Supranational Assessment of the Quality of Probiotics: Collaborative Initiative between Independent Accredited Testing Laboratories
Abstract
:1. Quality of Probiotic Preparations
2. Regulatory Framework of Probiotic Preparations in Europe
3. Quality Labeling: The ESLP (European Scientific League for Probiotics) Initiative and Expertise
- Three levels of denomination for a specific strain: genus, species, strain.
- Registration number in a “strain bank”.
- The quantity of bacteria announced is exact: ESLP allows a maximum 1 log difference from the amount stated on the packaging and in the instructions for the proprietary medicinal product for both of these quantitative measures:
- (a)
- First Quantitative measure at day 0
- (b)
- Second Quantitative measure after 6 months
- The strains announced are present in the product
4. Relevance of the Accreditation in Probiotic Testing
5. Supranational Collaborations in Probiotics Testing
6. Sharing the Experience and Developing Common Approaches
- the ESPGHAN [7] working group for Probiotics and Prebiotics recommending the minimum criteria for probiotics:
- a
- being sufficiently characterized,
- b
- safe,
- c
- supported by at least one positive trial according to generally accepted scientific standards—the beneficial effects of probiotics being strain specific; not all the positive results of one strain or association can be generalized to other strains or associations
- d
- alive in adequate numbers in the product throughout shelf-life and when consumed [7]
- The ISAPP and IPA Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements with respect to commercial communications defending the same principles [6].
7. Future Perspectives of Quality Assessment of Probiotics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Cordoba, Argentina, 1–4 October 2001. Probiotics in Food Health and Nutritional Properties and Guidelines for Evaluation. FAO Food and Nutrition Paper 85 ISSN 0254 – 4725. Available online: www.fao.org/3/a0512e/a0512e.pdf (accessed on 15 January 2021).
- Salminen, S.; Nybom, S.; Meriluoto, J.; Collado, M.C.; Vesterlund, S.; El-Nezami, H. Interaction of probiotics and pathogens—benefits to human health? Curr. Opin. Biotechnol. 2010, 21, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- IPA, International Probiotics Association; IPA Manifesto. Available online: www.ipaeurope.org (accessed on 15 January 2021).
- Jackson, S.A.; Schoeni, J.L.; Vegge, C.; Pane, M.; Stahl, B.; Bradley, M.; Goldman, V.S.; Burguière, P.; Atwater, J.B.; Sanders, M.E. Improving End-User Trust in the Quality of Commercial Probiotic Products. Front. Microbiol. 2019, 10, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolaček, S.; Hojsak, I.; Canani, R.B.; Guarino, A.; Indrio, F.; Orel, R.; Pot, B.; Shamir, R.; Szajewska, H.; Vandenplas, Y.; et al. Commercial Probiotic Products: A Call for Improved Quality Control. A Position Paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Fredua-Agyeman, M.; Parab, S.; Gaisford, S. Evaluation of commercial Probiotics Products B. J. Pharm. 2016, 1, 84–89. [Google Scholar]
- Caselli, M.; Cassol, F.; Calò, G.; Holton, J.; Zuliani, G.; Gasbarrini, A. Actual concept of "probiotics": Is it more functional to science or business? World J. Gastroenterol. 2013, 19, 1527–1540. [Google Scholar] [CrossRef]
- Aureli, P.; Fiore, A.; Scalfaro, C.; Casale, M.; Franciosa, G. National survey outcomes on commercial probiotic food supplements in Italy. Int. J. Food Microbiol. 2010, 137, 265–273. [Google Scholar] [CrossRef]
- Klein, G.; Pack, A.; Bonaparte, C.; Reuter, G. Taxonomy and physiology of probiotic lactic acid bacteria. Int. J. Food Microbiol. 1998, 41, 103–125. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M.T.; Shah, S.; Winkler, J.T. Public health issues arising from microbiological and labelling quality of foods and supplements containing probiotic microorganims. Public Health Nutr. 1999, 2, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Shah, N. Probiotic Bacteria: Selective Enumeration and Survival in Dairy Foods. J. Dairy Sci. 2000, 83, 894–907. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M.T.; Shah, S. Deficiencies in microbiological quality and labelling of probiotic supplements. Int. J. Food Microbiol. 2002, 72, 175–176. [Google Scholar] [CrossRef]
- Temmerman, R.; Pot, B.; Huys, G.; Swings, J. Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int. J. Food Microbiol. 2003, 81, 1–10. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- IPA Manifesto. Available online: www.ipaeurope.org (accessed on 15 January 2021).
- Neunez, M.; Goldman, M.; Ghezzi, P. Online Information on Probiotics: Does It Match Scientific Evidence? Front. Med. 2020, 6. [Google Scholar] [CrossRef] [Green Version]
- Guidelines on Probiotics and Prebiotics—Ministry of Health (Italy). March 2018. Available online: www.salute.gov.it/MinisterodellaSalute,Lineeguidasuprobioticieprebiotici,revizionemarzo2018.C_17publiccazioni_1016allegato-2 (accessed on 15 January 2021).
- EFSA Europa—European Food Safety Authority: Guidance on the Implementation of Regulation N° 1924/2006 on Nutrition and Health Claims Made on Foods Conclusions of the Standing Committee on the Food Chain and Animal Health. 14 December 2007. Available online: www.EFSA.europa.eu (accessed on 15 January 2021).
- AESAN—Agencia Española de Seguridad Alimentaria y Nutrición. Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/subdetalle/probioticos.htm (accessed on 15 January 2021).
- ESLP—European Scientific League for Probiotics. Available online: www.probioleague.org (accessed on 15 January 2021).
- International Standards Organisation [ISO]. General Requirements for the Competence of Testing and Calibration Laboratories; ISO/IEC 17025:2017; International Standards Organisation: Geneva, Switzerland, 2017. [Google Scholar]
- International Standards Organisation [ISO]. Protocol for Method Validation in a Single Laboratory; ISO 16140-4:2020; International Standards Organisation: Geneva, Switzerland, 2020. [Google Scholar]
- International Standards Organisation [ISO]. Microbiology of the Food Chain—Estimation of Measurement Uncertainty for Quantitative Determinations; ISO 19036:2019; International Standards Organisation: Geneva, Switzerland, 2019. [Google Scholar]
- International Standards Organisation [ISO]; International Dairy Federation. Milk and Milk Products—Starter Cultures, Probiotics and Fermented Products—Quantification of Lactic Acid Bacteria by Flow Cytometry; International Standard, ISO 19344:2015 (IDF 232:2015); International Standards Organisation: Geneva, Switzerland, 2015. [Google Scholar]
- Chiron, C.; Tompkins, T.A.; Burguière, P. Flow cytometry: A versatile technology for specific quantification and viability assessment of micro-organisms in multistrain probiotic products. J. Appl. Microbiol. 2018, 124, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Pane, M.; Allesina, S.; Amoruso, A.; Nicola, S.; Deidda, F.; Mogna, L. Flow cytometry: Evolution of Microbiological Methods for Probiotics Enumeration. J. Clin. Gastroenterol. 2018, 52 (Suppl. S1), S41–S45. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- International Standards Organisation [ISO]. Microbiology of Food and Animal Feeding Stuffs—General Requirements and Guidance fro Microbiological Examinations; International Standard, ISO 7218:2007/Amd.1:2013 (E); International Standards Organisation: Geneva, Switzerland, 2017. [Google Scholar]
- International Standards Organisation [ISO]. Statistical Methods for Use in Proficiency Testing by Interlaboratory Comparison; International Standard, ISO 13528:2015; International Standards Organisation: Geneva, Switzerland, 2015. [Google Scholar]
- International Standards Organisation [ISO]. Milk Products—Enumeration of Presumptive Lactobacillus acidophilus on a Selective Medium—Colony Count Technique at 37 Degrees C; ISO 20128:2006 (IDF 192:2006); International Standards Organisation: Geneva, Switzerland, 2006. [Google Scholar]
- International Standards Organisation [ISO]. Milk Products—Enumeration of Presumptive Bifidobacteria—Colony Count Technique at 37 Degrees C; ISO 29981:2010 (IDF 220:2010); International Standards Organisation: Geneva, Switzerland, 2010. [Google Scholar]
- Rapporti ISTISAN 08/36; Aureli, P.; Fiore, A.; Scalfaro, C.; Franciosa, G. Metodi Microbiologici Tradizionali e Metodi Molecolari per L’analisi Degli Integratori Alimentari a Base di o con Probiotici per uso Umano; Istituto Superiore di Sanità: Rome, Italy, 2008. [Google Scholar]
- ISAPP—International Scientific Association for Probiotics and Prebiotics. Available online: www.isappscience.org (accessed on 15 May 2021).
- Collado, M.C.; Vinderola, G.; Salminen, S. Postbiotics: Facts and open questions. A position paper on the need for a consen-sus definition. Benef. Microbes 2019, 14, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 1–19. [Google Scholar] [CrossRef]

| Market | Probiotic | Packaging | Label Composition a | Expiration Date a |
|---|---|---|---|---|
| Belgium | A | Capsule | 12.5 billion CFU/cps of L. acidophilus, 12.5 billion CFU/cps of B. animalis subsp. lactis | 01/2021 |
| B | Capsule | 5 billion CFU/cps of L. acidophilus, 5 billion CFU/cps of B. animalis subsp. lactis | 06/2021 | |
| Italy | C | Sachet | 1.5 billion CFU/sachet of L. acidophilus, 1.5 billion CFU/sachet of B. animalis subsp. lactis | 10/2020 |
| D | Capsule | 2 billion CFU/cps of L. acidophilus, 1 billion CFU/cps of L. casei | 07/2020 |
| Bacterial Target | Method of Analysis | Medium Culture and Supplement | Diluent | Growth Condition |
|---|---|---|---|---|
| L. acidophilus | ISO 20128:2006 [32] | MRS Clindamycin 0.1 µg/mL and Ciprofloxacin 10 µg/mL | MRD (Maximum Recovery Diluent) | Anaerobic incubation at 37 °C for 72 h |
| B. animalis lactis | ISO 29981:2010 [33] | TOS Propionate Mupirocin 50 µg/mL | ||
| L. casei | Rapporti ISTISAN 2008/36 [34] | MRS Vancomycin 10 µg/mL |
| Product | Sample A | Sample B | Sample C | Sample D | |||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Lactobacillus acidophilus | Bifidobacterium lactis | Lactobacillus acidophilus | Bifidobacterium lactis | Lactobacillus acidophilus | Bifidobacterium lactis | Lactobacillus acidophilus | Lactobacillus casei | |
| Value stated by producer (Log10 CFU/g) | 10.67 | 10.67 | 10.52 | 10.52 | 9.10 | 9.10 | 9.78 | 9.48 | |
| LAB 1 | Operator 1 (log10 CFU/g) | 10.56 | 10.18 | 10.60 | 10.15 | 9.43 | 8.58 | 10.15 | 10.74 |
| Operator 2 (log10 CFU/g) | 9.92 | 9.65 | 10.08 | 9.83 | 9.88 | 9.04 | 10.08 | 10.08 | |
| Operator 3 (log10 CFU/g) | 10.85 | 9.40 | 10.48 | 10.26 | 9.67 | 9.56 | 10.68 | 10.58 | |
| LAB 2 | Operator 1 (log10 CFU/g) | 10.69 | 10.74 | 10.18 | 10.36 | 9.36 | 9.73 | 10.77 | 10.28 |
| Operator 2 (log10 CFU/g) | 10.65 | 10.71 | 10.15 | 10.32 | 9.28 | 9.79 | 10.73 | 10.18 | |
| Operator 3 (log10 CFU/g) | 10.69 | 10.68 | 10.26 | 10.36 | 9.30 | 9.78 | 10.76 | 10.18 | |
| Precision | LAB 1 Mean | 10.44 | 9.74 | 10.39 | 10.08 | 9.66 | 9.06 | 10.30 | 10.47 |
| LAB 1 SD | 0.47 | 0.40 | 0.27 | 0.22 | 0.22 | 0.49 | 0.33 | 0.34 | |
| LAB 1 Standard Error | 0.08 | 0.07 | 0.05 | 0.04 | 0.04 | 0.09 | 0.06 | 0.06 | |
| K2 uncertainty with 95% confidence | 9.08% | 8.14% | 5.26% | 4.43% | 4.60% | 10.78% | 6.41% | 6.59% | |
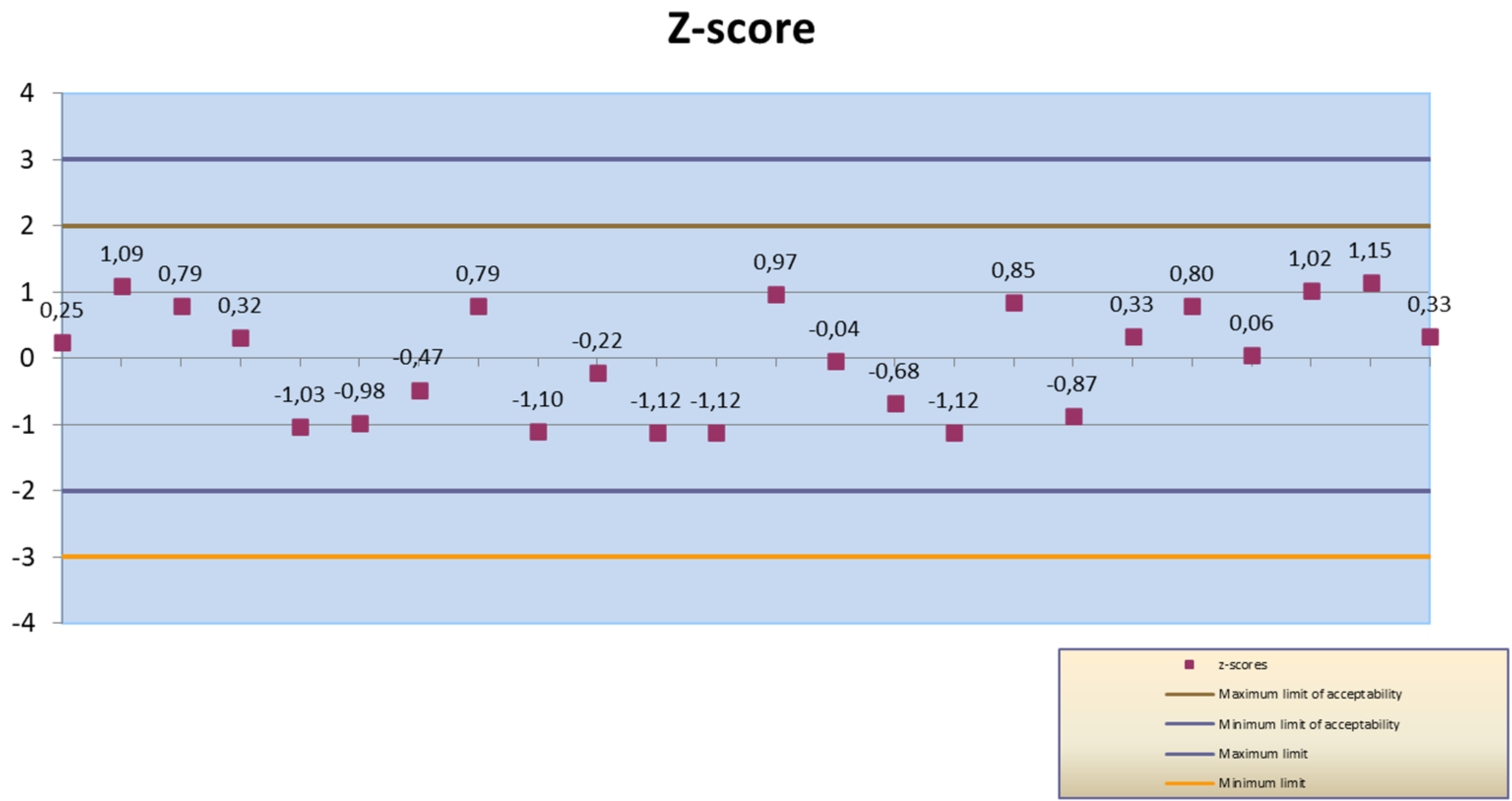
| Z-score 1 | 0.25 | 1.09 | 0.79 | 0.32 | −1.03 | −0.98 | −0.47 | 0.79 | |
| Z-score 2 | −1.10 | −0.22 | −1.12 | −1.12 | 0.97 | −0.04 | −0.68 | −1.12 | |
| Z-score 3 | 0.85 | −0.87 | 0.33 | 0.80 | 0.06 | 1.02 | 1.15 | 0.33 | |
| Variation coefficient | 4.538% | 4.072% | 2.629% | 2.214% | 2.299% | 5.392% | 3.203% | 3.295% | |
| LAB 2 Mean | 10.68 | 10.71 | 10.19 | 10.35 | 9.31 | 9.77 | 10.76 | 10.21 | |
| LAB 2 SD | 0.02 | 0.03 | 0.06 | 0.02 | 0.04 | 0.03 | 0.02 | 0.06 | |
| LAB 2 Standard Error | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | |
| K2 uncertainty with 95% confidence | 0.40% | 0.55% | 1.11% | 0.44% | 0.92% | 0.59% | 0.38% | 1.16% | |
| Z-score 1 | 0.58 | 1.03 | −0.29 | 0.58 | 1.12 | −1.15 | 0.75 | 1.15 | |
| Z-score 2 | −1.15 | −0.07 | −0.82 | −1.15 | −0.82 | 0.70 | −1.14 | −0.58 | |
| Z-score 3 | 0.58 | −0.96 | 1.11 | 0.58 | −0.30 | 0.45 | 0.39 | −0.58 | |
| Variation coefficient | 0.2000% | 0.2766% | 0.5533% | 0.2204% | 0.4611% | 0.2941% | 0.1897% | 0.5805% | |
| Accuracy | Accuracy LAB 1 | −0.23 | −0.92 | −0.14 | −0.45 | 0.56 | −0.04 | 0.52 | 0.98 |
| Accuracy LAB 2 | 0.01 | 0.04 | −0.33 | −0.17 | 0.22 | 0.67 | 0.97 | 0.73 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warzée, J.-P.; Elli, M.; Fall, A.; Cattivelli, D.; François, J.-Y. Supranational Assessment of the Quality of Probiotics: Collaborative Initiative between Independent Accredited Testing Laboratories. Microorganisms 2021, 9, 1456. https://doi.org/10.3390/microorganisms9071456
Warzée J-P, Elli M, Fall A, Cattivelli D, François J-Y. Supranational Assessment of the Quality of Probiotics: Collaborative Initiative between Independent Accredited Testing Laboratories. Microorganisms. 2021; 9(7):1456. https://doi.org/10.3390/microorganisms9071456
Chicago/Turabian StyleWarzée, Jean-Pol, Marina Elli, Abdoulaye Fall, Daniela Cattivelli, and Jean-Yves François. 2021. "Supranational Assessment of the Quality of Probiotics: Collaborative Initiative between Independent Accredited Testing Laboratories" Microorganisms 9, no. 7: 1456. https://doi.org/10.3390/microorganisms9071456
APA StyleWarzée, J.-P., Elli, M., Fall, A., Cattivelli, D., & François, J.-Y. (2021). Supranational Assessment of the Quality of Probiotics: Collaborative Initiative between Independent Accredited Testing Laboratories. Microorganisms, 9(7), 1456. https://doi.org/10.3390/microorganisms9071456





