Low C/N Ratios Promote Dissimilatory Nitrite Reduction to Ammonium in Pseudomonas putida Y-9 under Aerobic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Media
2.2. Evaluation of the Effects of Chloramphenicol on the Growth and Nitrite Reduction Performance of Strain Y-9
2.3. Single-Factor Affecting Dissimilatory Nitrite Reduction
2.4. Analytical Methods
2.5. Statistical Analysis and Graphical Work
3. Results
3.1. Effect of Chloramphenicol on Strain Growth and Nitrite Reduction
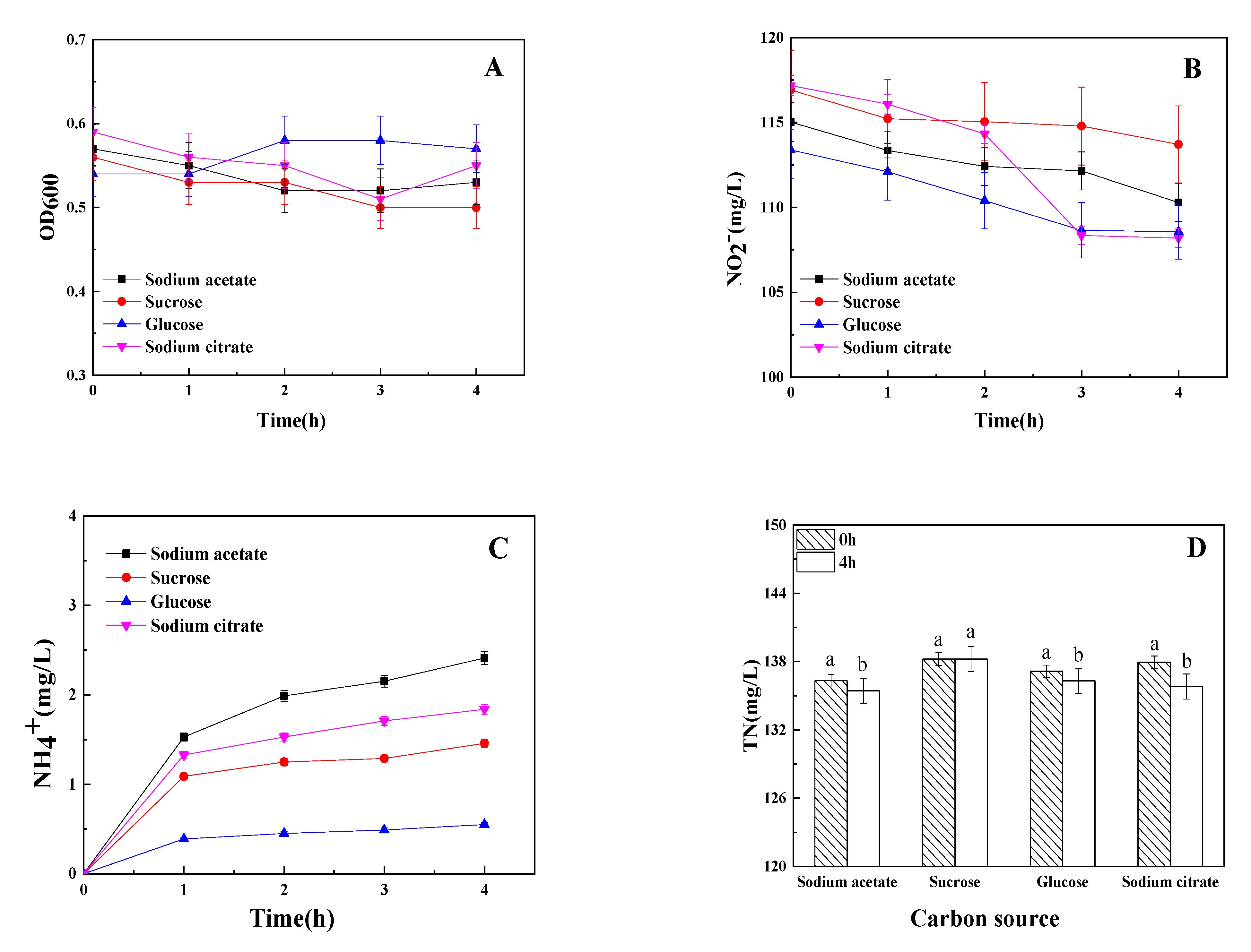
3.2. The Influence of Carton Source on Dissimilatory Nitrite Reduction
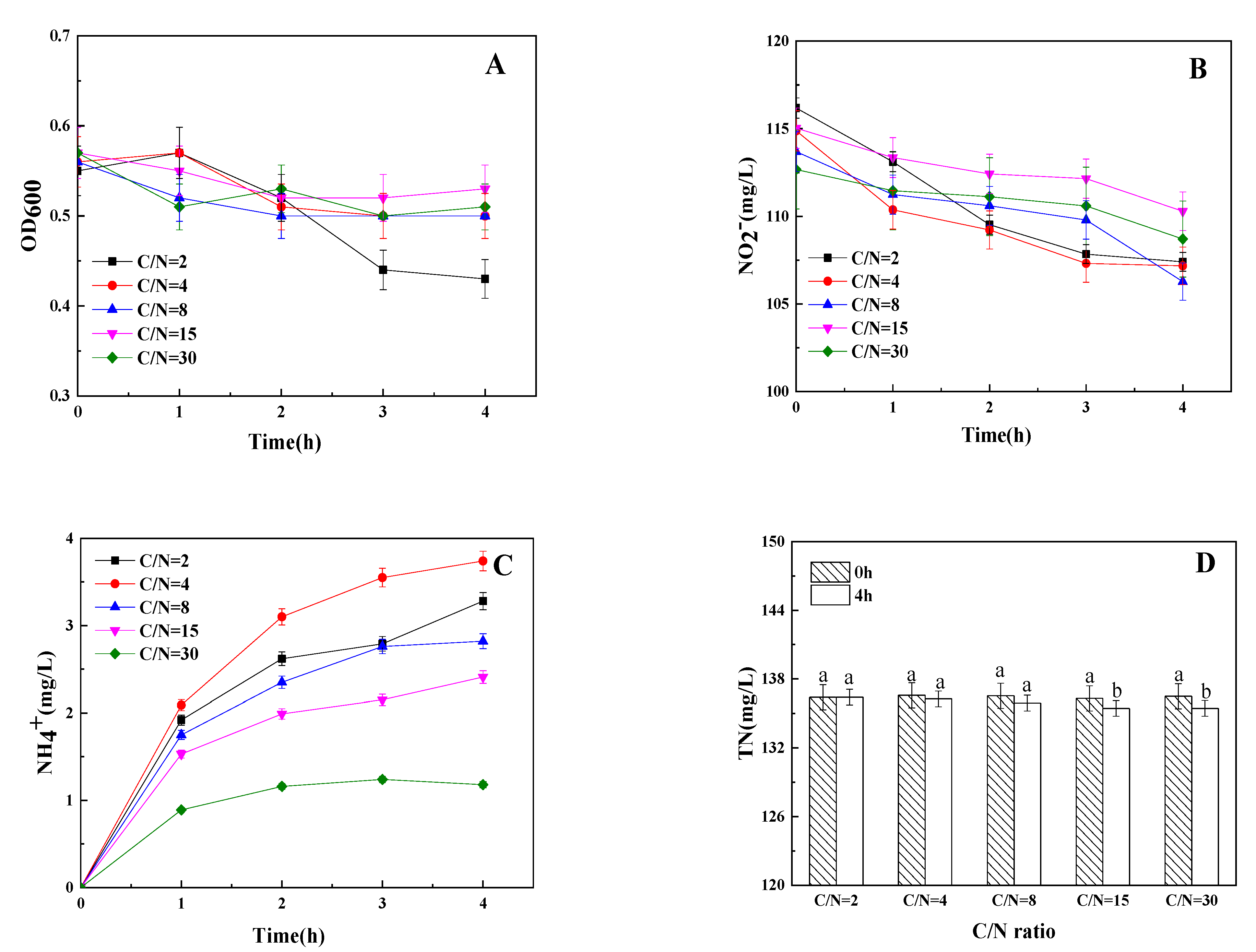
3.3. The Influence of C/N Ratio on Dissimilatory Nitrite Reduction
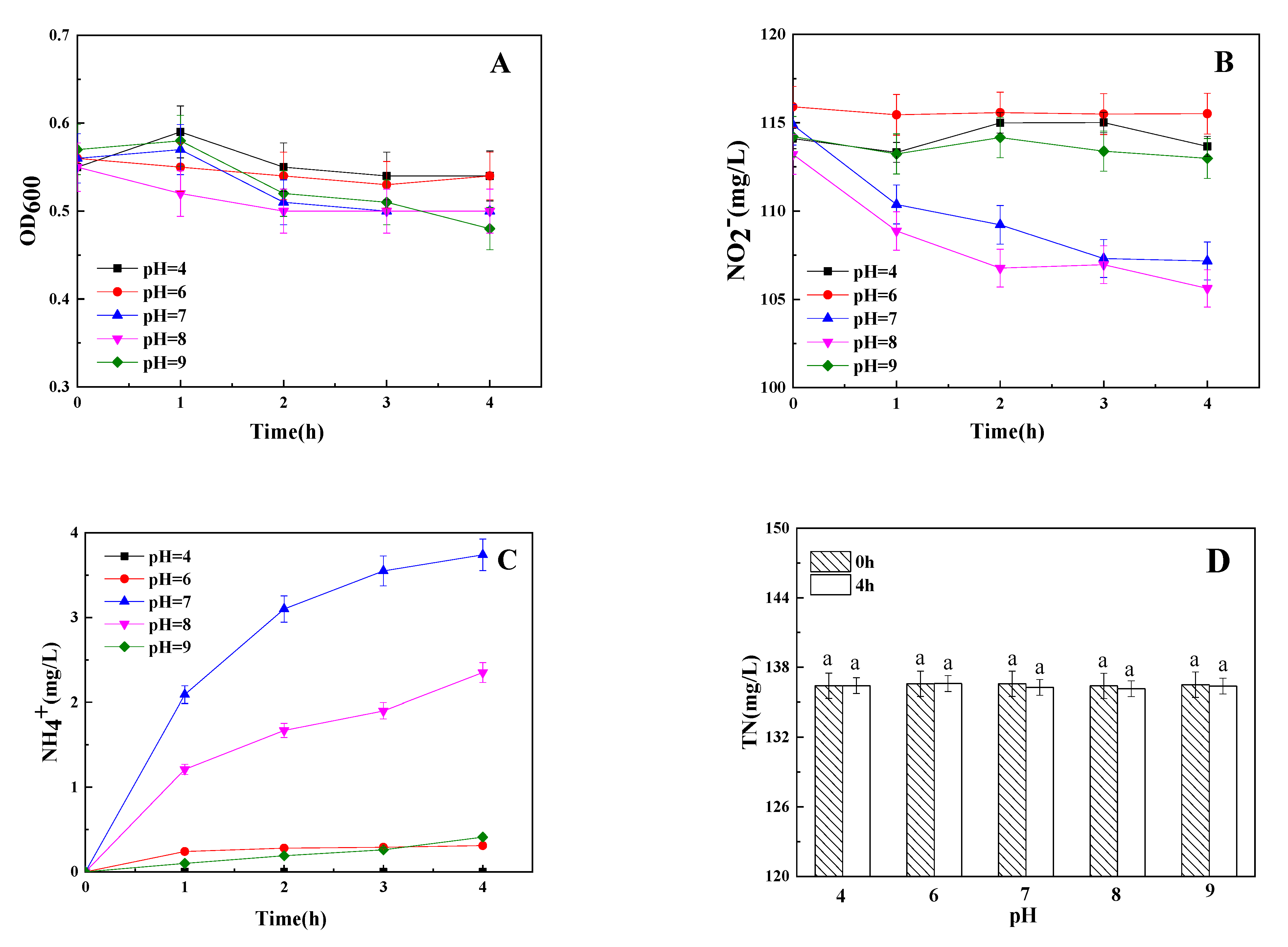
3.4. The Influence of Initial pH on Dissimilatory Nitrite Reduction
3.5. The Influence of Dissolved Oxygen on Dissimilatory Nitrite Reduction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ju, X.T.; Kou, C.L.; Zhang, F.S.; Christie, P. Nitrogen balance and groundwater nitrate contamination: Comparison among three intensive cropping systems on the North China Plain. Environ. Pollut. 2006, 143, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Di, H.J.; Cameron, K.C. Nitrate leaching losses and pasture yields as affected by different rates of animal urine nitrogen returns and application of a nitrification inhibitor—A lysimeter study. Nutr. Cycl. Agroecosyst. 2007, 79, 281–290. [Google Scholar] [CrossRef]
- Zhao, C.S.; Hu, C.X.; Hang, W.; Sun, X.C.; Tan, Q.L.; Di, H.J. A lysimeter study of nitrate leaching and optimum nitrogen application rates for intensively irrigated vegetable production systems in Central China. J. Soils Sediments 2010, 10, 9–17. [Google Scholar] [CrossRef]
- Su, W.T.; Zhang, L.X.; Li, D.P.; Zhan, G.Q.; Qian, J.W.; Tao, Y. Dissimilatory nitrate reduction by Pseudomonas alcaliphila with an electrode as the sole electron donor. Biotechnol. Bioeng. 2012, 109, 2904–2910. [Google Scholar] [CrossRef]
- Lu, W.W.; Zhang, H.L.; Shi, W.M. Dissimilatory nitrate reduction to ammonium in an anaerobic agricultural soil as affected by glucose and free sulfide. Eur. J. Soil Biol. 2013, 58, 98–104. [Google Scholar] [CrossRef]
- Templer, P.H.; Silver, W.L.; Pett Ridge, J.; Deangelis, K.M.; Firestone, M.K. Plant and microbial controls on nitrogen retention and loss in a humid tropical forest. Ecology 2008, 89, 3030–3040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, J.; Klotz, M.G. Diversity and evolution of bioenergetic systems involved in microbial nitrogen compound transformations. BBA Bioenergy 2013, 1827, 114–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Berg, E.M.; Rombouts, J.L.; Gijs Kuenen, J.; Kleerebezem, R.; Van Loosdrecht, M.C.M. Role of nitrite in the competition between denitrification and DNRA in a chemostat enrichment culture. AMB Express 2017, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Fazzolari, É.; Nicolardot, B.; Germon, J.C. Simultaneous effects of increasing levels of glucose and oxygen partial pressures on denitrification and dissimilatory nitrate reduction to ammonium in repacked soil cores. Eur. J. Soil Biol. 1998, 34, 47–52. [Google Scholar] [CrossRef]
- Tugtas, A.E.; Pavlostathis, S.G. Effect of sulfide on nitrate reduction in mixed methanogenic cultures. Biotechnol. Bioeng. 2007, 97, 1448–1459. [Google Scholar] [CrossRef]
- Koop Jakobsen, K.; Giblin, A.E. The effect of increased nitrate loading on nitrate reduction via denitrification and DNRA in salt marsh sediments. Limnol. Oceanogr. 2010, 55, 789–802. [Google Scholar] [CrossRef]
- Matheson, F.E.; Nguyen, M.L.; Cooper, A.B.; Burt, T.P.; Bull, D.C. Fate of 15N nitrate in unplanted, planted and harvested riparian wetland soil microcosms. Ecol. Eng. 2002, 19, 249–264. [Google Scholar] [CrossRef]
- Yin, S.X.; Shen, Q.R.; Tang, Y.; Cheng, L.M. Reduction of nitrate to ammonium in selected paddy soils of China. Pedosphere 1998, 8, 221–228. [Google Scholar]
- Strohm, T.O.; Griffin, B.; Zumft, W.G.; Schink, B. Growth yields in bacterial denitrification and nitrate ammonification. Appl. Environ. Microbiol. 2007, 73, 1420–1424. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.J.; Weisener, C.G.; Ni, J.P.; He, B.H.; Xie, D.T.; Li, Z.L. Nitrate assimilation, dissimilatory nitrate reduction to ammonium, and denitrification coexist in Pseudomonas putida Y-9 under aerobic conditions. Bioresour. Technol. 2020, 312, 123597. [Google Scholar] [CrossRef]
- Huang, X.J.; Jiang, D.H.; Ni, J.P.; Xie, D.T.; Li, Z.L. Removal of ammonium and nitrate by the hypothermia bacterium Pseudomonas putida Y-9 mainly through assimilation. Environ. Technol. Innov. 2021, 22, 101458. [Google Scholar] [CrossRef]
- Tiedje, J.M. Denitrifiers. In Methods of Soil Analysis, 1st ed.; Weaver, R.W., Angle, S., Eds.; Soil Science Society of America Journal: Madison, WI, USA, 1994; pp. 245–267. [Google Scholar]
- Ren, Y.X.; Yang, L.; Liang, X. The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB. Bioresour. Technol. 2014, 171, 1–9. [Google Scholar] [CrossRef]
- Lei, X.; Jia, Y.T.; Chen, Y.C.; Hu, Y.Y. Simultaneous nitrification and denitrification without nitrite accumulation by a novel isolated Ochrobactrum anthropic LJ81. Bioresour. Technol. 2019, 272, 442–450. [Google Scholar] [CrossRef] [PubMed]
- State Environmental Protection Administration of China. Water and Wastewater Analysis Methods; China Environmental Science Press: Beijing, China, 2002; pp. 132–286. [Google Scholar]
- He, T.X.; Li, Z.L. Identification and denitrification characterization of a psychrotrophic and aerobic nitrite-bacterium. Biotechnol. Bull. 2015, 31, 191–198. (In Chinese) [Google Scholar]
- Zhao, B.; Cheng, D.Y.; Tan, P.; An, Q.; Guo, J.S. Characterization of an aerobic denitrifier Pseudomonas stutzeri strain XL-2 to achieve efficient nitrate removal. Bioresour. Technol. 2018, 250, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Mania, D.; Heylen, K.; Van Spanning, R.J.M.; Frostegård, A. The nitrate-ammonifying and nosZ-carrying bacterium Bacillus vireti is a potent source and sink for nitric and nitrous oxide under high nitrate conditions. Environ. Microbiol. 2014, 16, 3196–3210. [Google Scholar] [CrossRef]
- Bykov, D.; Neese, F. Six-electron reduction of nitrite to ammonia by cytochrome c nitrite reductase: Insights from density functional theory studies. Inorg. Chem. 2015, 54, 9303–9316. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.J.; Laughlin, R.J.; Malone, J.P. Soil pH affects the processes reducing nitrate to nitrous oxide and di-nitrogen. Soil Biol. Biochem. 1998, 30, 1119–1126. [Google Scholar] [CrossRef]
- Šimek, M.; Cooper, J.E. The influence of soil pH on denitrification: Progress towards the understanding of this interaction over the last 50 years. Eur. J. Soil. Sci. 2002, 53, 345–354. [Google Scholar] [CrossRef]
- Schmidt, C.S.; Richardson, D.J.; Baggs, E.M. Constraining the conditions conducive to dissimilatory nitrate reduction to ammonium in temperate arable soils. Soil Biol. Biochem. 2011, 43, 1607–1611. [Google Scholar] [CrossRef]
- Tiedje, J.M. Ecology of denitrification and dissimilatory nitrate reduction to ammonia. In Biology of Anaerobic Microorganisms; Zehnder, A.J.B., Ed.; John Wiley & Sons: New York, NY, USA, 1988; pp. 179–244. [Google Scholar]
- Padhi, S.K.; Tripathy, S.; Sen, R.; Mahapatra, A.S.; Mohanty, S.; Maiti, N.K. Characterisation of heterotrophic nitrifying and aerobic denitrifying Klebsiella pneumoniae CF-S9 strain for bioremediation of wastewater. Int. Biodeterior. Biodegrad. 2013, 78, 67–73. [Google Scholar] [CrossRef]
- Ji, B.; Wang, H.Y.; Yang, K. Tolerance of an aerobic denitrifier (Pseudomonas stutzeri) to high O2 concentrations. Biotechnol. Lett. 2014, 36, 719–722. [Google Scholar] [CrossRef]
- He, T.X.; Li, Z.L.; Sun, Q.; Xu, Y.; Ye, Q. Heterotrophic nitrification and aerobic denitrification by Pseudomonas tolaasii Y-11 without nitrite accumulation during nitrogen conversion. Bioresour. Technol. 2016, 200, 493–499. [Google Scholar] [CrossRef]
- Xu, Y.; He, T.X.; Li, Z.L.; Ye, Q.; Chen, Y.L.; Xie, E.Y.; Zhang, X. Nitrogen removal characteristics of Pseudomonas putida Y-9 capable of heterotrophic nitrification and aerobic denitrification at low temperature. BioMed Res. Int. 2017, 2017, 1429018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huygens, D.; Rütting, T.; Boeckx, P.; Van Cleemput, O.; Godoy, R.; Müller, C. Soil nitrogen conservation mechanisms in a pristine south Chilean Nothofagus forest ecosystem. Soil Biol. Biochem. 2007, 39, 2448–2458. [Google Scholar] [CrossRef]
- Rütting, T.; Boeckx, P.; Müller, C.; Klemedtsson, L. Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle. Biogeosciences 2011, 8, 1779–1791. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.W.; Riya, S.; Zhou, S.; Hosomi, M.; Zhang, H.L.; Shi, W.M. In situ dissimilatory nitrate reduction to ammonium in a paddy soil fertilized with liquid cattle waste. Pedosphere 2012, 22, 314–321. [Google Scholar] [CrossRef]
- Yin, S.X.; Chen, D.; Chen, L.M.; Edis, R. Dissimilatory nitrate reduction to ammonium and responsible microorganisms in two Chinese and Australian paddy soils. Soil Biol. Biochem. 2002, 34, 1131–1137. [Google Scholar] [CrossRef]
- Yoon, S.; Cruz García, C.; Sanford, R.; Ritalahti, K.M.; Löffler, F.E. Denitrification versus respiratory ammonification: Environmental controls of two competing dissimilatory NO3−/NO2− reduction pathways in Shewanella loihica strain PV-4. ISME J. 2015, 9, 1093–1104. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Suter, H.; He, J.Z.; Hu, H.W.; Chen, D. Dissimilatory nitrate reduction to ammonium dominates nitrate reduction in long-term low nitrogen fertilized rice paddies. Soil Biol. Biochem. 2019, 131, 149–156. [Google Scholar] [CrossRef]
- Kelso, B.H.L.; Smith, R.V.; Laughlin, R.J.; David Lennox, S. Dissimilatory nitrate reduction in anaerobic sediments leading to river nitrite accumulation. Appl. Environ. Microbiol. 1997, 63, 4679–4685. [Google Scholar] [CrossRef] [Green Version]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Tie, W.; Xie, D.; Li, Z. Low C/N Ratios Promote Dissimilatory Nitrite Reduction to Ammonium in Pseudomonas putida Y-9 under Aerobic Conditions. Microorganisms 2021, 9, 1524. https://doi.org/10.3390/microorganisms9071524
Huang X, Tie W, Xie D, Li Z. Low C/N Ratios Promote Dissimilatory Nitrite Reduction to Ammonium in Pseudomonas putida Y-9 under Aerobic Conditions. Microorganisms. 2021; 9(7):1524. https://doi.org/10.3390/microorganisms9071524
Chicago/Turabian StyleHuang, Xuejiao, Wenzhou Tie, Deti Xie, and Zhenlun Li. 2021. "Low C/N Ratios Promote Dissimilatory Nitrite Reduction to Ammonium in Pseudomonas putida Y-9 under Aerobic Conditions" Microorganisms 9, no. 7: 1524. https://doi.org/10.3390/microorganisms9071524





