Cyclosporiasis—Updates on Clinical Presentation, Pathology, Clinical Diagnosis, and Treatment
Abstract
:1. Introduction
2. Biology and Life Cycle
3. Pathogenesis
4. Clinical Presentation
5. Treatment
6. Diagnosis
7. Stool Microscopy
8. Wet Mounts
9. Permanent-Stained Smears
10. Histopathology
11. Molecular Diagnosis
12. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Eberhard, M.L.; da Silva, A.J.; Lilley, B.G.; Pieniazek, N.J. Morphologic and molecular characterization of new Cyclospora species from Ethiopian monkeys: C. cercopitheci sp.n. C. colobi sp.n. and C. papionis sp.n. Emerg. Infect. Dis. 1999, 5, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Marangi, M.; Koehler, A.V.; Zanzani, S.A.; Manfredi, M.T.; Brianti, E.; Giangaspero, A.; Gasser, R.B. Detection of Cyclospora in captive chimpanzees and macaques by a quantitative PCR-based mutation scanning approach. Parasit. Vectors 2015, 8, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeria, S.; Cinar, H.N.; Dubey, J.P. Cyclospora cayetanensis and Cyclosporiasis: An Update. Microorganisms 2019, 7, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, Y.R.; Sanchez, R. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin. Microbiol. Rev. 2010, 23, 218–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CFIA-PHAC Public Health Notice-Outbreak of Cyclospora Infections under Investigation, October 11, 2017-Final Update. Available online: https://www.canada.ca/en/public-health/services/public-health-notices/2017/public-health-notice-outbreak-cyclospora-infections-under-investigation.html (accessed on 30 April 2021).
- Dixon, B.; Mihajlovic, B.; Couture, H.; Farber, J.M. Qualitative risk assessment: Cyclospora cayetanensis on fresh raspberries and blackberries imported into Canada. Food Prot. Trends 2016, 36, 18–32. [Google Scholar]
- Nichols, G.L.; Freedman, J.; Pollock, K.G.; Rumble, C.; Chalmers, R.M.; Chiodini, P.; Hawkins, G.; Alexander, C.L.; Godbole, G.; Williams, C.; et al. Cyclospora infection linked to travel to Mexico, June to September 2015. Euro Surveill 2015, 20. [Google Scholar] [CrossRef]
- CFIA-PHAC Public Health Notice: Outbreak of Cyclospora Infections Linked to Salad Products and Fresh Herbs. Available online: https://www.canada.ca/en/public-health/services/public-health-notices/2020/outbreak-cyclospora-infections-salad-products.html (accessed on 30 April 2021).
- Bednarska, M.; Bajer, A.; Welc-Faleciak, R.; Pawelas, A. Cyclospora cayetanensis infection in transplant traveller: A case report of outbreak. Parasit. Vectors 2015, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Abanyie, F.; Harvey, R.R.; Harris, J.R.; Wiegand, R.E.; Gaul, L.; Desvignes-Kendrick, M.; Irvin, K.; Williams, I.; Hall, R.L.; Herwaldt, B.; et al. 2013 multistate outbreaks of Cyclospora cayetanensis infections associated with fresh produce: Focus on the Texas investigations. Epidemiol. Infect. 2015, 143, 3451–3458. [Google Scholar] [CrossRef] [Green Version]
- Casillas, S.M.; Bennett, C.; Straily, A. Notes from the Field: Multiple Cyclosporiasis Outbreaks-United States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1101–1102. [Google Scholar] [CrossRef] [Green Version]
- Casillas, S.M.; Hall, R.L.; Herwaldt, B.L. Cyclosporiasis Surveillance-United States, 2011–2015. MMWR Surveill. Summ. 2019, 68, 1–16. [Google Scholar] [CrossRef] [Green Version]
- CDC. U.S. Foodborne Outbreaks of Cyclosporiasis-2000–2017. Available online: https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/foodborneoutbreaks.html (accessed on 30 April 2021).
- CDC. Domestically Acquired Cases of Cyclosporiasis-United States, May–August 2018. Available online: https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/2018/c-082318/index.html (accessed on 30 April 2021).
- CDC. Multistate Outbreak of Cyclosporiasis Linked to Fresh Express Salad Mix Sold at McDonald’s Restaurants—United States, 2018: Final Update. Available online: https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/2018/b-071318/index.html (accessed on 30 April 2021).
- CDC. Multistate Outbreak of Cyclosporiasis Linked to Del Monte Fresh Produce Vegetable Trays—United States, 2018: Final Update. Available online: https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/2018/a-062018/index.html (accessed on 30 April 2021).
- CDC. Outbreak of Cyclospora Infections Linked to Bagged Salad Mix. Available online: https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/2020/ (accessed on 30 April 2021).
- CDC. Cyclosporiasis-Outbreak Investigations and Updates. Available online: https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/index.html (accessed on 22 July 2021).
- FDA. Cyclospora Prevention, Response and Research Action Plan. Available online: https://www.fda.gov/food/foodborne-pathogens/cyclospora-prevention-response-and-research-action-plan (accessed on 22 July 2021).
- Cama, V.A.; Mathison, B.A. Infections by Intestinal Coccidia and Giardia duodenalis. Clin. Lab. Med. 2015, 35, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.V.; Paton, C.A.; Mitambo, M.M.; Girdwood, R.W. Sporulation of Cyclospora sp. oocysts. Appl. Environ. Microbiol. 1997, 63, 1631–1632. [Google Scholar] [CrossRef] [Green Version]
- de Gorgolas, M.; Fortes, J.; Guerrero, M.L.F. Cyclospora cayetanensis Cholecystitis in a Patient with AIDS. Ann. Intern. Med. 2001, 134, 166. [Google Scholar] [CrossRef]
- Sifuentes-Osornio, J.; Porras-Cortés, G.; Bendall, R.P.; Morales-Villarreal, F.; Reyes-Terán, G.; Ruiz-Palacios, G.M. Cyclospora cayetanensis Infection in Patients with and Without AIDS: Biliary Disease as Another Clinical Manifestation. Clin. Infect. Dis. 1995, 21, 1092–1097. [Google Scholar] [CrossRef]
- Zar, F.A.; El-Bayoumi, E.; Yungbluth, M.M. Histologic Proof of Acalculous Cholecystitis Due to Cyclospora cayetanensis. Clin. Infect. Dis. 2001, 33, e140–e141. [Google Scholar] [CrossRef] [Green Version]
- Giangaspero, A.; Gasser, R.B. Human cyclosporiasis. Lancet Infect. Dis. 2019, 19, e226–e236. [Google Scholar] [CrossRef]
- Sun, T.; Ilardi, C.F.; Asnis, D.; Bresciani, A.R.; Goldenberg, S.; Roberts, B.; Teichberg, S. Light and electron microscopic identification of Cyclospora species in the small intestine. Evidence of the presence of asexual life cycle in human host. Am. J. Clin. Pathol. 1996, 105, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Fleming, C.A.; Caron, D.; Gunn, J.E.; Barry, M.A. A foodborne outbreak of Cyclospora cayetanensis at a wedding: Clinical features and risk factors for illness. Arch. Intern. Med. 1998, 158, 1121–1125. [Google Scholar] [CrossRef] [Green Version]
- Herwaldt, B.L.; Ackers, M.L. An outbreak in 1996 of cyclosporiasis associated with imported raspberries. The Cyclospora Working Group. N. Engl. J. Med. 1997, 336, 1548–1556. [Google Scholar] [CrossRef]
- Hoge, C.W.; Shlim, D.R.; Ghimire, M.; Rabold, J.G.; Pandey, P.; Walch, A.; Rajah, R.; Gaudio, P.; Echeverria, P. Placebo-controlled trial of co-trimoxazole for Cyclospora infections among travellers and foreign residents in Nepal. Lancet 1995, 345, 691–693. [Google Scholar] [CrossRef]
- Huang, P.; Weber, J.T.; Sosin, D.M.; Griffin, P.M.; Long, E.G.; Murphy, J.J.; Kocka, F.; Peters, C.; Kallick, C. The first reported outbreak of diarrheal illness associated with Cyclospora in the United States. Ann. Intern. Med. 1995, 123, 409–414. [Google Scholar] [CrossRef]
- CDC, Treatment for Cyclosporiasis. Available online: https://www.cdc.gov/parasites/cyclosporiasis/health_professionals/tx.html (accessed on 22 July 2021).
- Medical Letter. The Medical Letter: Drugs for Parasitic Infections, 3rd ed.; The Medical Letter, Inc.: New Rochelle, NY, USA, 2013. [Google Scholar]
- Verdier, R.I.; Fitzgerald, D.W.; Johnson, W.D., Jr.; Pape, J.W. Trimethoprim-sulfamethoxazole compared with ciprofloxacin for treatment and prophylaxis of Isospora belli and Cyclospora cayetanensis infection in HIV-infected patients. A randomized, controlled trial. Ann. Intern. Med. 2000, 132, 885–888. [Google Scholar] [CrossRef]
- Pape, J.W.; Verdier, R.-I.; Boncy, M.; Boncy, J.; Johnson, W.D. Cyclospora Infection in Adults Infected with HIV: Clinical Manifestations, Treatment, and Prophylaxis. Ann. Intern. Med. 1994, 121, 654–657. [Google Scholar] [CrossRef]
- CDC. Chapter 2-Preparing Interntional Travelers. In CDC Yellow Book; Oxford University Press: Oxford, UK. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2020/preparing-international-travelers/food-and-water-precautions (accessed on 22 July 2021).
- La Hoz, R.M.; Morris, M.I.; AST Infectious Diseases Community of Practice. Intestinal parasites including Cryptosporidium, Cyclospora, Giardia, and Microsporidia, Entamoeba histolytica, Strongyloides, Schistosomiasis, and Echinococcus: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant 2019, 33, e13618. [Google Scholar]
- NIH HIV.gov-Clinical Guidelines. Available online: https://clinicalinfo.hiv.gov/en/guidelines (accessed on 22 July 2021).
- Garcia, L.S.; Arrowood, M.; Kokoskin, E.; Paltridge, G.P.; Pillai, D.R.; Procop, G.W.; Ryan, N.; Shimizu, R.Y.; Visvesvara, G. Laboratory Diagnosis of Parasites from the Gastrointestinal Tract. Clin. Microbiol. Rev. 2018, 31, e00025-17. [Google Scholar] [CrossRef] [Green Version]
- Ash, L.R.; Orihel, T.C. Atlas of Human Parasitology, 5th ed.; ASCP Press: Chicago, IL, USA, 2007. [Google Scholar]
- Harrington, B.J. Microscopy of 4 Pathogenic Enteric Protozoan Parasites: A Review. Lab. Med. 2008, 39, 231–238. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Weiss, L.M. Cystoisospora, Cyclospora, and Sarcocystis. In Manual of Clinical Microbiology, 12th ed.; Carroll, K.C., Pfaller, M.A., Landry, M.L., McAdam, A.J., Patel, R., Richter, S.S., Warnock, D.W., Eds.; American Society for Microbiology: Washington, DC, USA, 2019; Volume 2, pp. 2526–2535. [Google Scholar]
- Parija, S.C.; Shivaprakash, M.R.; Jayakeerthi, S.R. Evaluation of lacto-phenol cotton blue (LPCB) for detection of Cryptosporidium, Cyclospora and Isospora in the wet mount preparation of stool. Acta Trop. 2003, 85, 349–354. [Google Scholar] [CrossRef]
- Visvesvara, G.S.; Moura, H.; Kovacs-Nace, E.; Wallace, S.; Eberhard, M.L. Uniform staining of Cyclospora oocysts in fecal smears by a modified safranin technique with microwave heating. J. Clin. Microbiol. 1997, 35, 730–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou El-Naga, I.F.; Gaafar, M.R. Auramine-phenol vs. modified Kinyoun’s acid-fast stains for detection of coccidia parasites. Lab. Med. 2014, 45, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Negm, A.Y. Identification of Cyclospora cayetanensis in stool using different stains. J. Egypt. Soc. Parasitol. 1998, 28, 429–436. [Google Scholar]
- Cann, K.; Chalmers, R.; Nichols, G.; O’Brien, S. Cyclospora infections in England and Wales: 1993 to 1998. Commun. Dis. Public Health/PHLS 2000, 3, 46–49. [Google Scholar]
- Mathison, B.A.; Pritt, B.S. Chapter 5: Parasitology. In Atlas of Fundamental Infectious Diseases Histopathology: A Guide for Daily Practice; Pritt, B.S., Ed.; College of American Pathologists: Northfield, IL, USA, 2018; pp. 193–288. [Google Scholar]
- Rowan, D.J.; Said, S.; Schuetz, A.N.; Pritt, B.S. A Case of Cystoisospora (Isospora) belli Infection With Multiple Life Stages Identified on Endoscopic Small Bowel Biopsies. Int. J. Surg. Pathol. 2020, 28, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.C.; Kim, Y.J.; Guaracao, A.I.; Mason, J.L.; Klos, R.F.; Warshauer, D.M. Performance and Impact of the BioFire FilmArray Gastrointestinal Panel on a Large Cyclospora Outbreak in Wisconsin, 2018. J. Clin. Microbiol. 2020, 58, e01415–e01419. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.N.; Alter, R.; Iwen, P.C.; Fey, P.D. Implications of culture-independent panel-based detection of Cyclospora cayetanensis. J. Clin. Microbiol. 2013, 51, 3909. [Google Scholar] [CrossRef] [Green Version]
- Amjad, M. An Overview of the Molecular Methods in the Diagnosis of Gastrointestinal Infectious Diseases. Int. J. Microbiol. 2020, 2020, 8135724. [Google Scholar] [CrossRef]
- Stark, D.; Roberts, T.; Ellis, J.T.; Marriott, D.; Harkness, J. Evaluation of the EasyScreen™ enteric parasite detection kit for the detection of Blastocystis spp. Cryptosporidium spp. Dientamoeba fragilis, Entamoeba complex, and Giardia intestinalis from clinical stool samples. Diagn. Microbiol. Infect. Dis. 2014, 78, 149–152. [Google Scholar]
- Mobidiag Novodiag–A Fully Automated Diagnostic Solution for Rapid and Reliable On-Demand Testing. Available online: https://mobidiag.com/products/novodiag/#bacterialge (accessed on 2 June 2021).
- Hofstetter, J.N.; Nascimento, F.S.; Park, S.; Casillas, S.; Herwaldt, B.L.; Arrowood, M.J.; Qvarnstrom, Y. Evaluation of Multilocus Sequence Typing of Cyclospora cayetanensis based on microsatellite markers. Parasite 2019, 26, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Roellig, D.M.; Li, N.; Tang, K.; Frace, M.; Ortega, Y.; Arrowood, M.J.; Feng, Y.; Qvarnstrom, Y.; Wang, L.; et al. Multilocus Sequence Typing Tool for Cyclospora cayetanensis. Emerg. Infect. Dis. 2016, 22, 1464–1467. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, F.S.; Barratt, J.; Houghton, K.; Plucinski, M.; Kelley, J.; Casillas, S.; Bennett, C.C.; Snider, C.; Tuladhar, R.; Zhang, J.; et al. Evaluation of an ensemble-based distance statistic for clustering MLST datasets using epidemiologically defined clusters of cyclosporiasis. Epidemiol. Infect. 2020, 148, e172. [Google Scholar] [CrossRef]
- Barratt, J.L.N.; Park, S.; Nascimento, F.S.; Hofstetter, J.; Plucinski, M.; Casillas, S.; Bradbury, R.S.; Arrowood, M.J.; Qvarnstrom, Y.; Talundzic, E. Genotyping genetically heterogeneous Cyclospora cayetanensis infections to complement epidemiological case linkage. Parasitology 2019, 146, 1275–1283. [Google Scholar] [CrossRef] [Green Version]
- ECDC Rapid Risk Assessment-Cyclospora Infections in European Travellers Returning from Mexico. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/rapid-risk-assessment-cyclospora-infections-in-travellers-to-Mexico-21-july-2017.pdf (accessed on 22 July 2021).

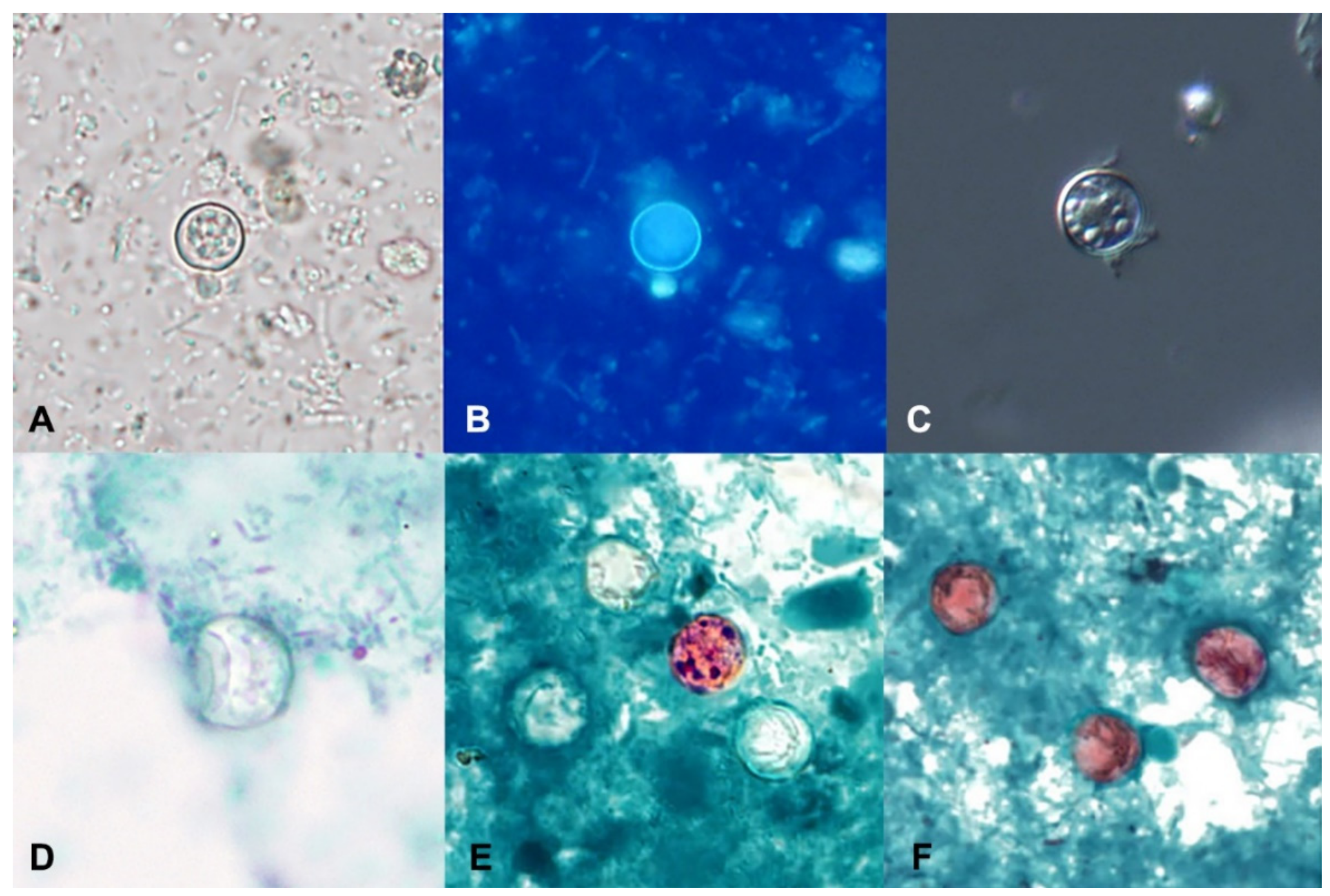
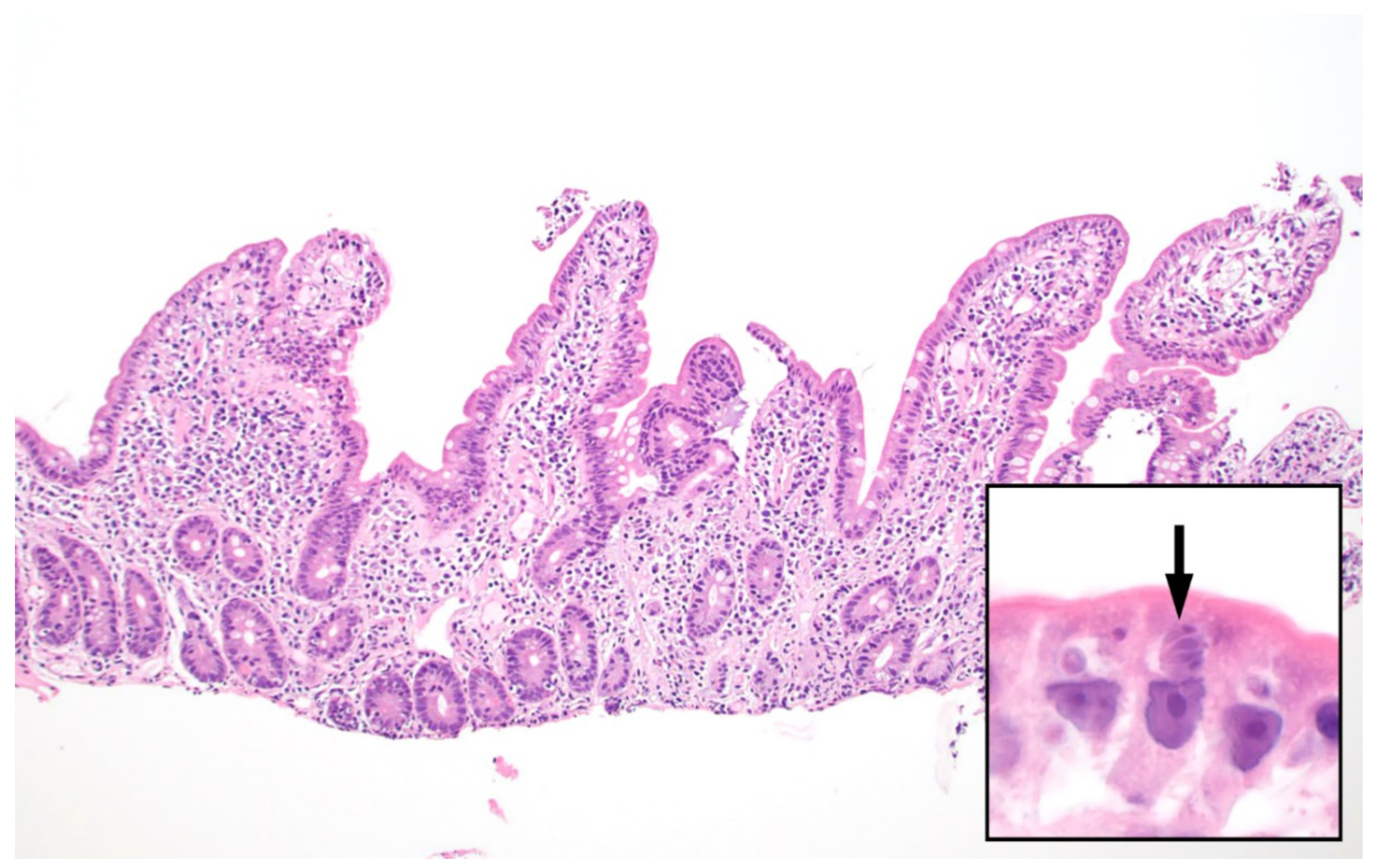
| Diagnostic Method | Advantages | Disadvantages |
|---|---|---|
| Stool Microscopy | ||
| Direct wet mount | Fast, inexpensive; simultaneous detection of other intestinal parasites | Lack of sensitivity without concentration step; lack of defined morphologic features might make detection difficult for microscopists |
| Concentrated wet mount | Fast, inexpensive; simultaneous detection of other intestinal parasites | Lack of defined morphologic features might make detection difficult for microscopists |
| Differential Interference Contrast (DIC) | Increased sensitivity by highlighting internal structures | Not routinely available in many diagnostic labs |
| Ultraviolet autofluorescence | More sensitive than permanent smears; simultaneous detection of other coccidian oocysts and several helminth eggs | Requires specific UV filters that may not be routinely present in diagnostic labs |
| Lacto-phenol cotton blue | Fast, inexpensive; may be advantageous in resource-poor areas where acid-fast staining is not available | Non-specific; likely false positives with fungal elements |
| Trichrome/iron hematoxylin stain | Simultaneous detection of other intestinal protozoans | Oocysts do not stain with trichrome |
| Modified Ziehl-Neelsen (ZN) stain | Increased sensitivity over traditional O&P exams | Inconsistent staining of oocysts |
| Kinyoun’s modified acid-fast (MAF) stain | Increased sensitivity over traditional O&P exams | Inconsistent staining of oocysts |
| Modified safranin | More consistent staining of oocysts over ZN and MAF | Requires heating of stain |
| Auramine O (auramine-phenol) | More sensitive than traditional O&P exams | May be less sensitive than MAF, ZN; requires fluorescent microscope |
| Histopathology | ||
| Hematoxylin-and-eosin (H&E), periodic acid Schiff (PAS) | Identify multiple developmental stages of C. cayetanensis | Not routinely ordered for C. cayetanensis; may be difficult to distinguish from Cystoisospora belli |
| Ziehl–Neelsen stain, Fite’s acid-fast stain | Can detect oocysts in tissues | Pre-oocyst stages may not stain |
| Assay | Manufacturer (Location) | Parasite and Microsporidial Targets | Approval * |
|---|---|---|---|
| BioFire® FilmArray® Gastrointestinal (GI) Panel | Biomérieux (Lyon, France) | Cyclospora cayetanensis, Cryptosporidium spp., Giardia duodenalis, Entamoeba histolytica | FDA, CE |
| Allplex™ Gastrointestinal Panel | Seegene (Seoul, South Korea) | C. cayetanensis, Blastocystis spp., Cryptosporidium spp., Dientamoeba fragilis, E. histolytica, and G. duodenalis | CE |
| QIAstat-Dx® Gastrointestinal Panel | Qiagen (Hilden, Germany) | Cyclospora cayetanensis, Cryptosporidium spp., Giardia duodenalis, Entamoeba histolytica | CE |
| EasyScreen™ Enteric Protozoan Extended Detection Kit | Genetic Signatures (Newtown, Australia) | C. cayetanensis, Blastocystis spp., Cryptosporidium spp., Dientamoeba fragilis, E. histolytica, and G. duodenalis, Enterocytozoon bieneusi, Encephalitozoon intestinalis | CE |
| Novodiag® Stool Parasites | Mobidiag (Espoo, Finland) | Ancylostoma duodenale, Ascaris lumbricoides, Balantioides coli, Blastocystis spp., Clonorchis/Opisthorchis/Metorchis, Cryptosporidium spp., C. cayetanensis, Cystoisospora belli, D. fragilis, Dibothriocephalus spp., Encephalitozoon spp., E. histolytica, Enterobius vermicularis, E. bieneusi, Fasciola spp., Fasciolopsis buski, G. duodenalis, Hymenolepis diminuta, H. nana, Necator americanus, Schistosoma mansoni, Schistosoma spp., Strongyloides stercoralis, Taenia saginata/suihominis (=asiatica), T. solium, Trichuris spp. | CE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathison, B.A.; Pritt, B.S. Cyclosporiasis—Updates on Clinical Presentation, Pathology, Clinical Diagnosis, and Treatment. Microorganisms 2021, 9, 1863. https://doi.org/10.3390/microorganisms9091863
Mathison BA, Pritt BS. Cyclosporiasis—Updates on Clinical Presentation, Pathology, Clinical Diagnosis, and Treatment. Microorganisms. 2021; 9(9):1863. https://doi.org/10.3390/microorganisms9091863
Chicago/Turabian StyleMathison, Blaine A., and Bobbi S. Pritt. 2021. "Cyclosporiasis—Updates on Clinical Presentation, Pathology, Clinical Diagnosis, and Treatment" Microorganisms 9, no. 9: 1863. https://doi.org/10.3390/microorganisms9091863
APA StyleMathison, B. A., & Pritt, B. S. (2021). Cyclosporiasis—Updates on Clinical Presentation, Pathology, Clinical Diagnosis, and Treatment. Microorganisms, 9(9), 1863. https://doi.org/10.3390/microorganisms9091863






