Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems: A Comprehensive Outlook on Current and Emerging Mitigation and Control Approaches
Abstract
:1. Introduction
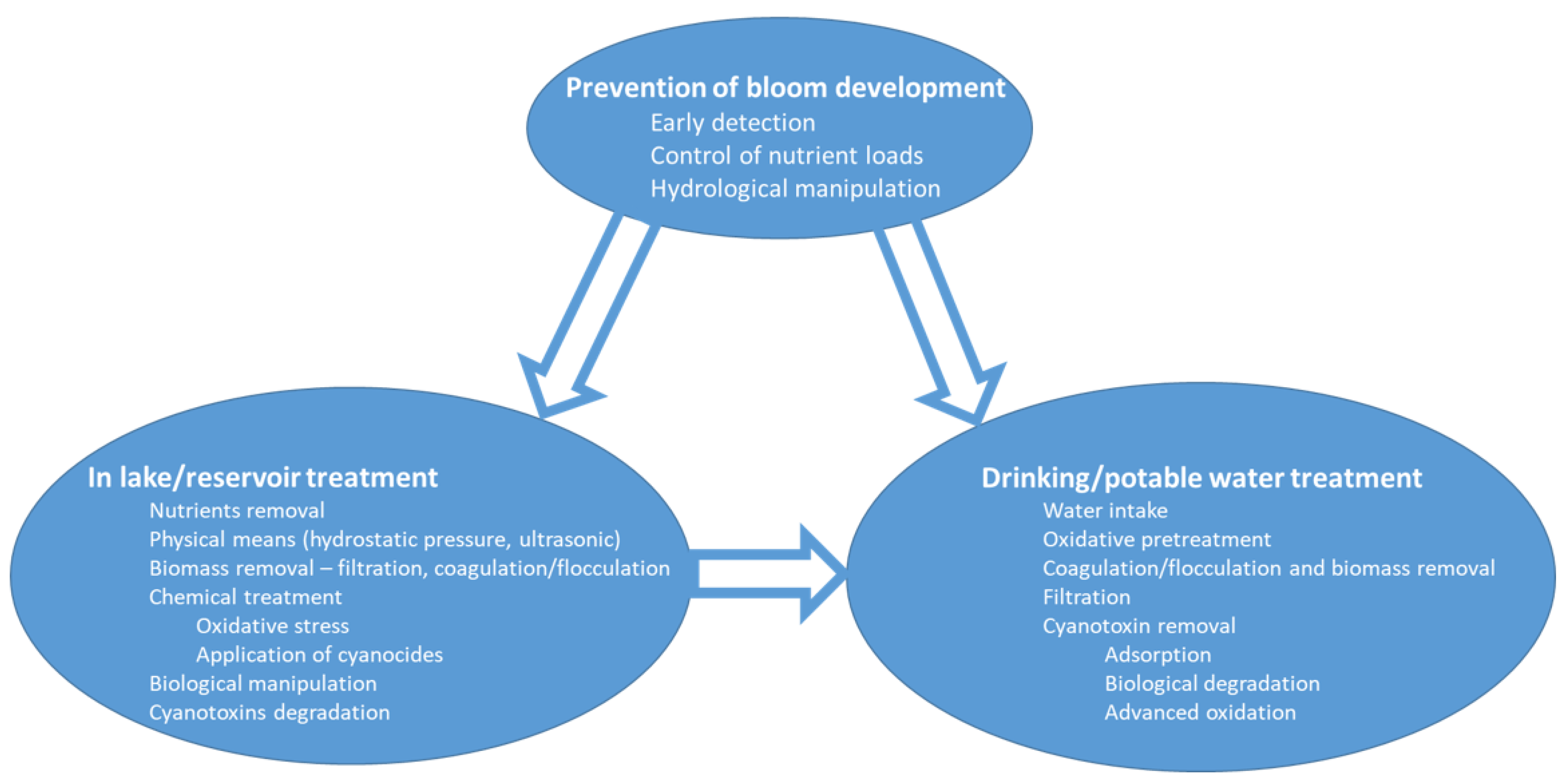
2. Prevention of Bloom Development
2.1. Early Detection
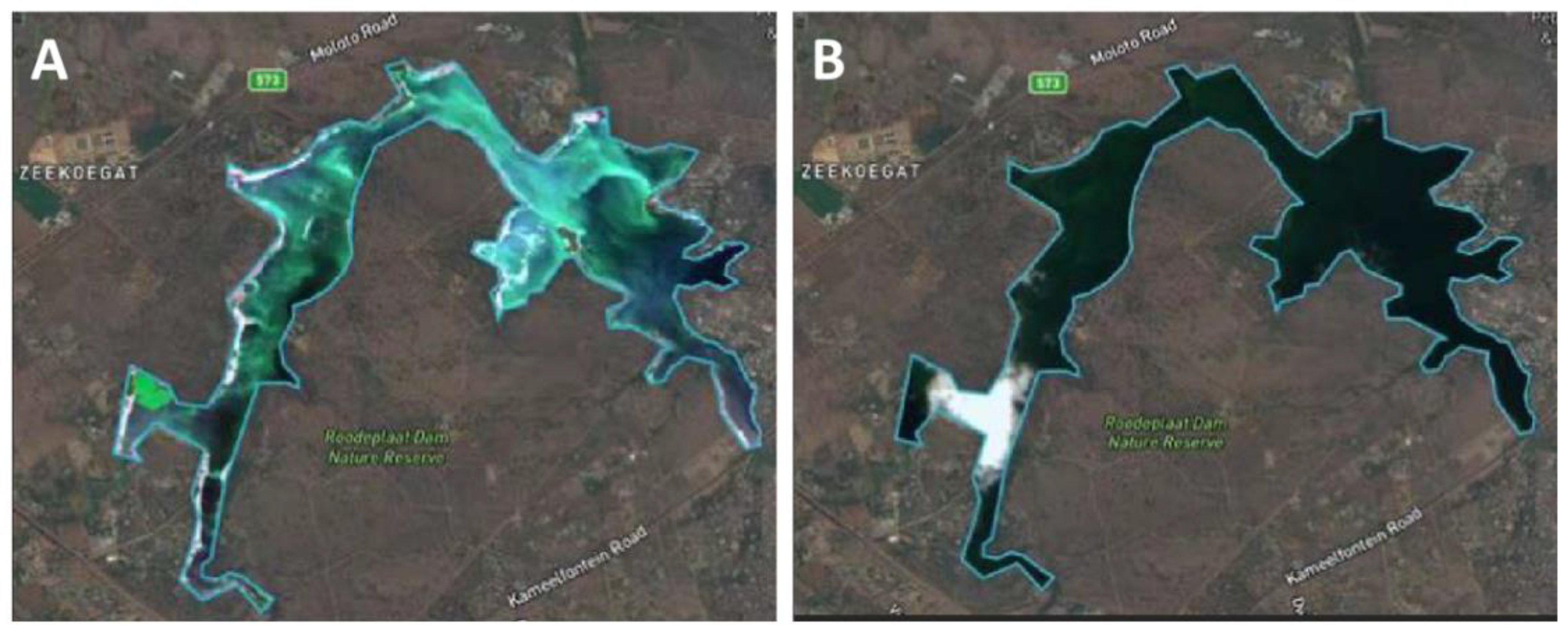
2.2. Water Shade Management—Nutrient Loads
2.3. Hydrological Manipulation
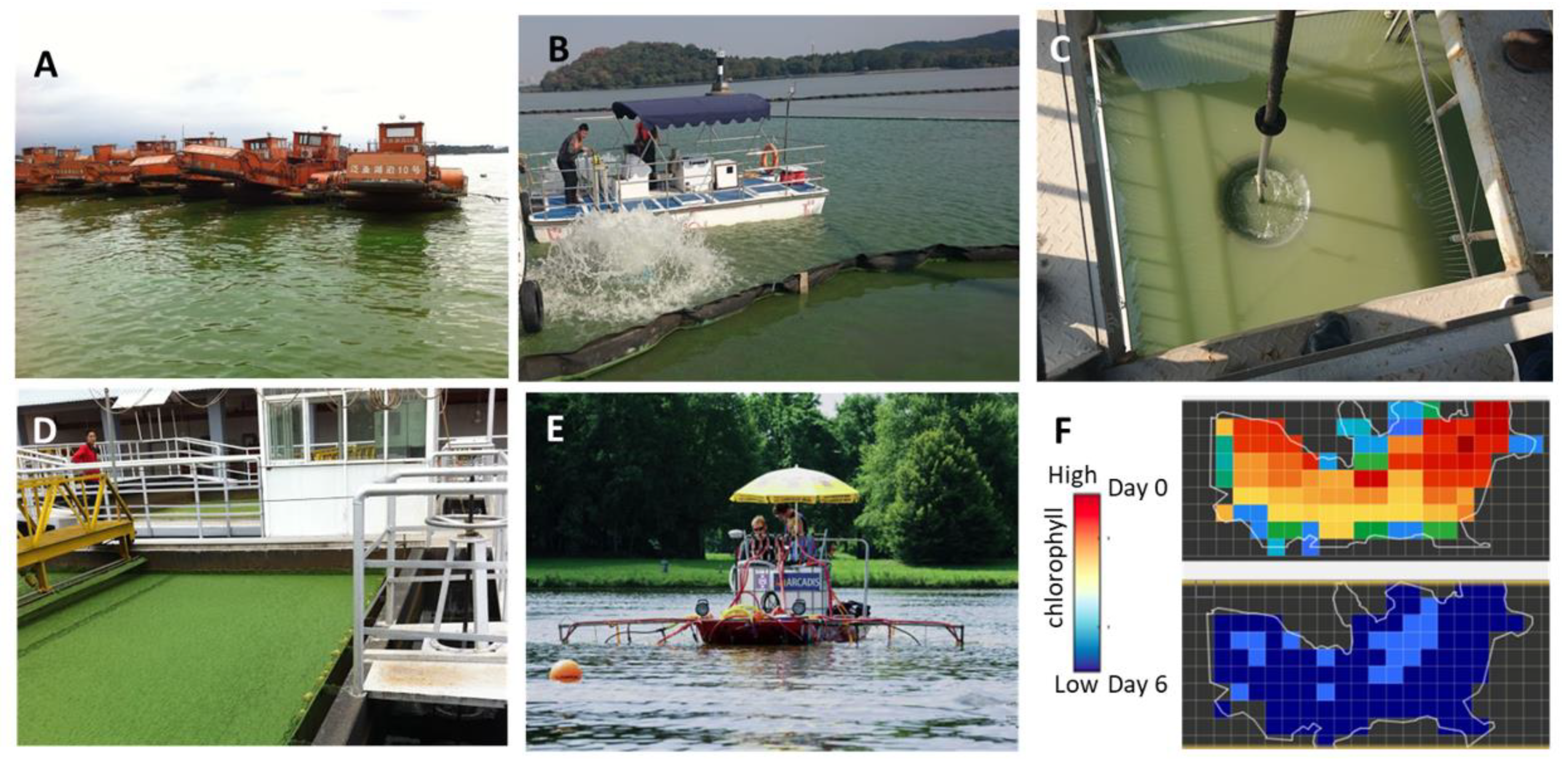
3. In-Lake Treatments
3.1. Harvesting of the Floating Cells
3.2. Nutrient Removal
3.3. Hydrophysical and Physical Control
Buoyancy Regulation
3.4. Chemical Treatments
3.4.1. Oxidative Stress Based Treatments
3.4.2. Other Algicides and Cyanocides
3.5. Removal of CyanoHAB Biomass by Chemical Treatment
3.6. Approaches toward Biological Treatments
4. Drinking/Potable Water Treatment—Removal of Cyanobacteria and Their Toxins
4.1. General Considerations
4.2. Water Intake
4.3. On-Line Pretreatment
4.4. Removal of Suspended Matter
4.5. Membrane Filtration
4.6. Removal and Degradation of Soluble Cyanotoxins
4.6.1. Toxin Adsorption
4.6.2. Chemical Processes and Advanced Oxidation
5. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasmussen, B.; Fletcher, I.R.; Brocks, J.J.; Kilburn, M.R. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 2008, 455, 1101–1104. [Google Scholar] [CrossRef]
- Bekker, A.; Holland, H.D.; Wang, P.L.; Rumble, D.; Stein, H.J.; Hannah, J.L.; Coetzee, L.L.; Beukes, N.J. Dating the rise of atmospheric oxygen. Nature 2004, 427, 117–120. [Google Scholar] [CrossRef]
- Knoll, A.H. Cyanobacteria and earth history. In The Cyanobacteria, Molecular Biology, Genetics and Evolution; Herrero, A., Flores, E., Eds.; Caister Academic Press: Norfolk, UK, 2008; pp. 1–20. [Google Scholar]
- Walsby, A.-E. Gas vesicles. Microbiol. Rev. 1994, 58, 94–144. [Google Scholar] [CrossRef]
- Sukenik, A.; Zohary, T.; Padisák, J. Cyanoprokaryota and other prokaryotic algae. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Elsevier: Oxford, UK, 2009; Volume 1, pp. 138–148. [Google Scholar]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Paerl, H.W.; Barnard, M.A. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human-and climatically-altered world. Harmful Algae 2020, 96, 101845. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.; Chorus, I.; Schaeffer, B.A.; Urquhart, E. Planning monitoring programmes for cyanobacteria and cyanotoxins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Welker, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 641–669. [Google Scholar]
- Bertone, E.; Burford, M.A.; Hamilton, D.P. Fluorescence probes for real-time remote cyanobacteria monitoring: A review of challenges and opportunities. Water Res. 2018, 141, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Choo, F.; Zamyadi, A.; Newton, K.; Newcombe, G.; Bowling, L.; Stuetz, R.; Henderson, R.K. Performance evaluation of in situ fluorometers for real-time cyanobacterial monitoring. H2Open J. 2018, 1, 26–46. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- Pahlevan, N.; Smith, B.; Schalles, J.; Binding, C.; Cao, Z.; Ma, R.; Alikas, K.; Kangro, K.; Gurlin, D.; Hà, N.; et al. Seamless retrievals of chlorophyll-a from sentinel-2 (MSI) and sentinel-3 (OLCI) in inland and coastal waters: A machine-learning approach. Remote Sens. Environ. 2020, 240, 111604. [Google Scholar] [CrossRef]
- Mishra, S.; Stumpf, R.P.; Schaeffer, B.A.; Werdell, P.J.; Loftin, K.A.; Meredith, A. Measurement of cyanobacterial bloom magnitude using satellite remote sensing. Sci. Rep. 2019, 9, 18310. [Google Scholar] [CrossRef]
- Feng, L.; Dai, Y.; Hou, X.; Xu, Y.; Liu, J.; Zheng, C. Concerns about phytoplankton bloom trends in global lakes. Nature 2021, 590, E35–E47. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Pyo, J.; Kwon, Y.-H.; Duan, H.; Cho, K.H.; Park, Y. Drone-based hyperspectral remote sensing of cyanobacteria using vertical cumulative pigment concentration in a deep reservoir. Remote Sens. Environ. 2020, 236, 111517. [Google Scholar] [CrossRef]
- Woolway, R.I.; Jennings, E.; Shatwell, T.; Golub, M.; Pierson, D.C.; Maberly, S.C. Lake heatwaves under climate change. Nature 2021, 589, 402–407. [Google Scholar] [CrossRef]
- Sha, J.; Xiong, H.; Li, C.; Lu, Z.; Zhang, J.; Zhong, H.; Zhang, W.; Yan, B. Harmful algal blooms and their eco-environmental indication. Chemosphere 2021, 274, 129912. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.C.; Zhang, Y.J.; Arhonditsis, G.B.; Gao, J.F.; Chen, Q.W.; Peng, J. The magnitude and drivers of harmful algal blooms in china’s lakes and reservoirs: A national-scale characterization. Water Res. 2020, 181, 115902. [Google Scholar] [CrossRef] [PubMed]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 1015902. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Zessner, M. Assessing and controlling the risk of cyanobacterial blooms. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: Boca Raton, FL, USA, 2021; p. 433. [Google Scholar]
- Burford, M.; Carey, C.; Hamilton, D.; Huisman, J.; Paerl, H.; Wood, S.; Wulff, A. Perspective: Advancing the research agenda for improving understanding of cyanobacteria in a future of global change. Harmful Algae 2020, 91, 101601. [Google Scholar] [CrossRef]
- Bar-Yosef, Y.; Sukenik, A.; Hadas, O.; Viner-Mozzini, Y.; Kaplan, A. Enslavement in the water body by toxic aphanizomenon ovalisporum, inducing alkaline phosphatase in phytoplanktons. Curr. Biol. 2010, 20, 1557–1561. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.M.; Tang, X.M.; Qin, B.Q.; Gao, G.; Zhang, Y.L.; Zhu, G.W.; Gong, Z.J. Decreasing nitrogen loading and climate change promotes the occurrence of nitrogen-fixing cyanobacteria in a restored city lake. Hydrobiologia 2020, 847, 2963–2975. [Google Scholar] [CrossRef]
- Sukenik, A.; Quesada, A.; Salmaso, N. Global expansion of toxic and non-toxic cyanobacteria: Effect on ecosystem functioning. Biodivers. Conserv. 2015, 24, 889–908. [Google Scholar] [CrossRef]
- Guljamow, A.; Barchewitz, T.; Große, B.; Timm, S.; Hagemann, M.; Dittmann, E. Diurnal variations of extracellular microcystin influence the subcellular dynamics of RuBisCo in Microcystis aeruginosa pcc 7806. Microorganism 2021, 9, 1265. [Google Scholar] [CrossRef]
- Mantzouki, E.; Visser, P.M.; Bormans, M.; Ibelings, B.W. Understanding the key ecological traits of cyanobacteria as a basis for their management and control in changing lakes. Aquat. Ecol. 2016, 50, 333–350. [Google Scholar] [CrossRef]
- Romo, S.; Soria, J.; Fernandez, F.; Ouahid, Y.; Barón--Solá, Á. Water residence time and the dynamics of toxic cyanobacteria. Freshw. Biol. 2013, 58, 513–522. [Google Scholar] [CrossRef]
- Bormans, M.; Marsalek, B.; Jancula, D. Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 407–422. [Google Scholar] [CrossRef]
- Soares, M.C.S.; Marinho, M.M.; Azevedo, S.M.O.F.; Branco, C.W.C.; Huszar, V.L.M. Eutrophication and retention time affecting spatial heterogeneity in a tropical reservoir. Limnologica 2012, 42, 197–203. [Google Scholar] [CrossRef]
- Burch, M.; Brookes, J.; Chorus, I. Assessing and controlling the risk of cyanobacterial blooms. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Welker, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 505–562. [Google Scholar]
- Geada, P.; Oliveira, F.; Loureiro, L.; Esteves, D.; Teixeira, J.A.; Vasconcelos, V.; Vicente, A.A.; Fernandes, B.D. Comparison and optimization of different methods for Microcystis aeruginosa’s harvesting and the role of zeta potential on its efficiency. Environ. Sci. Pollut. Res. 2019, 26, 16708–16715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druga, B.; Buda, D.M.; Szekeres, E.; Chis, C.; Chis, I.; Sicora, C. The impact of cation concentration on Microcystis (cyanobacteria) scum formation. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.M.; Zhang, H.Y.; Liu, Q.L.; Li, L.L.; Li, L.; Zhang, X.Z. Harvesting of Microcystis flos-aquae using chitosan coagulation: Influence of proton-active functional groups originating from extracellular and intracellular organic matter. Water Res. 2020, 185, 116272. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.S.; Zang, X.M.; Zhang, X.Z. Harvesting of Microcystis aeruginosa using membrane filtration: Influence of pore structure on fouling kinetics, algogenic organic matter retention and cake formation. Algal Res. Biomass Biofuels Bioprod. 2020, 52, 102112. [Google Scholar] [CrossRef]
- Labeeuw, L.; Commault, A.S.; Kuzhiumparambil, U.; Emmerton, B.; Nguyen, L.N.; Nghiem, L.D.; Ralph, P.J. A comprehensive analysis of an effective flocculation method for high quality microalgal biomass harvesting. Sci. Total Environ. 2021, 752, 141708. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Shi, X.L.; Zhang, M.; Liu, C.Q.; Chen, K.N. Comparison of algal harvest and hydrogen peroxide treatment in mitigating cyanobacterial blooms via an in situ mesocosm experiment. Sci. Total Environ. 2019, 694, 133721. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Hou, J.; Wang, P.F.; Wang, C.; Miao, L.Z.; Ao, Y.H.; Xu, Y.; Wang, X.; Lv, B.W.; You, G.X.; et al. Interpretation of the disparity in harvesting efficiency of different types of Microcystis aeruginosa using polyethylenimine (pei)-coated magnetic nanoparticles. Algal Res. Biomass Biofuels Bioprod. 2018, 29, 257–265. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.Y.; Hou, J.; Wang, P.F.; Miao, L.Z.; Wang, X.; Guo, L.D. Optimization of cyanobacterial harvesting and extracellular organic matter removal utilizing magnetic nanoparticles and response surface methodology: A comparative study. Algal Res. Biomass Biofuels Bioprod. 2020, 45, 101756. [Google Scholar] [CrossRef]
- Hao, J.C.; Lian, B.; Liu, H.F.; Lu, X.Z. The release of phosphorus from sediment to lake water induced by cyanobacterial blooms and phosphorus removal by cell harvesting. Geomicrobiol. J. 2016, 33, 347–353. [Google Scholar] [CrossRef]
- del Arco, A.; Alvarez-Manzaneda, I.; Funes, A.; Perez-Martinez, C.; de Vicente, I. Assessing the toxic effects of magnetic particles used for lake restoration on phytoplankton: A community-based approach. Ecotoxicol. Environ. Saf. 2021, 207, 111288. [Google Scholar] [CrossRef]
- Lürling, M.; Mucci, M.; Waajen, G. Removal of positively buoyant Planktothrix rubescens in lake restoration. Toxins 2020, 12, 700. [Google Scholar] [CrossRef]
- Wang, C.H.; Wu, Y.; Wang, Y.Q.; Bai, L.L.; Jiang, H.L.; Yu, J.H. Lanthanum-modified drinking water treatment residue for initial rapid and long-term equilibrium phosphorus immobilization to control eutrophication. Water Res. 2018, 137, 173–183. [Google Scholar] [CrossRef] [PubMed]
- van Oosterhout, F.; Goitom, E.; Roessink, I.; Lurling, M. Lanthanum from a modified clay used in eutrophication control is bioavailable to the marbled crayfish (procambarus fallax f. Virginalis). PLoS ONE 2014, 9, e102410. [Google Scholar] [CrossRef]
- Waajen, G.; van Oosterhout, F.; Lurling, M. Bio-accumulation of lanthanum from lanthanum modified bentonite treatments in lake restoration. Environ. Pollution 2017, 230, 911–918. [Google Scholar] [CrossRef]
- van Oosterhout, F.; Waajen, G.; Yasseri, S.; Manzi Marinho, M.; Pessoa Noyma, N.; Mucci, M.; Douglas, G.; Lürling, M. Lanthanum in water, sediment, macrophytes and chironomid larvae following application of lanthanum modified bentonite to lake rauwbraken (the netherlands). Sci. Total Environ. 2020, 706, 135188. [Google Scholar] [CrossRef]
- Behets, G.J.; Mubiana, K.V.; Lamberts, L.; Finsterle, K.; Traill, N.; Blust, R.; D’Haese, P.C. Use of lanthanum for water treatment a matter of concern? Chemosphere 2020, 239, 124780. [Google Scholar] [CrossRef]
- Chen, C.; Kong, M.; Wang, Y.Y.; Shen, Q.S.; Zhong, J.C.; Fan, C.X. Dredging method effects on sediment resuspension and nutrient release across the sediment-water interface in Lake Taihu, China. Environ. Sci. Pollut. Res. 2020, 27, 25861–25869. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Ding, S.M.; Gao, S.S.; Fu, Z.; Tang, W.Y.; Wu, Y.X.; Gong, M.D.; Wang, D.; Wang, Y. Efficacy of dredging engineering as a means to remove heavy metals from lake sediments. Sci. Total Environ. 2019, 665, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Porat, R.; Teltesch, B.; Dubinsky, Z.; Walsby, A.E. Effects of light and pressure on gas vesicle formation and buoyancy in Aphanizomenon ovalisporum Forti (cyanobacteria) from Lake Kinneret, Israel. Arch. Hydrobiol. 2000, 55, 333–348. [Google Scholar]
- Porat, R. The Cyanobacterium Aphanizomenon Ovalisporum in Lake Kinneret and in the Israeli National Water Carrier-Photoacclimation, Bouyancy Mechanism and Cells’ and Cyanotoxin Fate in the Drinking Water Distribution System. Ph.D. thesis, Bar-Ilan University, Ramat Gan, Israel, 2001; p. 287. [Google Scholar]
- Li, J.; Liao, R.; Tao, Y.; Zhuo, Z.; Liu, Z.; Deng, H.; Ma, H. Probing the cyanobacterial Microcystis gas vesicles after static pressure treatment: A potential in situ rapid method. Sensors 2020, 20, 4170. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Sun, F.; Wu, J.; Zhou, Y.; Yan, Q.; Ren, A.; Xu, H. Study on method and mechanism of deep well circulation for the growth control of Microcystis in aquaculture pond. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2017, 75, 2692–2701. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.-Y.; Park, M.-H.; Joung, S.-H.; Kim, H.-S.; Jang, K.-Y.; Oh, H.-M. Growth inhibition of cyanobacteria by ultrasonic radiation: Laboratory and enclosure studies. Environ. Sci. Technol. 2003, 37, 3031–3037. [Google Scholar] [CrossRef]
- Wu, X.; Joyce, E.M.; Mason, T.J. The effects of ultrasound on cyanobacteria. Harmful Algae 2011, 10, 738–743. [Google Scholar] [CrossRef]
- Haocai, H.; Wu, G.; Sheng, C.; Wu, J.; Li, D.; Wang, H. Improved cyanobacteria removal from harmful algae blooms by two-cycle, low-frequency, low-density, and short-duration ultrasonic radiation. Water 2020, 12, 2431. [Google Scholar]
- Park, C.B.; Baik, S.; Kim, S.; Choi, J.W.; Lee, S.H.; Kim, Y.J. The use of ultrasonic frequencies to control the bloom formation, regrowth, and eco-toxicity in Microcystis aeruginosa. Int. J. Environ. Sci. Technol. 2017, 14, 923–932. [Google Scholar] [CrossRef]
- Rodriguez-Molares, A.; Dickson, S.; Hobson, P.; Howard, C.; Zander, A.; Burch, M. Quantification of the ultrasound induced sedimentation of Microcystis aeruginosa. Ultrason. Sonochem. 2014, 21, 1299–1304. [Google Scholar] [CrossRef]
- LaLiberte, G.; Haber, E. Literature Review of the Effects of Ultrasonic Waves on Cyanobacteria, Other Aquatic Organisms, and Water Quality; Volume 195 of Research report, Wisconsin Department of Natural Resources. 2014. [Google Scholar]
- Asadi, A.; Soltani, N.; Asadi, A. Effect of various microwave frequencies on the physiology of a cyanobacterium, Schizothrix mexicana. Acta Physiol. Plant 2013, 35, 1367–1372. [Google Scholar] [CrossRef]
- Singh, S.P.; Rai, S.; Rai, A.K.; Tiwari, S.P.; Singh, S.S.; Abraham, J. Athermal physiological effects of microwaves on a cynobacterium Nostoc muscorum: Evidence for EM-memory bits in water. Med. Biol. Eng. Comput. 1994, 32, 175–180. [Google Scholar] [CrossRef]
- Marsalek, B.; Zezulka, S.; Marsalkova, E.; Pochyly, F.; Rudolf, P. Synergistic effects of trace concentrations of hydrogen peroxide used in a novel hydrodynamic cavitation device allows for selective removal of cyanobacteria. Chem. Eng. J. 2020, 382, 122383. [Google Scholar] [CrossRef]
- Lürling, M.; Tolman, Y. Effects of commercially available ultrasound on the zooplankton grazer Daphnia and consequent water greening in laboratory experiments. Water 2014, 6, 3247–3263. [Google Scholar] [CrossRef] [Green Version]
- Matthijs, H.C.P.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef]
- Qian, Y.P.; Li, X.T.; Tian, R.N. Effects of aqueous extracts from the rhizome of Pontederia cordata on the growth and interspecific competition of two algal species. Ecotoxicol. Environ. Saf. 2019, 168, 401–407. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.; Selyutina, O.Y.; Polyakov, N.E.; Kontoghiorghes, G.J. Effective inhibition of copper-catalyzed production of hydroxyl radicals by deferiprone. J. Biol. Inorg. Chem. 2019, 24, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, L.; Ke, Z.; Li, G.; Shi, R.; Tan, Y. Beneficial effects of aluminum enrichment on nitrogen-fixing cyanobacteria in the south china sea. Mar. Pollut. Bull. 2018, 129, 142–150. [Google Scholar] [CrossRef]
- Brattebo, S.K.; Welch, E.B.; Gibbons, H.L.; Burghdoff, M.K.; Williams, G.N.; Oden, J.L. Effectiveness of alum in a hypereutrophic lake with substantial external loading. Lake Reserv. Manag. 2017, 33, 108–118. [Google Scholar] [CrossRef]
- Barroin, G.; Feuillade, M. Hydrogen peroxide as a potential algicide for Oscillatoria rubescens DC. Water Res. 1986, 20, 619–623. [Google Scholar] [CrossRef]
- Drábková, M.; Matthijs, H.C.P.; Admiraal, W.; Maršálek, B. Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica 2007, 45, 363–369. [Google Scholar] [CrossRef]
- Barrington, D.J.; Ghadouani, A. Application of hydrogen peroxide for the removal of toxic cyanobacteria and other phytoplankton from wastewater. Environ. Sci. Technol. 2008, 42, 8916–8921. [Google Scholar] [CrossRef]
- Weenink, E.; Luimstra, V.; Schuurmans, J.; Van Herk, M.; Visser, P.; Matthijs, H.C.P. Combatting cyanobacteria with hydrogen peroxide: A laboratory study on the consequences for phytoplankton community and diversity. Front. Microbiol. 2015, 6, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Chen, Z.W.; Chen, H.; Wen, Y.Z. Effect of hydrogen peroxide on Microcystic aeruginosa: Role of cytochromes p450. Sci. Total Environ. 2018, 626, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lusty, M.W.; Gobler, C.J. The efficacy of hydrogen peroxide in mitigating cyanobacterial blooms and altering microbial communities across four lakes in NY, USA. Toxins 2020, 12, 428. [Google Scholar] [CrossRef]
- Sandrini, G.; Piel, T.; Xu, T.S.; White, E.; Qin, H.J.; Slot, P.C.; Huisman, J.; Visser, P.M. Sensitivity to hydrogen peroxide of the bloom-forming cyanobacterium Microcystis pcc 7806 depends on nutrient availability. Harmful Algae 2020, 99, 101916. [Google Scholar] [CrossRef]
- Xu, H.; Pang, Y.; Li, Y.; Zhang, S.; Pei, H. Using sodium percarbonate to suppress vertically distributed filamentous cyanobacteria while maintaining the stability of microeukaryotic communities in drinking water reservoirs. Water Res. 2021, 197, 117111. [Google Scholar] [CrossRef]
- Weenink, E.F.J.; Matthijs, H.C.P.; Schuurmans, J.M.; Piel, T.; van Herk, M.J.; Sigon, C.A.M.; Visser, P.M.; Huisman, J. Interspecific protection against oxidative stress: Green algae protect harmful cyanobacteria against hydrogen peroxide. Environ. Microbiol. 2021, 23, 2404–2419. [Google Scholar] [CrossRef]
- Tichy, M.; Vermaas, W. In vivo role of catalase-peroxidase in Synechocystis sp. strain pcc 6803. J. Bacteriol. 1999, 181, 1875–1882. [Google Scholar] [CrossRef] [Green Version]
- Helman, Y.; Tchernov, D.; Reinhold, L.; Shibata, M.; Ogawa, T.; Schwarz, R.; Ohad, I.; Kaplan, A. Genes encoding a-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr. Biol. 2003, 13, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, N.E. The C2 oxidative photosynthetic carbon cycle. Annu. Rev. Plant Physiol. Plant Molec. Biol. 1997, 48, 1–23. [Google Scholar] [CrossRef]
- Burnap, R.L.; Hagemann, M.; Kaplan, A. Regulation of the CO2 concentrating mechanism in cyanobacteria. Life 2015, 5, 348–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piel, T.; Sandrini, G.; Muyzer, G.; Brussaard, C.P.D.; Slot, P.C.; van Herk, M.J.; Huisman, J.; Visser, P.M. Resilience of microbial communities after hydrogen peroxide treatment of a eutrophic lake to suppress harmful cyanobacterial blooms. Microorganism 2021. [Google Scholar]
- Thoo, R.; Siuda, W.; Jasser, I. The effects of sodium percarbonate generated free oxygen on Daphnia—Implications for the management of harmful algal blooms. Water 2020, 12, 1304. [Google Scholar] [CrossRef]
- Matthijs, H.C.; Jančula, D.; Visser, P.M.; Maršálek, B. Existing and emerging cyanocidal compounds: New perspectives for cyanobacterial bloom mitigation. Aquat. Ecol. 2016, 50, 443–460. [Google Scholar] [CrossRef] [Green Version]
- Keliri, E.; Paraskeva, C.; Sofokleous, A.; Sukenik, A.; Dziga, D.; Chernova, E.; Brient, L.; Antoniou, M.G. Occurrence of a single-species cyanobacterial bloom in a lake in Cyprus: Monitoring and treatment with hydrogen peroxide-releasing granules. Environ. Sci. Eur. 2021, 33, 31. [Google Scholar] [CrossRef]
- Buley, R.P.; Adams, C.; Belfiore, A.P.; Fernandez-Figueroa, E.G.; Gladfelter, M.F.; Garner, B.; Wilson, A.E. Field evaluation of seven products to control cyanobacterial blooms in aquaculture. Environ. Sci. Pollut. Res. 2021, 28, 29971–29983. [Google Scholar] [CrossRef]
- Yang, Z.; Buley, R.P.; Fernandez-Figueroa, E.G.; Barros, M.U.G.; Rajendran, S.; Wilson, A.E. Hydrogen peroxide treatment promotes chlorophytes over toxic cyanobacteria in a hyper-eutrophic aquaculture pond. Environ. Pollut. 2018, 240, 590–598. [Google Scholar] [CrossRef]
- Daniel, E.; Weiss, G.; Murik, O.; Sukenik, A.; Lieman-Hurwitz, J.; Kaplan, A. The response of Microcystis aeruginosa strain MGK to a single or two consecutive H2O2 applications. Environ. Microbiol. 2019, 11, 621–629. [Google Scholar]
- Lin, L.Z.; Shan, K.; Xiong, Q.; Zhou, Q.C.; Li, L.; Gan, N.Q.; Song, L.R. The ecological risks of hydrogen peroxide as a cyanocide: Its effect on the community structure of bacterioplankton. J. Oceanol. Limnol. 2018, 36, 2231–2242. [Google Scholar] [CrossRef]
- Sinha, A.K.; Eggleton, M.A.; Lochmann, R.T. An environmentally friendly approach for mitigating cyanobacterial bloom and their toxins in hypereutrophic ponds: Potentiality of a newly developed granular hydrogen peroxide-based compound. Sci. Total Environ. 2018, 637, 524–537. [Google Scholar] [CrossRef]
- Reichwaldt, E.S.; Zheng, L.; Barrington, D.J.; Ghadouani, A. Acute toxicological response of Daphnia and Moina to hydrogen peroxide. J. Environ. Eng. 2012, 138, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Murik, O.; Kaplan, A. Paradoxically, prior acquisition of antioxidant activity enhances oxidative stress--induced cell death. Environ. Microbiol. 2009, 11, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Murik, O.; Elboher, A.; Kaplan, A. Dehydroascorbate: A possible surveillance molecule of oxidative stress and programmed cell death in the green alga Chlamydomonas reinhardtii. New Phytol. 2014, 202, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Berman-Frank, I.; Bidle, K.D.; Haramaty, L.; Falkowski, P.G. The demise of the marine cyanobacterium, Trichodesmium spp., via an autocatalyzed cell death pathway. Limnol. Oceanogr. 2004, 49, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhou, Q.H.; Liu, B.Y.; Cheng, L.; Tian, Y.; Zhang, Y.Y.; Wu, Z.B. Programmed cell death in the cyanobacterium Microcystis aeruginosa induced by allelopathic effect of submerged macrophyte Myriophyllum spicatum in co-culture system. J. Appl. Phycol. 2016, 28, 2805–2814. [Google Scholar] [CrossRef]
- Ross, C.; Santiago-Vazquez, L.; Paul, V. Toxin release in response to oxidative stress and programmed cell death in the cyanobacterium Microcystis aeruginosa. Aqu. Toxicol. 2006, 78, 66–73. [Google Scholar] [CrossRef]
- Ding, Y.; Gan, N.Q.; Li, J.; Sedmak, B.; Song, L.R. Hydrogen peroxide induces apoptotic-like cell death in Microcystis aeruginosa (chroococcales, cyanobacteria) in a dose-dependent manner. Phycologia 2012, 51, 567–575. [Google Scholar] [CrossRef]
- Piel, T.; Sandrini, G.; White, E.; Xu, T.S.; Schuurmans, J.M.; Huisman, J.; Visser, P.M. Suppressing cyanobacteria with hydrogen peroxide is more effective at high light intensities. Toxins 2020, 12, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.C.; Li, L.; Huang, L.C.; Guo, L.L.; Song, L.R. Combining hydrogen peroxide addition with sunlight regulation to control algal blooms. Environ. Sci. Pollut. Res. 2018, 25, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.R.; Cao, H.S.; Zheng, J.; Teng, F.; Wang, X.J.; Lou, K.; Zhang, X.H.; Tao, Y. Suppression of water-bloom cyanobacterium Microcystis aeruginosa by algaecide hydrogen peroxide maximized through programmed cell death. J. Hazard Mat. 2020, 393, 122394. [Google Scholar] [CrossRef]
- Zhou, T.R.; Zheng, J.; Cao, H.S.; Wang, X.J.; Lou, K.; Zhang, X.H.; Tao, Y. Growth suppression and apoptosis-like cell death in Microcystis aeruginosa by H2O2: A new insight into extracellular and intracellular damage pathways. Chemosphere 2018, 211, 1098–1108. [Google Scholar] [CrossRef]
- Franklin, D.J. Examining the evidence for regulated and programmed cell death in cyanobacteria. How significant are different forms of cell death in cyanobacteria population dynamics? Front. Microbiol. 2021, 12, 633954. [Google Scholar] [CrossRef]
- Hu, C.; Rzymski, P. Programmed cell death-like and accompanying release of microcystin in freshwater bloom-forming cyanobacterium Microcystis: From identification to ecological relevance. Toxins 2019, 11, 706. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.Q.; Dao, G.H.; Tao, Y.; Zhan, X.M.; Hu, H.Y. A review on control of harmful algal blooms by plant-derived allelochemicals. J. Hazard Mat. 2021, 401, 123403. [Google Scholar] [CrossRef] [PubMed]
- Di Nica, V.; Gallet, J.; Villa, S.; Mezzanotte, V. Toxicity of quaternary ammonium compounds (QACs) as single compounds and mixtures to aquatic non-target microorganisms: Experimental data and predictive models. Ecotoxicol. Environ. Saf. 2017, 142, 567–577. [Google Scholar] [CrossRef]
- Jing, G.; Zhou, Z.; Zhuo, J. Quantitative structure–activity relationship (QSAR) study of toxicity of quaternary ammonium compounds on Chlorella pyrenoidosa and Scenedesmus quadricauda. Chemosphere 2012, 86, 76–82. [Google Scholar] [CrossRef]
- Wu, X.; Viner-Mozzini, Y.; Jia, Y.; Song, L.; Sukenik, A. Alkyltrimethylammonium (ATMA) surfactants as cyanocides-effects on photosynthesis and growth of cyanobacteria. Chemosphere 2021, 274, 129778. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.; Wuest, W.M. Quaternary ammonium compounds: An antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Fan, C.; Zhong, J.; Zhang, L.; Ding, S.; Yan, S.; Han, S. Using hexadecyl trimethyl ammonium bromide (CTAB) modified clays to clean the Microcystis aeruginosa blooms in Lake Taihu, China. Harmful Algae 2010, 9, 413–418. [Google Scholar] [CrossRef]
- Sukenik, A.; Viner-Mozzini, Y.; Tavassi, M.; Nir, S. Removal of cyanobacteria and cyanotoxins from lake water by composites of bentonite with micelles of the cation octadecyltrimethyl ammonium (ODTMA). Water Res. 2017, 120, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M.; Kang, L.; Mucci, M.; van Oosterhout, F.; Noyma, N.P.; Miranda, M.; Huszar, V.L.; Waajen, G.; Marinho, M.M. Coagulation and precipitation of cyanobacterial blooms. Ecol. Eng. 2020, 158, 106032. [Google Scholar] [CrossRef]
- Shi, W.; Tan, W.; Wang, L.; Pan, G. Removal of Microcystis aeruginosa using cationic starch modified soils. Water Res. 2016, 97, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Pan, G. Cyanobacterial bloom mitigation using proteins with high isoelectric point and chitosan-modified soil. J. Appl. Phycol. 2016, 28, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Pan, G.; Zou, H.; Chen, H.; Yuan, X. Removal of harmful cyanobacterial blooms in Taihu Lake using local soils iii. Factors affecting the removal efficiency and an in situ field experiment using chitosan-modified local soils. Environ. Pollut. 2006, 141, 206–212. [Google Scholar] [CrossRef]
- Kaplan, A.; Harel, M.; Kaplan-Levy, R.N.; Hadas, O.; Sukenik, A.; Dittmann, E. The languages spoken in the water body (or the biological role of cyanobacterial toxins). Front. Aquat. Microbiol. 2012, 3, 138. [Google Scholar] [CrossRef] [Green Version]
- Harel, M.; Weiss, G.; Lieman-Hurwitz, J.; Gun, J.; Lev, O.; Lebendiker, M.; Temper, V.; Block, C.; Sukenik, S.; Zohary, T.; et al. Interactions between Scenedesmus and Microcystis may be used to clarify the role of secondary metabolites. Environ. Microbiol. Rep. 2012, 5, 97–104. [Google Scholar] [CrossRef]
- Vardi, A.; Schatz, D.; Beeri, K.; Motro, U.; Sukenik, A.; Levine, A.; Kaplan, A. Dinoflagellate-cyanobacterium communication may determine the composition of phytoplankton assemblage in a mesotrophic lake. Curr. Biol. 2002, 12, 1767–1772. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, K.C.; Jackrel, S.L.; Smith, D.J.; Dick, G.J.; Denef, V.J. Genotype and host microbiome alter competitive interactions between Microcystis aeruginosa and Chlorella sorokiniana. Harmful Algae 2020, 99, 101939. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Eshkol, R.; Livne, A.; Hadas, O.; Rom, M.; Tchernov, D.; Vardi, A.; Kaplan, A. Inhibition of growth and photosynthesis of the dinoflagellate Peridinium gatunense by Microcystis sp. (cyanobacteria): A novel allelopathic mechanism. Limnol. Oceanogr. 2002, 47, 1656–1663. [Google Scholar] [CrossRef]
- Hulot, F.D.; Huisman, J. Allelopathic interactions between phytoplankton species: The roles of heterotrophic bacteria and mixing intensity. Limnol. Oceanogr. 2004, 49, 1424–1434. [Google Scholar] [CrossRef]
- Zhang, M.; Kong, F.X.; Xing, P.; Tan, X. Effects of interspecific interactions between Microcystis aeruginosa and Chlorella pyrenoidosa on their growth and physiology. Int. Rev. Hydrobiol. 2007, 92, 281–290. [Google Scholar] [CrossRef]
- Qian, S.Q.; Kong, F.X.; Shi, X.L.; Zhang, M.; Tan, X.; Yang, Z. Interspecific interaction between Microcystis aeruginosa and Chlorella pyrenoidosa in different phosphate media. J. Freshw. Ecol. 2008, 23, 635–642. [Google Scholar] [CrossRef]
- Chang, X.X.; Eigemann, F.; Hilt, S. Do macrophytes support harmful cyanobacteria? Interactions with a green alga reverse the inhibiting effects of macrophyte allelochemicals on Microcystis aeruginosa. Harmful Algae 2012, 19, 76–84. [Google Scholar] [CrossRef]
- Bittencourt-Oliveira, M.D.; Chia, M.A.; de Oliveira, H.S.B.; Araujo, M.K.C.; Molica, R.J.R.; Dias, C.T.S. Allelopathic interactions between microcystin-producing and non-microcystin-producing cyanobacteria and green microalgae: Implications for microcystins production. J. Appl. Phycol. 2015, 27, 275–284. [Google Scholar] [CrossRef]
- Gao, Y.N.; Dong, J.; Fu, Q.Q.; Wang, Y.P.; Chen, C.; Li, J.H.; Li, R.; Zhou, C.J. Allelopathic effects of submerged macrophytes on phytoplankton. Allelopath. J. 2017, 40, 1–22. [Google Scholar]
- Wu, Y.H.; Wang, F.W.; Xiao, X.; Liu, J.Z.; Wu, C.X.; Chen, H.; Kerr, P.; Shurin, J. Seasonal changes in phosphorus competition and allelopathy of a benthic microbial assembly facilitate prevention of cyanobacterial blooms. Environ. Microbiol. 2017, 19, 2483–2494. [Google Scholar] [CrossRef]
- Chia, M.A.; Jankowiak, J.G.; Kramer, B.J.; Goleski, J.A.; Huang, I.S.; Zimba, P.V.; Bittencourt-Oliveira, M.D.; Gobler, C.J. Succession and toxicity of Microcystis and Anabaena (Dolichospermum) blooms are controlled by nutrient-dependent allelopathic interactions. Harmful Algae 2018, 74, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.P.; Cardinale, B.J. Species diversity of resident green algae slows the establishment and proliferation of the cyanobacterium Microcystis aeruginosa. Limnologica 2019, 74, 23–27. [Google Scholar] [CrossRef]
- Yang, K.; Chen, Q.L.; Zhang, D.Y.; Zhang, H.J.; Lei, X.Q.; Chen, Z.R.; Li, Y.; Hong, Y.L.; Ma, X.H.; Zheng, W.; et al. The algicidal mechanism of prodigiosin from Hahella sp. KA22 against Microcystis aeruginosa. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Pal, M.; Yesankar, P.J.; Dwivedi, A.; Qureshi, A. Biotic control of harmful algal blooms (HABs): A brief review. J. Environ. Manag. 2020, 268, 110687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.J.; Pan, R.J.; Luo, Z.; Zhang, T.Q.; Fan, J.J. Interspecific competition between Microcystis aeruginosa and Pseudanadaena and their production of T&O compounds. Chemosphere 2020, 252, 126509. [Google Scholar] [PubMed]
- Bittner, M.; Stern, A.; Smutna, M.; Hilscherova, K.; Zegura, B. Cytotoxic and genotoxic effects of cyanobacterial and algal extracts-microcystin and retinoic acid content. Toxins 2021, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Chao, J.J.; McKay, R.M.L.; Xu, R.B.; Wang, T.; Xu, J.; Zhang, J.L.; Chang, X.X. Antibiotic pollution promotes dominance by harmful cyanobacteria: A case study examining norfloxacin exposure in competition experiments. J. Phycol. 2021, 57, 677–688. [Google Scholar] [CrossRef]
- Savic, G.B.; Bormans, M.; Edwards, C.; Lawton, L.; Briand, E.; Wiegand, C. Cross talk: Two way allelopathic interactions between toxic Microcystis and Daphnia. Harmful Algae 2020, 94, 101803. [Google Scholar] [CrossRef]
- Omidi, A.; Esterhuizen-Londt, M.; Pflugmacher, S. Desmodesmus subspicatus co-cultured with microcystin producing (PCC 7806) and the non-producing (PCC 7005) strains of Microcystis aeruginosa. Ecotoxicology 2019, 28, 834–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, Y.; Liu, L.; Hilt, S.; Xu, R.B.; Wang, B.L.; Li, C.B.; Chang, X.X. Root exudated algicide of Eichhornia crassipes enhances allelopathic effects of cyanobacteria Microcystis aeruginosa on green algae. Hydrobiologia 2018, 823, 67–77. [Google Scholar] [CrossRef]
- Wang, B.L.; Song, Q.Y.; Long, J.J.; Song, G.F.; Mi, W.J.; Bi, Y.H. Optimization method for Microcystis bloom mitigation by hydrogen peroxide and its stimulative effects on growth of chlorophytes. Chemosphere 2019, 228, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Lurling, M.; Roessink, I. On the way to cyanobacterial blooms: Impact of the herbicide metribuzin on the competition between a green alga (Scenedesmus) and a cyanobacterium (Microcystis). Chemosphere 2006, 65, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Dunker, S.; Jakob, T.; Wilhelm, C. Contrasting effects of the cyanobacterium Microcystis aeruginosa on the growth and physiology of two green algae, Oocystis marsonii and Scenedesmus obliquus, revealed by flow cytometry. Freshw. Biol. 2013, 58, 1573–1587. [Google Scholar] [CrossRef]
- Jin, X.Q.; Jiang, J.; Sheng, L.X.; Jin, M.H. Interspecies competition between Microcystis aeruginosa and Scenedesmus obliquus under phenanthrene stress. Pol. J. Environ. Stud. 2014, 23, 1609–1616. [Google Scholar]
- Chen, J.Q.; Guo, R.X. Inhibition effect of green alga on cyanobacteria by the interspecies interactions. Int. J. Environ. Sci. Technol. 2014, 11, 839–842. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.C.; Zi, J.M.; Xu, R.B.; Hilt, S.; Hou, X.L.; Chang, X.X. Allelopathic effects of Microcystis aeruginosa on green algae and a diatom: Evidence from exudates addition and co-culturing. Harmful Algae 2017, 61, 56–62. [Google Scholar] [CrossRef]
- Zhao, M.M.; Chen, X.Y.; Ma, N.; Zhang, Q.Y.; Qu, D.; Li, M. Overvalued allelopathy and overlooked effects of humic acid-like substances on Microcystis aeruginosa and Scenedesmus obliquus competition. Harmful Algae 2018, 78, 18–26. [Google Scholar] [CrossRef]
- Bai, F.; Shi, J.Q.; Yang, S.Q.; Yang, Y.J.; Wu, Z.X. Interspecific competition between Cylindrospermopsis raciborskii and Microcystis aeruginosa on different phosphorus substrates. Environ. Sci. Poll Res. 2020, 27, 42264–42275. [Google Scholar] [CrossRef]
- Jia, N.N.; Yang, Y.M.; Yu, G.L.; Wang, Y.L.; Qiu, P.F.; Li, H.; Li, R.H. Interspecific competition reveals Raphidiopsis raciborskii as a more successful invader than Microcystis aeruginosa. Harmful Algae 2020, 97, 101858. [Google Scholar] [CrossRef]
- Xiao, M.; Adams, M.P.; Willis, A.; Burford, M.A.; O’Brien, K.R. Variation within and between cyanobacterial species and strains affects competition: Implications for phytoplankton modelling. Harmful Algae 2017, 69, 38–47. [Google Scholar] [CrossRef]
- Wang, Z.C.; Zhang, Y.; Huang, S.; Peng, C.R.; Hao, Z.X.; Li, D.H. Nitrogen limitation significantly reduces the competitive advantage of toxic Microcystis at high light conditions. Chemosphere 2019, 237, 124508. [Google Scholar] [CrossRef]
- Marinho, M.M.; Souza, M.B.G.; Lurling, M. Light and phosphate competition between Cylindrospermopsis raciborskii and Microcystis aeruginosa is strain dependent. Mic. Ecol. 2013, 66, 479–488. [Google Scholar] [CrossRef]
- Alexova, R.; Dang, C.; Fujii, M.; Raftery, M.J.; Waite, T.D.; Ferrari, B.C.; Neilan, B.A. Specific global responses to N and Fe nutrition in toxic and non-toxic Microcystis aeruginosa. Environ. Microbiol. 2016, 18, 401–413. [Google Scholar] [CrossRef]
- Lei, L.M.; Li, C.L.; Peng, L.; Han, B.P. Competition between toxic and non-toxic Microcystis aeruginosa and its ecological implication. Ecotoxicology 2015, 24, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Mello, M.M.; Soares, M.C.S.; Roland, F.; Lurling, M. Growth inhibition and colony formation in the cyanobacterium Microcystis aeruginosa induced by the cyanobacterium Cylindrospermopsis raciborskii. J. Plankton Res. 2012, 34, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Gross, E.M.; Wolk, C.P.; Juettner, F. Fischerellin, a new allelochemical from the freshwater cyanobacterium Fischerella muscicola. J. Phycol. 1991, 27, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Leao, P.N.; Pereira, A.R.; Liu, W.T.; Ng, J.; Pevzner, P.A.; Dorrestein, P.C.; Konig, G.M.; Vasconcelosa, V.M.; Gerwick, W.H. Synergistic allelochemicals from a freshwater cyanobacterium. Proc. Natl. Acad. Sci. USA 2010, 107, 11183–11188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, J.P.; Gantar, M.; Perez, M.H.; Berry, G.; Noriega, F.G. Cyanobacterial toxins as allelochemicals with potential applications as algaecides, herbicides and insecticides. Mar. Drugs 2008, 6, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Hou, X.P.; Wu, D.H.; Chang, W.Y.; Zhang, X.; Dai, X.Z.; Du, H.X.; Zhang, X.H.; Igarashi, Y.; Luo, F. The characteristics and algicidal mechanisms of cyanobactericidal bacteria, a review. World J. Microbiol. Biotechnol. 2020, 36, 188. [Google Scholar] [CrossRef] [PubMed]
- Nishu, S.D.; Kang, Y.; Han, I.; Jung, T.Y.; Lee, T.K. Nutritional status regulates algicidal activity of Aeromonas sp. L23 against cyanobacteria and green algae. PLoS ONE 2019, 14, e0213370. [Google Scholar]
- Weiss, G.; Kovalerchick, D.; Lieman-Hurwitz, J.; Murik, O.; De Philippis, R.; Carmeli, S.; Sukenik, A.; Kaplan, A. Increased algicidal activity of Aeromonas veronii in response to Microcystis aeruginosa: Inter-species crosstalk and secondary metabolites synergism. Environ. Microbiol. 2019, 21, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Kovalerchick, D.; Murik, O.; Sukenik, A.; Kaplan, A.; Carmeli, S. Secondary metabolites of Aeromonas veronii strain A134 isolated from a microcystis aeruginosa bloom. Metabolites 2019, 9, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Cyanobacterial Toxins: Anatoxin-A and Analogues. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; WHO: Geneva, Switzerland, 2020; p. 21. [Google Scholar]
- World Health Organization. Cyanobacterial Toxins: Microcystins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; WHO: Geneva, Switzerland, 2020; p. 55. [Google Scholar]
- World Health Organization. Cyanobacterial Toxins: Cylindrospermopsins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; WHO: Geneva, Switzerland, 2020; p. 31. [Google Scholar]
- World Health Organization. Cyanobacterial Toxins: Saxitoxins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; WHO: Geneva, Switzerland, 2020; p. 24. [Google Scholar]
- Hozumi, A.; Ostrovsky, I.; Sukenik, A.; Gildor, H. Turbulence regulation of Microcystis surface scum formation and dispersion during a cyanobacteria bloom event. Inland Waters 2020, 10, 51–70. [Google Scholar] [CrossRef]
- Paerl, H.W. Mitigating toxic planktonic cyanobacterial blooms in aquatic ecosystems facing increasing anthropogenic and climatic pressures. Toxins 2018, 10, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wynne, T.T.; Stumpf, R.P.; Tomlinson, M.C.; Dyble, J. Characterizing a cyanobacterial bloom in western lake erie using satellite imagery and meteorological data. Limnol. Oceanogr. 2010, 55, 2025–2036. [Google Scholar] [CrossRef] [Green Version]
- Vander Woude, A.; Ruberg, S.; Johengen, T.; Miller, R.; Stuart, D. Spatial and temporal scales of variability of cyanobacteria harmful algal blooms from NOAA GLERL airborne hyperspectral imagery. J. Great Lakes Res. 2019, 45, 536–546. [Google Scholar] [CrossRef]
- Wilkinson, A.; Hondzo, M.; Guala, M. Vertical heterogeneities of cyanobacteria and microcystin concentrations in lakes using a seasonal in situ monitoring station. Glob. Ecol. Conserv. 2020, 21, e00838. [Google Scholar] [CrossRef]
- Recknagel, F.; Orr, P.T.; Bartkow, M.; Swanepoel, A.; Cao, H. Early warning of limit-exceeding concentrations of cyanobacteria and cyanotoxins in drinking water reservoirs by inferential modelling. Harmful Algae 2017, 69, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Westrick, J.A.; Szlag, D.C.; Southwell, B.J.; Sinclair, J. A review of cyanobacteria and cyanotoxins removal/inactivation in drinking water treatment. Anal. Bioanal. Chem. 2010, 397, 1705–1714. [Google Scholar] [CrossRef]
- McKindles, K.; Manes, M.; DeMarco, J.; McClure, A.; McKay, R.; Davis, T.; Bullerjahn, G. Dissolved microcystin release coincident with lysis of a Microcystis-dominated bloom in western LAKE ERIE attributed to a novel cyanophage. Appl. Environ. Microbiol. 2020, 86, e01397-20. [Google Scholar] [CrossRef]
- Saker, M.L.; Griffiths, D.J. The effect of temperature on growth and cylindrospermopsin content of seven isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from water bodies in northern Australia. Phycologia 2000, 39, 349–354. [Google Scholar] [CrossRef]
- Kokociński, M.; Cameán, A.M.; Carmeli, S.; Guzmán-Guillén, R.; Jos, Á.; Mankiewicz-Boczek, J.; Metcalf, J.S.; Moreno, I.M.; Prieto, A.I.; Sukenik, A. Cylindrospermopsin and congeners. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, L.S.J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 127–137. [Google Scholar]
- Kinley-Baird, C.; Calomeni, A.; Berthold, D.E.; Lefler, F.W.; Barbosa, M.; Rodgers, J.H.; Laughinghouse, H.D. Laboratory-scale evaluation of algaecide effectiveness for control of microcystin-producing cyanobacteria from lake okeechobee, florida (USA). Ecotoxicol. Environ. Saf. 2021, 207, 111233. [Google Scholar] [CrossRef]
- Hu, J.; Chu, W.; Sui, M.; Xu, B.; Gao, N.; Ding, S. Comparison of drinking water treatment processes combinations for the minimization of subsequent disinfection by-products formation during chlorination and chloramination. Chem. Eng. J. 2018, 335, 352–361. [Google Scholar] [CrossRef]
- Zamyadi, A.; Ho, L.; Newcombe, G.; Bustamante, H.; Prévost, M. Fate of toxic cyanobacterial cells and disinfection by-products formation after chlorination. Water Res. 2012, 46, 1524–1535. [Google Scholar] [CrossRef]
- He, X.; Liu, Y.-L.; Conklin, A.; Westrick, J.; Weavers, L.K.; Dionysiou, D.D.; Lenhart, J.J.; Mouser, P.J.; Szlag, D.; Walker, H.W. Toxic cyanobacteria and drinking water: Impacts, detection, and treatment. Harmful Algae 2016, 54, 174–193. [Google Scholar] [CrossRef] [PubMed]
- Coral, L.A.; Zamyadi, A.; Barbeau, B.; Bassetti, F.J.; Lapolli, F.R.; Prevost, M. Oxidation of Microcystis aeruginosa and Anabaena flos-aquae by ozone: Impacts on cell integrity and chlorination by-product formation. Water Res. 2013, 47, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qu, F.; Chen, W.; Liang, H.; Wang, T.; Cheng, X.; Yu, H.; Li, G.; Van der Bruggen, B. Microcystis aeruginosa-laden water treatment using enhanced coagulation by persulfate/Fe (II), ozone and permanganate: Comparison of the simultaneous and successive oxidant dosing strategy. Water Res. 2017, 125, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Barešová, M.; Načeradská, J.; Novotná, K.; Čermáková, L.; Pivokonský, M. The impact of preozonation on the coagulation of cellular organic matter produced by Microcystis aeruginosa and its toxin degradation. J. Environ. Sci. 2020, 98, 124–133. [Google Scholar] [CrossRef]
- Naceradska, J.; Pivokonsky, M.; Pivokonska, L.; Baresova, M.; Henderson, R.K.; Zamyadi, A.; Janda, V. The impact of pre-oxidation with potassium permanganate on cyanobacterial organic matter removal by coagulation. Water Res. 2017, 114, 42–49. [Google Scholar] [CrossRef]
- Dixon, M.B.; Ho, L.; Antoniou, M.G. Removal of cyanobacteria and cyanotoxins by membrane processes. In Water Treatment for Purification from Cyanobacteria and Cyanotoxins; Hiskia, A.E., Triantis, T.M., Antoniou, M.G., Kaloudis, T., Dionysiou, D.D., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 99–116. [Google Scholar]
- Chow, C.W.; Drikas, M.; House, J.; Burch, M.D.; Velzeboer, R.M. The impact of conventional water treatment processes on cells of the cyanobacterium Microcystis aeruginosa. Water Res. 1999, 33, 3253–3262. [Google Scholar] [CrossRef]
- Drikas, M.; Chow, C.W.; House, J.; Burch, M.D. Using coagulation, flocculation, and settling to remove toxic cyanobacteria. J. Am. Water Works Assoc. 2001, 93, 100–111. [Google Scholar] [CrossRef]
- Gonzalez-Torres, A.; Putnam, J.; Jefferson, B.; Stuetz, R.; Henderson, R. Examination of the physical properties of Microcystis aeruginosa flocs produced on coagulation with metal salts. Water Res. 2014, 60, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, J.; Bornmann, K.; Schmidt, W. Relevance of intra--and extracellular cyanotoxins for drinking water treatment. Acta Hydrochim. Hydrobiol. 2002, 30, 7–15. [Google Scholar] [CrossRef]
- Han, J.; Jeon, B.-S.; Park, H.-D. Microcystin release and Microcystis cell damage mechanism by alum treatment with long-term and large dose as in-lake treatment. J. Environ. Sci. Health Part A 2016, 51, 455–462. [Google Scholar] [CrossRef]
- Bolto, B.; Gregory, J. Organic polyelectrolytes in water treatment. Water Res. 2007, 41, 2301–2324. [Google Scholar] [CrossRef]
- Mucci, M.; Guedes, I.A.; Faassen, E.J.; Lürling, M. Chitosan as a coagulant to remove cyanobacteria can cause microcystin release. Toxins 2020, 12, 711. [Google Scholar] [CrossRef]
- Lürling, M.; Noyma, N.P.; de Magalhães, L.; Miranda, M.; Mucci, M.; van Oosterhout, F.; Huszar, V.L.; Marinho, M.M. Critical assessment of chitosan as coagulant to remove cyanobacteria. Harmful Algae 2017, 66, 1–12. [Google Scholar] [CrossRef]
- Ahmad, I.; Ali, F.; Rahim, F. Clay based nanocomposites and their environmental applications. In Development and Prospective Applications of Nanoscience and Nanotechnology, Volume 2—Nanomaterials for Environmental Applications and Their Fascinating Attributes; Khan, S.B., Asiri, A.M., Akhtar, K., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2018; pp. 166–190. [Google Scholar]
- Rytwo, G. Securing the future: Clay-based solutions for a comprehensive and sustainable potable-water supply system. Clays Clay Miner. 2018, 66, 315–328. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Rosa, M.J.; Sorlini, S.; Biasibetti, M.; Christophoridis, C.; Edwards, C. Removal of cyanobacteria and cyanotoxins by conventional physical-chemical treatment. In Water Treatment for Purification from Cyanobacteria and Cyanotoxins; Hiskia, A.E., Triantis, T.M., Antoniou, M.G., Kaloudis, T., Dionysiou, D.D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 69–97. [Google Scholar]
- Teixeira, M.R.; Sousa, V.; Rosa, M.J. Investigating dissolved air flotation performance with cyanobacterial cells and filaments. Water Res. 2010, 44, 3337–3344. [Google Scholar] [CrossRef]
- Edzwald, J.K. Dissolved air flotation and me. Water Res. 2010, 44, 2077–2106. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, G.; Ho, L.; Capelo-Neto, J. Controlling cyanotoxin occurrence: Drinking-water treatment. In Toxic Cyanobacteria in Water; CRC Press, on behalf of the World Health Organization, Geneva: Boca Raton, FL, USA, 2021; pp. 591–639. [Google Scholar]
- Newcombe, G.; Cook, D.; Brooke, S.; Ho, L.; Slyman, N. Treatment options for microcystin toxins: Similarities and differences between variants. Environ. Technol. 2003, 24, 299–308. [Google Scholar] [CrossRef]
- Sukenik, A.; Viner-Mozzini, Y.; Mizrahi, D.; Tamam, I.; Benitez, A.R.; Nir, S. Removal of cyanotoxins-microcystins from water by filtration through granulated composites of bentonite with micelles of the cation octadecyltrimethyl ammonium (ODTMA). Appl. Nano 2021, 2, 67–81. [Google Scholar] [CrossRef]
- Wang, H.; Ho, L.; Lewis, D.M.; Brookes, J.D.; Newcombe, G. Discriminating and assessing adsorption and biodegradation removal mechanisms during granular activated carbon filtration of microcystin toxins. Water Res. 2007, 41, 4262–4270. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Li, J. Current research scenario for microcystins biodegradation–a review on fundamental knowledge, application prospects and challenges. Sci. Total Environ. 2017, 595, 615–632. [Google Scholar] [CrossRef]
- Ho, L.; Onstad, G.; Von Gunten, U.; Rinck-Pfeiffer, S.; Craig, K.; Newcombe, G. Differences in the chlorine reactivity of four microcystin analogues. Water Res. 2006, 40, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Banker, R.; Carmeli, S.; Werman, M.; Teltsch, B.; Porat, R.; Sukenik, A. Uracil moiety is required for toxicity of the cyanobacterial hepatotoxin cylindrospermopsin. J. Toxicol. Environ. Health Part A 2001, 62, 281–288. [Google Scholar] [CrossRef]
- Rodríguez, E.; Onstad, G.D.; Kull, T.P.; Metcalf, J.S.; Acero, J.L.; von Gunten, U. Oxidative elimination of cyanotoxins: Comparison of ozone, chlorine, chlorine dioxide and permanganate. Water Res. 2007, 41, 3381–3393. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Bláha, L. Advanced oxidation processes for the removal of cyanobacterial toxins from drinking water. Environ. Sci. Eur. 2020, 32, 94. [Google Scholar] [CrossRef]
- Zhang, G.; He, X.; Duan, X.; Huang, Y.; Han, C.; Nadagouda, M.N.; O’Shea, K.; Kim, D.K.; Sharma, V.K.; Johnson, N.; et al. Advanced oxidation processes. In Water Treatment for Purification from Cyanobacteria and Cyanotoxins; Hiskia, A.E., Triantis, T.M., Antoniou, M.G., Triantafyllos, K., Dionysios, D.D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 173–206. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukenik, A.; Kaplan, A. Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems: A Comprehensive Outlook on Current and Emerging Mitigation and Control Approaches. Microorganisms 2021, 9, 1472. https://doi.org/10.3390/microorganisms9071472
Sukenik A, Kaplan A. Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems: A Comprehensive Outlook on Current and Emerging Mitigation and Control Approaches. Microorganisms. 2021; 9(7):1472. https://doi.org/10.3390/microorganisms9071472
Chicago/Turabian StyleSukenik, Assaf, and Aaron Kaplan. 2021. "Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems: A Comprehensive Outlook on Current and Emerging Mitigation and Control Approaches" Microorganisms 9, no. 7: 1472. https://doi.org/10.3390/microorganisms9071472
APA StyleSukenik, A., & Kaplan, A. (2021). Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems: A Comprehensive Outlook on Current and Emerging Mitigation and Control Approaches. Microorganisms, 9(7), 1472. https://doi.org/10.3390/microorganisms9071472





