or immunological markers involved in mental disorders. Three distinct effects on oral dysbiosis associated with mental disorders emerged: modulation of the oral microbiome [38,39,40,41,42,43,44,45,46,47,48,49,50,51], antimicrobial activity [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69], and modulation of immunological or inflammatory factors [43,44,46,56,70,71,72,73,74,75].
3.2. Prebiotics
From our search, three recent studies on prebiotics were identified [38,39,40] (Table 1). Each demonstrated an oral microbiome modulation. In an in vitro study, Slomka et al. [38] identified two bacterial nutritional compounds, beta-methyl-d-galactoside and N-acetyl-d-mannosamine, that induced a beneficial composition in dual-species biofilm communities (beneficial and pathogen). Beta-methyl-d-galactoside decreased F. nucleatum and P. gingivalis in the biofilm by stimulating Streptococcus salivarius. N-acetyl-d-mannosamine stimulated S. mitis and Streptococcus sanguinis resulting in a reduction of A. actinomycetemcomitans and Streptococcus sobrinus. Rosier et al. [39] demonstrated that in vitro exposure to the ecological factors such as Nitrate favored the flora associated with oral health (Genera Neisseria and Rothia). This is achieved by limiting periodontitis-associated genera (Porphyromonas, Fusobacterium, Leptotrichia, Prevotella, and Alloprevotella) without affecting biofilm growth. In 2019, Jiménez-Hernández et al. show that consumption of a mixture of bacterial growth substrates (short-chain galacto-oligosaccharides (5 g), long-chain fructo-oligosaccharides (10 g), and glutamine (5 g) for six weeks by 32 patients change the structure of the oral microbiota. This is characterized by a decrease in alpha diversity and a change in beta diversity, with no clear orientation towards a healthy microbiota [40].

Table 1.
Prebiotics: Summary of selected studies.
3.3. Probiotics
In 35 studies published between 2008 and 2020, we identified the probiotics strains and their effects on the bacterial species potentially involved in mental disorders (Table 2).

Table 2.
Probiotics: Summary of selected studies.
Table 3 shows the different probiotic strains investigated alone or in combination in 12 in vitro studies [41,42,43,44,45,46,52,53,54,55,56,57,58,59,60] and 23 in vivo studies [46,47,48,49,50,51,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75] including three using animal models [46,70,71]. The experiments in patients were performed with small samples (n = 12 to 108) and a short follow-up period (1 day to 36 weeks).

Table 3.
Probiotics involved in modulating oral bacterial species associated with mental disorders.
Various Galenic forms were explored in addition to the classic tablets and lozenges, such as milk drinks [48,50,63], yogurts [49], cheese [64], and mouthwashes [66,67].
3.3.1. Modulation of the Oral Microbiome
The development and degradation of oral biofilms rely on adhesion and cooperation or competition between oral bacteria [76]. Several in vitro and in vivo studies demonstrated the ability of various probiotics strains to modulate these mechanisms. In vitro models, the Lactobacillus reuteri introduction results in changes to the nascent and developed biofilm ecosystem with an increase in exogenous lactobacilli (L.), streptococci (S.) and Gram-negative anaerobes [41]. Lactobacillus rhamnosus reduces the biofilm-forming capacity of F. nucleatum, integrates into all oral biofilms [42], and shows higher adhesive properties than P. gingivalis on gingival stromal cells [43].
Several probiotic strains (L. reuteri, L. rhamnosus, Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium longum, Bifidobacterium animalis, Bifidobacterium breve, Bifidobacterium pseudolongum, Bifidobacterium bifidum) can modulate the ability of P. gingivalis to adhere to and invade gingival epithelial cells [44]. The lipase enzyme from lactobacilli strains used as probiotics (Lactobacillus acidophilus, Lactobacillus casei) might be an influential factor in the biofilm degradation integrating A. actinomycetemcomitans [45].
In rats, Bifidobacterium reduces the proportion of some Gram-negative anaerobic bacteria-like species involved in the pathogenesis of periodontal diseases in the subgingival biofilm (Veillonella parvula, Capnocytophaga sputigena, Eikenella corrodens, and Prevotella intermedia) [46].
Similar results are found in several in vivo studies. Thus, Bifidobacterium animalis subsp. lactis HN019 used for 30 days (lozanges) reduce the adhesion of P. gingivalis to buccal epithelial cells [47]. Short-term consumption (1 day) of milk fermented with probiotic strains (Streptococcus thermophilus, Lactobacillus delbrueckii, and Lactobacillus paracasei) supplemented with vitamin B6 and vitamin D increases the overall diversity of the oral cavity microbiome with an increase in the genera Steptococcus and Actinomyces. However, no substantial change in the microbiome structure is noted [48,49]. The effects of L. acidophilus, B. animalis, and L. rhamnosus combined in a probiotic milk drink on periodontopathogenic bacteria in subgingival plaque is lower than those in supragingival plaque [50]. According to Tada et al., L. reuteri fails to improve the microbial flora of the peri-implant sulcus in patients with peri-implantitis [51]
3.3.2. Antibacterial Activity
The antimicrobial activity of probiotics controls and inhibits the growth of certain microorganisms and bacteria [77]. In many vitro studies, human oral lactobacilli (L. acidophilus, Lactobacillus crispatus, L. delbrueckii, Lactobacillus gasseri, Lactobacillus salivarius, L. paracasei, Lactobacillus plantarum, L. rhamnosus, Lactobacillus fermentum, L. caseï) or Bifidobacterium (B. animalis) possesses an antimicrobial activity against oral pathogens. It also has a strong inhibitory effect against S. mutans, S. sobrinus, Gram-negative periodontal pathogens P. gingivalis, A. actinomycetemcomitans, P. intermedia, and F. nucleatum [52,53,54,55]. Shin et al. showed that Lactococcus lactis possesses the antimicrobial activity against T. forsythia, T. denticola, F. nucleatum, and P. gingivalis [56]. Higuchi et al. also observed P. gingivalis demonstrated a retarded growth when L. salivarius and green tea catechins were combined [57]. Zhu et al. confirmed that the probiotic strains (L. delbrueckii, S.thermophilus, L. acidophilus, Bifidobacterium) inhibited periodontal pathogens. However, no effect on the “protective bacteria”, S. sanguinis, was observed, and competition between probiotics and periodontal pathogens depended on the inoculation sequence [60]. According to Koll et al., L. plantarum is unsafe because it is different from the natural resistance pattern of lactobacilli [52].
In addition, this antimicrobial activity is observed in vivo. According to Invernici et al., B. animalis inhibits the growth of P. gingivalis, P. intermedia, F. nucleatum, and A. actinomycetemcomitans [47]. A decrease in the number of P. gingivalis was also reported after oral administration of L. crispatus for four weeks [59]. Administration of L. reuteri tablets for 28 days reduced the number of P. intermedia and P. gingivalis in the subgingival microbiota [60]. In combination with scaling and root planning (SRP), the consumption of L. reuteri tablets for 12 weeks showed a limited difference in the number of P. gingivalis [61]. The association between L. plantarum, L. brevis, and Pediococcus acidilactici demonstrated a significant microbiological impact after reducing the counts of T. forsythia in patients with gingivitis [62]. From Imran et al., daily consumption of L. casei commercial fermented milk (Yakult©) for one month reduced the numerical sum of P. gingivalis, A. actinomycetemcomitans, and P. intermedia in patients with chronic generalized mild to moderate periodontitis [63]. Consuming petit-suisse cheese for nine days, L. casei reduces the density of A. actinomycetemcomitans and maintain lowers density of P. gingivalis two weeks later [64]. Four weeks after intake of L. salivarius tablets for eight days, a significant reduction is noted in the number of A. actinomycetemcomitans, P. intermedia, P. gingivalis, T. denticola, and T. forsythia in the subgingival plaque. This reduction disappears after eight weeks [65]. Sajedinejad et al. found that oral application of L. salivarius as a mouthwash for 28 days serves as the antimicrobial activity against A. actinomycetemcomitans [66]. In a pilot study, a commercial probiotic mouthwash containing natural oral bacteria (S. oralis, S. uberis, S. rattus) shows a trend towards the reduction of periodontal pathogens in subgingival plaque (P. gingivalis and C. rectus) [67]. Daily consumption of probiotic lozenges that combined L. rhamnosus and B. animalis for four weeks decreases the bacterial load of A. actinomycetemcomitans and F. nucleatum in both saliva and plaque. The consumption also decreases the number of P. gingivalis in the plaque [68]. In patients who received non-surgical treatment (SRP), the administration of L. rhamnosus sachets (30 days) or azithromycin pills (five days) offered microbiological effects similar to SRP alone for the treatment of chronic periodontitis [69].
3.3.3. Modulation of Immunological or Inflammatory Mediators of Oral Dysbiosis
The bacterial exposure induces the synthesis of cytokine as Interleukins (IL) or TNF α and chemokine as CXCL8. This synthesis regulates inflammation and modulates cellular activities associated with the host immune response [78]. Some probiotics demonstrated in vitro ability to modulate immunoinflammatory parameters. According to Shin et al., Lactococcus lactis neutralizes and inhibits the production of IL-6 or TNF-α induced by lipopolysaccharides from F. nucleatum, P. gingivalis, and T. forsythia [56]. CXCL8 secretions from gingival stromal stem cells increase when pretreated with L. rhamnosus before P. gingivalis stimulation [43]. In gingival epithelial cells, IL-1β and TNF-α synthesis stimulation decreases when co-culture with P. gingivalis and L. rhamnosus or bifidobacteria (B. longum, B. animalis, B. pseudolongum, B. bifidum) or L. salivarius. CXCL8 secretion increases when co-cultured with P. gingivalis and L. salivarius or L. rhamnosus [45]. In mice, L. gasseri and L. brevis decrease the expression and secretion in gingival tissue of inflammatory cytokine (IL-1β, IL-6, IL-17A, and TNF α) [70,71]. B. lactis reduces levels of IL-1b and IL-1b/IL-10 ratios in rats using experimental periodontitis [46]. These observations are different from those observed in humans. In a group of 47 individuals, 4-week consumption of tablets containing a mixture of L. rhamnosus and L. curvatus showed no effect on the concentration of selected cytokines (IL1β, IL6, IL8, IL10, TNF-α) in gingival crevicular fluid [72]. In two randomized, double-blind, placebo-controlled crossover trials, three-week ingestion of L. reuteri tablets twice a day offers no difference in cytokine saliva levels (IL1β, IL6, IL8, and IL10) [73,74]. Following the management of peri-implant mucositis, probiotic supplementation with L. reuteri offered no difference in inflammatory mediator level in the crevicular fluid for 89 patients at a 26-week follow-up [75].
4. Discussion
Many studies suggest beneficial effects of prebiotics and probiotics on brain function through the gut–brain axis [79]. In contrast, no studies demonstrate a use of prebiotics compounds or probiotics strains to prevent or treat brain disorders through oral-brain axis.
We identified 38 studies, and only three explored the effects of prebiotic compounds on the growth of beneficial bacteria. Prebiotics are a recent field of research [80], and scanty studies publish their effects on the oral microbiome. Our review shows that nutritional stimulation of the oral microbiome using various prebiotic compounds (Nitrate, beta-methyl-d-galactoside, N-acetyl-d-mannosamine, etc.) may induce the composition of the dental biofilm and growth of beneficial oral bacteria at the expense of pathogenic bacteria (A. actinomycetemcomitans, F. nucleatum, P. gingivalis). Anxiolytic and antidepressant effects or enhancement in cognitive deficit and social functioning were observed with the rebalancing of gut flora through the consumption of prebiotics by patients suffering from depression, Alzheimer’s disease, or autism spectrum disorders [81]. Thus, in the context of the oral-brain axis, it can be assumed that the use of prebiotics in modulating the oral microbiome could lead to improvements in mental health. As the beneficial effects of prebiotics have already been studied for the modulation of the gut microbiome [82,83], it can be assumed that research on the effects of prebiotics on the oral flora will represent an area with growing interest.
Since the 1980s, studies have explored probiotics, particularly lactobacilli of which the most popular are L. rhamnosus, L. reuteri, L. casei, and L. acidophilus [84]. Probiotic microorganisms that offer health benefits for humans are Lactobacilli and Bifidobacterium species [85]. The major probiotic mechanisms of the action of probiotics include improved adhesion to bacterial colonization sites, competing with pathogenic microorganisms, production of antimicrobial substances, and modulating the host’s immune response [86].
The in vitro and in vivo confirm the effects of lactobacilli and bifidobacterium on modulating the oral microbiota associated with mental disorders (B. animalis, L. paracaseï, L. acidophilus, L. rhamnosus, L. delbrueckii). Bacterial competition excludes some pathogens without biofilm structural disruption (F. nucleatum, P. gingivalis, etc.). The biofilm enzymatic degradation capacity appears as a mechanism of action implicated in this competition.
The specific dysbiotic signature associated with patients with mental disorders suggests an influence of the central nervous system in the development of oral pathologies [12]. While the use of probiotics may appear as a complementary therapeutic means for patients with mental disorders, it is necessary to keep in mind the multifactorial character of the oral microbiome homeostasis [87]. Frequent oral alterations affect patients with mental disorders [88]. These alterations are correlated with a psychomotor impairment that prevents an adequate hygiene routine and reduces salivary flow due to various psychoactive substances (drugs, medication) and difficulty in accessing dental health services [89,90]. These mental disorders promote oral dysbiosis that can lead to dental caries and periodontal disease [91].
P. gingivalis, T. forsythia, and T. denticola represent the red complex polymicrobial community involved in the development of periodontal disease [92]. Some of these periodontopathogen bacteria (P. gingivalis, T. denticola) or their toxic proteases (gingipaïn) detected by postmortem analysis of Alzheimer’s disease patients’ brains suggest their pathogenesis involvement of this mental disorder [93]. Thus, the antimicrobial activity of probiotic strains against periodontal pathogens could represent an axis of prevention of oral dysbiosis and its potential implication in the development of mental disorders.
The modulation of the immune system induced by prebiotics and probiotics is one of the health benefits of increasing interest. Although the mechanisms of action are not yet clearly understood, this stimulation of the immune system can be direct by altering cytokine expression or indirect by altering the composition and population of bacterial species. The direct beneficial effects on the immune system are generally associated with an increase in the expression of anti-inflammatory cytokines (IL 4, IL 10, IL 11, IL 13) and a reduction in pro-inflammatory cytokines (IL 1β, IL 6, TNFα) [94]. Their immunomodulatory effects have been extensively studied in inflammatory diseases in the gastrointestinal tract [95,96,97] but remain poorly evaluated in periodontal pathogen-induced inflammatory diseases.
Chronic inflammation and latent infections can cause cognitive and behavioral problems [98]. Cytokines produced outside the central nervous system such as IL 1β and TNF α cause brain neurotoxicity [78]. The inflammation induced by periodontopathogen bacteria is associated with dementia and neurodegenerative lesions in patients with Alzheimer’s disease [21,22]. In in vitro and animal studies, while probiotic strains seem to decrease in cytokines induced by the main periodontopathogens species (P. gingivalis, F. nucleatum, T. forsythia), the improvement of the inflammatory condition is not observed in human studies. The probiotics are not demonstrated in preventing the neurological consequences of inflammation associated with periodontal disease.
5. Limits and Perspectives
In this review, studies suggest that prebiotics and probiotics can prevent and treat oral dysbiosis involved in the oral-brain axis. However, several limitations are noted. These studies only use human small samples with short intake and/or follow-up, which fails to define their long-term effects. In addition, the diversity of probiotic species studied according to different modes of administration neither support the standardization of a probiotic formulation nor the definition of an adapted delivery system.
Prebiotics and/or probiotics are not shown to treat or prevent mental disorders using modulating oral dysbiosis. In addition, their effects are preventive approaches for periodontal disease. Human studies using different galenic forms (milk drinks, yogurts, and mouthwashes) should be explored in future studies for more suitable use of prebiotics and/or probiotics in patients with reduced oral hygiene habits [98]. Longitudinal studies should define a formulation of prebiotics and/or probiotics and a mode of administration in preventing oral dysbiosis and evaluating their safety.
6. Conclusions
Our review highlights the limited research regarding the use of prebiotics and/or probiotics in modulating oral dysbiosis in mental disorders. However, the studies confirm their interest in preventing or treating mental disorders through global or targeted modulation of the oral microbiome. Research is emerging on prebiotic compounds and probiotics for the treatment of oral dysbiosis and mental disorders. In this review, the probiotic strains belong to the genus Lactobacilli and Bifidobacterium commonly studied for the rebalancing of intestinal flora. While prebiotics and probiotics are part of the gut–brain axis, it is still difficult to envisage their preventive or therapeutic application for managing mental disorders through the oral-brain axis. Modulating oral dysbiosis can improve the oral health of patients with mental disorders.
Author Contributions
Conceptualization, Y.M. and F.D.; Methodology, F.D., Y.M. and G.A.; Resources, F.D. and G.A.; Data curation, Y.M.; Writing—Original Draft Preparation, F.D., Y.M. and M.G.; Writing—Review and Editing, Y.M. and G.A.; Visualization, R.M.; Supervision, A.D., R.M., P.M. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krishnan, K.; Chen, T.; Paster, B.J. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Marsh, P.D. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent. Clin. N. Am. 2010, 54, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.; Lee, J.B.; Kim, K.H.; Kim, S.; Seol, Y.J.; Lee, Y.M.; Rhyu, I.C. Comparison of Periodontopathic Bacterial Profiles of Different Periodontal Disease Severity Using Multiplex Real-Time Polymerase Chain Reaction. Diagnostics 2020, 10, 965. [Google Scholar] [CrossRef]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease—Is There Cause for Consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavigne, S.E.; Forrest, J.L. An umbrella review of systematic reviews of the evidence of a causal relationship between perio-dontal disease and cardiovascular diseases: Position paper from the Canadian Dental Hygienists Association. Can. J. Dent. Hyg. 2020, 54, 32–41. [Google Scholar] [PubMed]
- Lafon, A.; Pereira, B.; Dufour, T.; Rigouby, V.; Giroud, M.; Béjot, Y.; Tubert-Jeannin, S. Periodontal disease and stroke: A me-ta-analysis of cohort studie. Eur. J. Neurol. 2014, 9, 1155–1161. [Google Scholar] [CrossRef]
- Lavigne, S.E.; Forrest, J.L. An umbrella review of systematic reviews of the evidence of a causal relationship between perio-dontal disease and adverse pregnancy outcomes: A position paper from the Canadian Dental Hygienists Association. Can. J. Dent. Hyg. 2020, 54, 92–100. [Google Scholar]
- Genco, R.J.; Graziani, F.; Hasturk, H. Effects of periodontal disease on glycemic control, complications, and incidence of dia-betes mellitus. Periodontol. 2000 2020, 83, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Awano, S.; Ansai, T.; Takata, Y.; Soh, I.; Akifusa, S.; Hamasaki, T.; Yoshida, A.; Sonoki, K.; Fujisawa, K.; Takehara, T. Oral health and mortality risk from pneumonia in the elderly. J. Dent. Res. 2008, 87, 334–339. [Google Scholar] [CrossRef]
- Karpiński, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Ding, H.; Wei, W.; Liu, J.; Wang, J.; Ren, J.; Chan, W.; Wang, M.; Hao, L.; Li, J.; et al. Periodontitis aggravates kidney injury by upregulating STAT1 expression in a mouse model of hypertension. FEBS Open Bio 2021, 11, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Maitre, Y.; Micheneau, P.; Delpierre, A.; Mahalli, R.; Guerin, M.; Amador, G.; Denis, F. Did the brain and oral microbiota talk to each other? A review of the literature. J. Clin. Med. 2020, 9, 3876. [Google Scholar] [CrossRef] [PubMed]
- Engevik, M.A.; Luk, B.; Chang-Graham, A.L.; Hall, A.; Herrmann, B.; Ruan, W.; Endres, B.T.; Shi, Z.; Garey, K.W.; Hyser, J.M.; et al. Bifidobacterium dentium Fortifies the Intestinal Mucus Layer via Autophagy and Calcium Signaling Pathways. mBio 2019, 10, e01087-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Callaghan, A.; Van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consen-sus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, H.F.; Lamont, R.J. Oral microbial communities in sickness and in health. Trends Microbiol. 2005, 13, 589–595. [Google Scholar] [CrossRef]
- Corby, P.M.; Lyons-Weiler, J.; Bretz, W.A.; Hart, T.C.; Aas, J.A.; Boumenna, T.; Goss, J.; Corby, A.L.; Junior, H.M.; Weyant, R.J.; et al. Microbial risk indicators of early childhood caries. J. Clin. Microbiol. 2005, 43, 5753–5759. [Google Scholar] [CrossRef] [Green Version]
- Belda-Ferre, P.; Alcaraz, L.D.; Cabrera-Rubio, R.; Romero, H.; Simón-Soro, A.; Pignatell, M.; Mira, A. The oral metagenome in health and disease. ISME J. 2012, 6, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Rai, B.; Kaur, J.; Anand, S.C. Possible relationship between periodontitis and dementia in a North Indian old age population: A pilot study. Gerodontology 2010, 29, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Sobczyński, M.; Sender-Janeczek, A.; Zwolinska, K.; Błachowicz, O.; Tomczyk, T.; Leszek, J. Association between Periodontal Health Status and Cognitive Abilities. The Role of Cytokine Profile and Systemic Inflammation. Curr. Alzheimer Res. 2017, 14, 978–990. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Wu, M.; Feng, Y.; Zhou, Z.; Chen, L.; Chen, F. Alterations of oral microbiota distinguish children with autism spec-trum disorders from healthy controls. Sci. Rep. 2018, 8, 1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.C.; Paiva, S.M.; Campos, M.R.; Czeresnia, D. Factors associated with malocclusions in children and adolescents with Down syndrome. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 489.e1–489.e8. [Google Scholar] [CrossRef]
- Kuboniwa, M.; Tribble, G.D.; James, C.E.; Kilic, A.O.; Tao, L.; Herzberg, M.C.; Shizukuishi, S.; Lamont, R.J. Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol. Microbiol. 2006, 60, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.C.; Paster, B.J.; Lu, S.C.; Kanasi, E.; Kent, R., Jr.; Van Dyke, T.; Sonis, S.T. Subgingival and tongue microbiota during early periodontitis. J. Dent. Res. 2006, 85, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Ledder, R.G.; Gilbert, P.; Huws, S.A.; Aarons, L.; Ashley, M.P.; Hull, P.S.; McBain, A.J. Molecular analysis of the subgingival microbiota in health and disease. Appl. Environ. Microbiol. 2007, 73, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.P.; Boches, S.K.; Cotton, S.L.; Goodson, J.M.; Kent, R.; Haffajee, A.D.; Socransky, S.S.; Hasturk, H.; Van Dyke, T.E.; Dewhirst, F.; et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J. Periodontol. 2009, 80, 1421–1432. [Google Scholar] [CrossRef]
- Khocht, A.; Yaskell, T.; Janal, M.; Turner, B.F.; Rams, T.E.; Haffajee, A.D.; Socransky, S.S. Subgingival microbiota in adult Down syndrome periodontitis. J. Periodontal Res. 2012, 47, 500–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, F.A.; Cota, L.O.M.; Cortelli, S.C.; Miranda, T.B.; Neves, F.S.; Cortelli, J.R.; Costa, F.O. Periodontal condition and levels of bacteria associated with periodontitis in individuals with bipolar affective disorders: A case-control study. J. Periodontal Res. 2018, 54, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Castro-Nallar, E.; Bendall, M.L.; Pérez-Losada, M.; Sabuncyan, S.; Severance, E.G.; Dickerson, F.B.; Schroeder, J.R.; Yolken, R.H.; Crandall, K.A. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ 2015, 3, e1140. [Google Scholar] [CrossRef]
- Riviere, G.R.; Riviere, K.H.; Smith, K.S. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol. Immunol. 2002, 17, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kamer, A.R.; Craig, R.G.; Pirraglia, E.; Dasanayake, A.P.; Norman, R.G.; Boylan, R.J.; Nehorayoff, A.; Glodzik, L.; Brys, M.; De Leon, M.J. TNF-α and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal sub-jects. J. Neuroimmunol. 2009, 216, 92–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and metaanalyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Hooks, K.B.; Konsman, J.P.; O’Malley, M.A. Microbiota-gut-brain research: A critical analysis. Behav. Brain Sci. 2018, 42, 1–40. [Google Scholar] [CrossRef]
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics History. J. Clin. Gastroenterol. 2016, 50, 13–15. [Google Scholar] [CrossRef]
- Slomka, V.; Hernandez-Sanabria, E.; Herrero, E.R.; Zaidel, L.; Bernaerts, K.; Boon, N.; Quirynen, M.; Teughels, W. Nutritional stimulation of commensal oral bacteria suppresses pathogens: The prebiotic concept. J. Clin. Periodontol. 2017, 44, 344–352. [Google Scholar] [CrossRef]
- Rosier, B.T.; Buetas, E.; Moya-Gonzalvez, E.M.; Artacho, A.; Mira, A. Nitrate as a potential prebiotic for the oral microbiome. Sci. Rep. 2020, 10, 12895. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Hernández, N.; Serrano-Villar, S.; Domingo, A.; Pons, X.; Artacho, A.; Estrada, V.; Moya, A.; Gosalbes, M.J. Modu-lation of Saliva Microbiota through Prebiotic Intervention in HIV-Infected Individuals. Nutrients 2019, 11, 1346. [Google Scholar] [CrossRef] [Green Version]
- Madhwani, T.; McBain, A.J. Bacteriological effects of a Lactobacillus reuteri probiotic on in vitro oral biofilms. Arch. Oral Biol. 2011, 56, 1264–1273. [Google Scholar] [CrossRef]
- Jiang, Q.; Stamatova, I.; Kainulainen, V.; Korpela, R.; Meurman, J.H. Interactions between Lactobacillus rhamnosus GG and oral micro-organisms in an in vitro biofilm model. BMC Microbiol. 2016, 16, 149. [Google Scholar] [CrossRef] [Green Version]
- Mendi, A.; Köse, S.; Uçkan, D.; Akca, G.; Yilmaz, D.; Aral, L.; Gültekin, S.E.; Eroğlu, T.; Kiliç, E.; Uçkan, S. Lactobacillus rhamnosus could inhibit Porphyromonas gingivalis derived CXCL8 attenuation. J. Appl. Oral Sci. 2016, 24, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque-Souza, E.; Balzarini, D.; Ando-Suguimoto, E.S.; Ishikawa, K.H.; Simionato, M.R.L.; Holzhausen, M.; Mayer, M.P.A. Probiotics alter the immune response of gingival epithelial cells challenged by Porphyromonas gingivalis. J. Periodontal Res. 2019, 54, 115–127. [Google Scholar] [CrossRef]
- Jaffar, N.; Ishikawa, Y.; Mizuno, K.; Okinaga, T.; Maeda, T. Mature Biofilm Degradation by Potential Probiotics: Aggregati-bacter actinomycetemcomitans versus Lactobacillus spp. PLoS ONE 2016, 11, e0159466. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Salvador, S.L.; Silva, P.H.; Furlaneto, F.A.; Figueiredo, L.; Casarin, R.; Ervolino, E.; Palioto, D.B.; Souza, S.L.; Taba, M., Jr.; et al. Benefits of Bifidobacterium animalis subsp. lactis Probiotic in Experimental Peri-odontitis. J. Periodontol. 2017, 88, 197–208. [Google Scholar] [CrossRef]
- Invernici, M.M.; Furlaneto, F.A.C.; Salvador, S.L.; Ouwehand, A.C.; Salminen, S.; Mantziari, A.; Vinderola, G.; Ervolino, E.; Santana, S.I.; Silva, P.H.F.; et al. Bifidobacterium animalis subsp lactis HN019 presents antimicrobial potential against periodontopathogens and modulates the immunological response of oral mucosa in periodontitis patients. PLoS ONE 2020, 15, e0238425. [Google Scholar]
- Dassi, E.; Ballarini, A.; Covello, G.; Quattrone, A.; Jousson, O.; De Sanctis, V.; Bertorelli, R.; Denti, M.A.; Segata, N. Enhanced microbial diversity in the saliva microbiome induced by short-term probiotic intake revealed by 16S rRNA sequencing on the IonTorrent PGM platform. J. Biotechnol. 2014, 190, 30–39. [Google Scholar] [CrossRef]
- Dassi, E.; Ferretti, P.; Covello, G.; Bertorelli, R.; Denti, M.A.; DeSanctis, V.; Tett, A.; Segata, N. The short-term impact of pro-biotic consumption on the oral cavity microbiome. Sci. Rep. 2018, 8, 10476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becirovic, A.; Abdi-Dezfuli, J.F.; Hansen, M.F.; Lie, S.A.; Vasstrand, E.N.; Bolstad, A.I. The effects of a probiotic milk drink on bacterial composition in the supra- and subgingival biofilm: A pilot study. Benef. Microbes 2018, 9, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Masaki, C.; Tsuka, S.; Mukaibo, T.; Kondo, Y.; Hosokawa, R. The effects of Lactobacillus reuteri probiotics com-bined with azithromycin on peri-implantitis: A randomized placebo-controlled study. J. Prosthodont. Res. 2018, 62, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kõll, P.; Mändar, R.; Marcotte, H.; Leibur, E.; Mikelsaar, M.; Hammarström, L. Characterization of oral lactobacilli as poten-tial probiotics for oral health. Oral Microbiol. Immunol. 2008, 23, 139–147. [Google Scholar] [CrossRef]
- Teanpaisan, R.; Piwat, S.; Dahlén, G. Inhibitory effect of oral Lactobacillus against oral pathogens. Lett. Appl. Microbiol. 2011, 53, 452–459. [Google Scholar] [CrossRef]
- Van Essche, M.; Loozen, G.; Godts, C.; Boon, N.; Pauwels, M.; Quirynen, M.; Teughels, W. Bacterial antagonism against peri-odontopathogens. J. Periodontol. 2013, 84, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Hsieh, P.S.; Ho, H.H.; Hsieh, S.H.; Kuo, Y.W.; Yang, S.F.; Lin, C.W. Antibacterial activity of viable and heat-killed probiotic strains against oral pathogens. Lett. Appl. Microbiol. 2020, 70, 310–317. [Google Scholar] [CrossRef]
- Shin, H.S.; Baek, D.H.; Lee, S.H. Inhibitory effect of Lactococcus lactis on the bioactivity of periodontopathogens. J. Gen. Appl. Microbiol. 2018, 64, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T.; Suzuki, N.; Nakaya, S.; Omagari, S.; Yoneda, M.; Hanioka, T.; Hirofuji, T. Effects of Lactobacillus salivarius WB21 combined with green tea catechins on dental caries, periodontitis, and oral malodor. Arch. Oral Biol. 2019, 98, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xiao, L.; Shen, D.; Hao, Y. Competition between yogurt probiotics and periodontal pathogens in vitro. Acta Odontol. Scand. 2010, 68, 261–268. [Google Scholar] [CrossRef]
- Tobita, K.; Watanabe, I.; Tomokiyo, M.; Saito, M. Effects of heat-treated Lactobacillus crispatus KT-11 strain consumption on improvement of oral cavity environment: A randomised double-blind clinical trial. Benef. Microbes 2018, 9, 585–592. [Google Scholar] [CrossRef]
- Iniesta, M.; Herrera, D.; Montero, E.; Zurbriggen, M.; Matos, A.R.; Marín, M.J.; Sánchez-Beltrán, M.C.; Llama-Palacio, A.; Sanz, M. Probiotic effects of orally administered Lactobacillus reuteri-containing tablets on the subgingival and salivary mi-crobiota in patients with gingivitis. A randomized clinical trial. J. Clin. Periodontol. 2012, 39, 736–744. [Google Scholar] [CrossRef]
- Teughels, W.; Durukan, A.; Ozcelik, O.; Pauwels, M.; Quirynen, M.; Haytac, M.C. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo-controlled study. J. Clin. Periodontol. 2013, 40, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Montero, E.; Iniesta, M.; Rodrigo, M.; Marín, M.J.; Figuero, E.; Herrera, D.; Sanz, M. Clinical and microbiological effects of the adjunctive use of probiotics in the treatment of gingivitis: A randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 708–716. [Google Scholar] [CrossRef]
- Imran, F.; Das, S.; Padmanabhan, S.; Rao, R.; Suresh, A.; Bharath, D. Evaluation of the efficacy of a probiotic drink contain-ing Lactobacillus casei on the levels of periodontopathic bacteria in periodontitis: A clinico-microbiologic study. Indian J. Dent. Res. 2015, 26, 462–468. [Google Scholar] [CrossRef]
- Sarmento, É.G.; Cesar, D.E.; Martins, M.L.; De Oliveira Góis, E.G.; Furtado Martins, E.M.; Da Rocha Campos, A.N.; Del’Duca, A.; De Oliveira Martins, A.D. Effect of probiotic bacteria in composition of children’s saliva. Food Res. Int. 2019, 116, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Mayanagi, G.; Kimura, M.; Nakaya, S.; Hirata, H.; Sakamoto, M.; Benno, Y.; Shimauchi, H. Probiotic effects of orally admin-istered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: A double-blinded, placebo-controlled, randomized clinical trial. J. Clin. Periodontol. 2009, 36, 506–513. [Google Scholar] [CrossRef]
- Sajedinejad, N.; Paknejad, M.; Houshmand, B.; Sharafi, H.; Jelodar, R.; Shahbani Zahiri, H.; Noghabi, K.A. Lactobacillus salivarius NK02: A Potent Probiotic for Clinical Application in Mouthwash. Probiotics Antimicrob. Proteins 2018, 10, 485–495. [Google Scholar] [CrossRef]
- Zahradnik, R.T.; Magnusson, I.; Walker, C.; McDonell, E.; Hillman, C.H.; Hillman, J.D. Preliminary assessment of safety and effectiveness in humans of ProBiora3, a probiotic mouthwash. J. Appl. Microbiol. 2009, 107, 682–690. [Google Scholar] [CrossRef]
- Alanzi, A.; Honkala, S.; Honkala, E.; Varghese, A.; Tolvanen, M.; Söderling, E. Effect of Lactobacillus rhamnosus and Bifidobacterium lactis on gingival health, dental plaque, and periodontopathogens in adolescents: A randomised placebo-controlled clinical trial. Benef. Microbes 2018, 9, 593–602. [Google Scholar] [CrossRef]
- Morales, A.; Gandolfo, A.; Bravo, J.; Carvajal, P.; Silva, N.; Godoy, C.; Garcia-Sesnich, J.; Hoare, A.; Diaz, P.; Gamonal, J. Microbiological and clinical effects of probiotics and antibiotics on nonsurgical treatment of chronic periodontitis: A randomized placebo- controlled trial with 9-month follow-up. J. Appl. Oral Sci. 2018, 26, e20170075. [Google Scholar] [CrossRef]
- Kobayashi, R.; Kobayashi, T.; Sakai, F.; Hosoya, T.; Yamamoto, M.; Kurita-Ochiai, T. Oral administration of Lactobacillus gasseri SBT2055 is effective in preventing Porphyromonas gingivalis-accelerated periodontal disease. Sci. Rep. 2017, 7, 545. [Google Scholar] [CrossRef]
- Maekawa, T.; Hajishengallis, G. Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. J. Periodontal Res. 2014, 49, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.K.; Brandsborg, E.; Holmstrøm, K.; Twetman, S. Effect of tablets containing probiotic candidate strains on gingival inflammation and composition of the salivary microbiome: A randomised controlled trial. Benef. Microbes 2018, 9, 487–494. [Google Scholar] [CrossRef]
- Braathen, G.; Ingildsen, V.; Twetman, S.; Ericson, D.; Jørgensen, M.R. Presence of Lactobacillus reuteri in saliva coincide with higher salivary IgA in young adults after intake of probiotic lozenges. Benef. Microbes 2017, 8, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, M.R.; Keller, M.K.; Kragelund, C.; Hamberg, K.; Ericson, D.; Nielsen, C.H.; Twetman, S. Lactobacillus reuteri supplements do not affect salivary IgA or cytokine levels in healthy subjects: A randomized, double-blind, placebo-controlled, cross-over trial. Acta Odontol. Scand. 2016, 74, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Hallström, H.; Lindgren, S.; Widén, C.; Renvert, S.; Twetman, S. Probiotic supplements and debridement of peri-implant mucositis: A randomized controlled trial. Acta Odontol. Scand. 2016, 74, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.; Orlandi Sardi, J.d.C.; Pitangui, N.d.S.; Roque, S.M.; Da Silva, B.A.C.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Ramesh, G.; MacLean, A.G.; Philipp, M.T. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegener-ation, and neuropathic pain. Mediat. Inflamm. 2013, 2013, 480739. [Google Scholar] [CrossRef] [Green Version]
- Suganya, K.; Koo, B.S. Gut-Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Ben-eficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol Sci. 2020, 21, 7551. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar]
- Ansari, F.; Pourjafar, H.; Tabrizi, A.; Homayouni, A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. [Google Scholar] [CrossRef]
- Roberfroid, M. Prebiotics: The concept revisited. J. Nutr. 2007, 137, 830–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Cornejo Ulloa, P.; Van der Veen, M.H.; Krom, B.P. Review: Modulation of the oral microbiome by the host to promote ecolog-ical balance. Odontology 2019, 107, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Kisely, S. No Mental Health without Oral Health. Can. J. Psychiatry. 2016, 61, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Coelho, J.M.F.; Miranda, S.S.; Da Cruz, S.S.; Dos Santos, D.N.; Trindade, S.C.; Cerqueira, E.M.M.; Passos-Soares, J.S.; Costa, M.D.C.N.; Figueiredo, A.C.M.G.; Hintz, A.M.; et al. Common mental disorder is associated with periodontitis. J. Periodontal Res. 2020, 55, 221–228. [Google Scholar] [CrossRef]
- Fratto, G.; Manzon, L. Use of psychotropic drugs and associated dental diseases. Int. J. Psychiatry Med. 2014, 48, 185–197. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Genet. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol. 2000 2005, 38, 72–122. [Google Scholar] [CrossRef] [PubMed]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokryazdan, P.; Faseleh Jahromi, M.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expres-sion. Med. Microbiol. Immunol. 2017, 206, 1–9. [Google Scholar] [CrossRef]
- Wan, L.Y.; Chen, Z.J.; Shah, N.P.; El-Nezami, H. Modulation of Intestinal Epithelial Defense Responses by Probiotic Bacteria. Crit. Rev. Food Sci. Nutr. 2016, 56, 2628–2641. [Google Scholar] [CrossRef]
- Pujari, R.; Banerjee, G. Impact of prebiotics on immune response: From the bench to the clinic. Immunol. Cell Biol. 2021, 99, 255–273. [Google Scholar] [CrossRef]
- Pape, K.; Tamouza, R.; Leboyer, M.; Zipp, F. Immunoneuropsychiatry-novel perspectives on brain disorders. Nat. Rev. Neurol. 2019, 15, 317–328. [Google Scholar] [CrossRef]
- Denis, F.; Siu-Paredes, F.; Maitre, Y.; Amador, G.; Rude, N. A qualitative study on experiences of persons with schizophrenia in oral-health-related quality of life. Braz. Oral Res. 2021, 35, e050. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).