Epidemiological Surveillance of Lyme Borreliosis in Bavaria, Germany, 2013–2020
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Definition of Variables
2.3. Statistical Analysis
2.4. LYDI Sentinel Network
2.5. Ethics
3. Results
3.1. Mandatory Notification
3.1.1. Burden of Disease
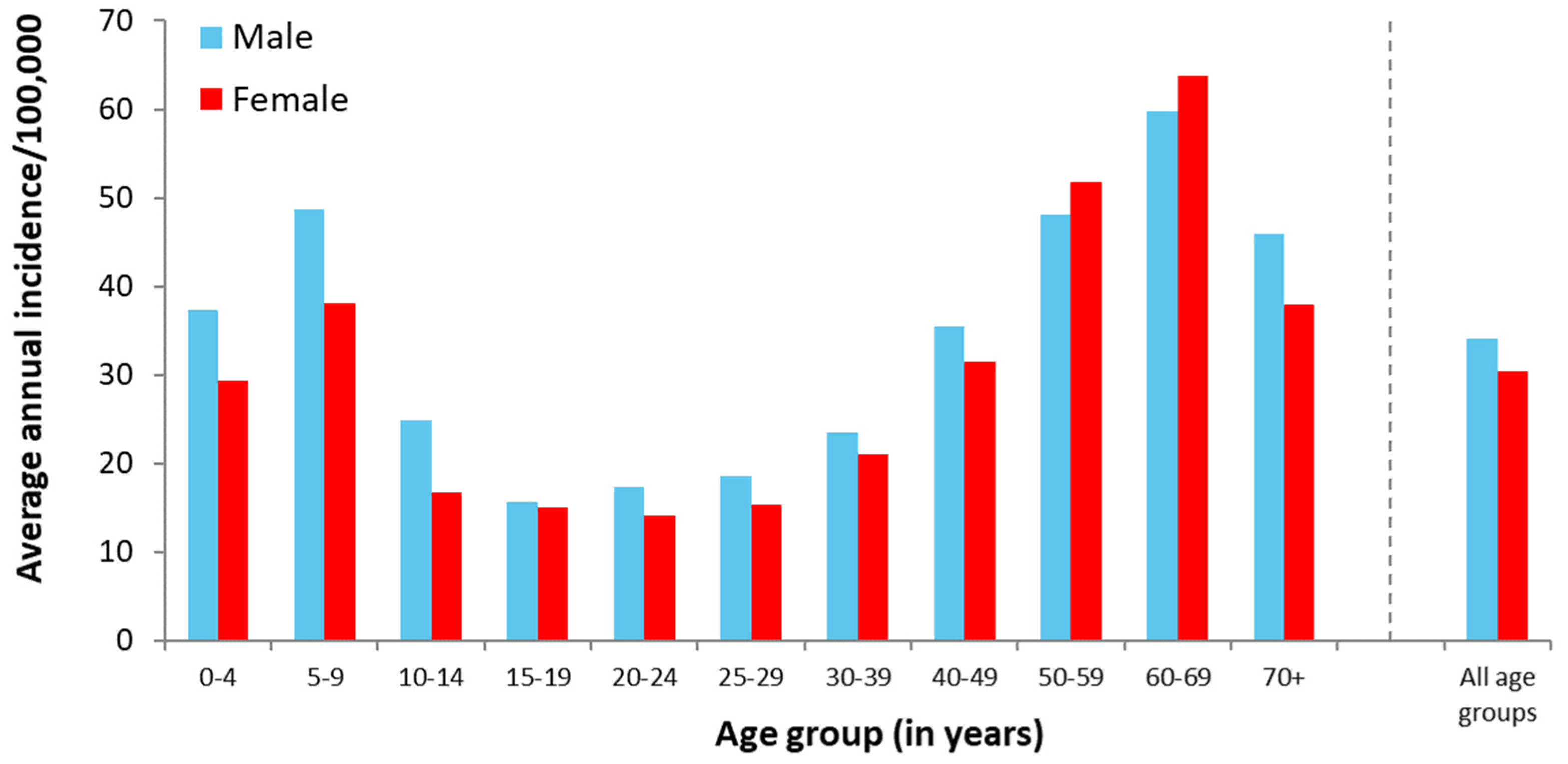
3.1.2. Demographic Aspects
3.1.3. Seasonality
3.1.4. Clinical Aspects
3.1.5. Geographic Aspects/Risk Areas
3.2. LYDI Sentinel Network
4. Discussion
4.1. Regional Distribution of LB
4.2. Demographic Aspects
4.3. Clinical Aspects
4.4. Seasonality
4.5. LB in Times of COVID-19
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Rizzoli, A.; Hauffe, H.; Carpi, G.; Vourc, H.G.; Neteler, M.; Rosa, R. Lyme borreliosis in Europe. Eurosurveillance 2011, 16, 19906. [Google Scholar] [CrossRef]
- Rauter, C.; Hartung, T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: A metaanalysis. Appl. Environ. Microbiol. 2005, 71, 7203–7216. [Google Scholar] [CrossRef] [Green Version]
- Wilske, B.; Steinhuber, R.; Bergmeister, H.; Fingerle, V.; Schierz, G.; Preac-Mursic, V.; Vanek, E.; Lorbeer, B. Lyme borreliosis in South Germany. Epidemiologic data on the incidence of cases and on the epidemiology of ticks (Ixodes ricinus) carrying Borrelia burgdorferi. Dtsch. Med. Wochenschr. 1987, 112, 1730–1736. [Google Scholar] [CrossRef]
- Michel, H.; Wilske, B.; Hettche, G.; Gottner, G.; Heimerl, C.; Reischl, U.; Schulte-Spechtel, U.; Fingerle, V. An ospA-polymerase chain reaction/restriction fragment length polymorphism-based method for sensitive detection and reliable differentiation of all European Borrelia burgdorferi sensu lato species and OspA types. Med. Microbiol. Immunol. 2004, 193, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Reimer, B.; Erbas, B.; Lobbichler, K.; Truckenbrodt, R.; Gartner-Kothe, U.; Kapeller, N.; Hansen, M.; Fingerle, V.; Wilske, B.; von Sonnenburg, F. Seroprevalence of Borrelia infection in occupational tick-exposed people in Bavaria (Germany). Int. J. Med. Microbiol. 2002, 291 (Suppl. 33), 215. [Google Scholar] [CrossRef]
- Raileanu, C.; Silaghi, C.; Fingerle, V.; Margos, G.; Thiel, C.; Pfister, K.; Overzier, E. Borrelia burgdorferi Sensu Lato in Questing and Engorged Ticks from Different Habitat Types in Southern Germany. Microorganisms 2021, 9, 1266. [Google Scholar] [CrossRef]
- Dehnert, M.; Fingerle, V.; Klier, C.; Talaska, T.; Schlaud, M.; Krause, G.; Wilking, H.; Poggensee, G. Seropositivity of Lyme borreliosis and associated risk factors: A population-based study in Children and Adolescents in Germany (KiGGS). PLoS ONE 2012, 7, e41321. [Google Scholar] [CrossRef] [PubMed]
- Wilking, H.; Fingerle, V.; Klier, C.; Thamm, M.; Stark, K. Antibodies against Borrelia burgdorferi sensu lato among Adults, Germany, 2008–2011. Emerg. Infect. Dis. 2015, 21, 107–110. [Google Scholar] [CrossRef]
- Stanek, G.; Fingerle, V.; Hunfeld, K.P.; Jaulhac, B.; Kaiser, R.; Krause, A.; Kristoferitsch, W.; O’Connell, S.; Ornstein, K.; Strle, F.; et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 2011, 17, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, H.; Fingerle, V.; Hunfeld, K.P.; Huppertz, H.I.; Krause, A.; Rauer, S.; Ruf, B.; Consensus Group. Cutaneous Lyme borreliosis: Guideline of the German Dermatology Society. Ger. Med. Sci. 2017, 15, Doc14. [Google Scholar] [CrossRef]
- Strle, F.; Lusa, L.; Ruzic-Sabljic, E.; Maraspin, V.; Lotric Furlan, S.; Cimperman, J.; Ogrinc, K.; Rojko, T.; Videcnik Zorman, J.; Stupica, D. Clinical characteristics associated with Borrelia burgdorferi sensu lato skin culture results in patients with erythema migrans. PLoS ONE 2013, 8, e82132. [Google Scholar] [CrossRef] [PubMed]
- Strle, F.; Stanek, G. Clinical manifestations and diagnosis of lyme borreliosis. Curr. Probl. Dermatol. 2009, 37, 51–110. [Google Scholar] [CrossRef]
- Rauer, S.; Kastenbauer, S.; Hofmann, H.; Fingerle, V.; Huppertz, H.I.; Hunfeld, K.P.; Krause, A.; Ruf, B.; Dersch, R.; Consensus Group. Guidelines for diagnosis and treatment in neurology—Lyme neuroborreliosis. Ger. Med. Sci. 2020, 18, Doc03. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, Y. Treatment and prevention of Lyme disease. Curr. Probl. Dermatol. 2009, 37, 111–129. [Google Scholar] [CrossRef]
- Koedel, U.; Fingerle, V.; Pfister, H.W. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat. Rev. Neurol. 2015, 11, 446–456. [Google Scholar] [CrossRef]
- Fingerle, V.; Schulte-Spechtel, U.C.; Ruzic-Sabljic, E.; Leonhard, S.; Hofmann, H.; Weber, K.; Pfister, K.; Strle, F.; Wilske, B. Epidemiological aspects and molecular characterization of Borrelia burgdorferi s.l. from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int. J. Med. Microbiol. 2008, 298, 279–290. [Google Scholar] [CrossRef]
- Vazquez, M.; Muehlenbein, C.; Cartter, M.; Hayes, E.B.; Ertel, S.; Shapiro, E.D. Effectiveness of personal protective measures to prevent Lyme disease. Emerg. Infect. Dis. 2008, 14, 210–216. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Factsheet for Health Professionals—Lyme Borreliosis. Available online: https://ecdc.europa.eu/en/publications-data/factsheet-lyme-borreliosis-healthcare-professionals (accessed on 11 July 2017).
- Bavarian State Office for Statistics. Demographic Statistics 2015. Available online: https://www.statistik.bayern.de/statistik/kreise/ (accessed on 5 April 2017).
- Fingerle, V.; Hautmann, W.; Liebl, B.; Nennstiel-Ratzel, U.; Wildner, M.; Zapf, A. Implementation of Mandatory Notification of Lyme Borreliosis in Bavaria. Bayerisches Ärzteblatt 2013, 68, 96–97. [Google Scholar]
- Heinzinger, S.; Böhmer, M.M.; Belting, A.; Liebl, B.; Wildner, M.; Sing, A.; Fingerle, V. LYDI-Sentinel and Mandatory Notification—Surveillance of Lyme Borreliosis in Bavaria, 2013–2016. Bayer. Ärzteblatt 2017, 72, 300–303. [Google Scholar]
- ECDC. ECDC Comment: European Commission Updates Communicable Disease Surveillance List—Lyme Neuroborreliosis Now under EU/EEA Surveillance. Available online: https://www.ecdc.europa.eu/en/news-events/ecdc-comment-european-commission-updates-communicable-disease-surveillance-list-lyme (accessed on 27 August 2021).
- Enkelmann, J.; Bohmer, M.; Fingerle, V.; Siffczyk, C.; Werber, D.; Littmann, M.; Merbecks, S.S.; Helmeke, C.; Schroeder, S.; Hell, S.; et al. Incidence of notified Lyme borreliosis in Germany, 2013–2017. Sci. Rep. 2018, 8, 14976. [Google Scholar] [CrossRef] [PubMed]
- Wilking, H.; Stark, K. Trends in surveillance data of human Lyme borreliosis from six federal states in eastern Germany, 2009–2012. Ticks Tick Borne Dis. 2014, 5, 219–224. [Google Scholar] [CrossRef]
- Fülöp, B.; Poggensee, G. Epidemiological situation of Lyme borreliosis in germany: Surveillance data from six Eastern German States, 2002 to 2006. Parasitol. Res. 2008, 103 (Suppl. 1), S117–S120. [Google Scholar] [CrossRef]
- Septfons, A.; Goronflot, T.; Jaulhac, B.; Roussel, V.; De Martino, S.; Guerreiro, S.; Launay, T.; Fournier, L.; De Valk, H.; Figoni, J.; et al. Epidemiology of Lyme borreliosis through two surveillance systems: The national Sentinelles GP network and the national hospital discharge database, France, 2005 to 2016. Eurosurveillance 2019, 24, 1800134. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.A.; Saha, S.; Kugeler, K.J.; Delorey, M.J.; Shankar, M.B.; Hinckley, A.F.; Mead, P.S. Incidence of Clinician-Diagnosed Lyme Disease, United States, 2005–2010. Emerg. Infect. Dis. 2015, 21, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Berglund, J.; Eitrem, R.; Ornstein, K.; Lindberg, A.; Ringer, A.; Elmrud, H.; Carlsson, M.; Runehagen, A.; Svanborg, C.; Norrby, R. An epidemiologic study of Lyme disease in southern Sweden. N. Engl. J. Med. 1995, 333, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.M.; Hinckley, A.F.; Mead, P.S.; Hook, S.A.; Kugeler, K.J. Surveillance for Lyme Disease—United States, 2008–2015. MMWR Surveill. Summ. 2017, 66, 1–12. [Google Scholar] [CrossRef]
- Lindgren, E.; Talleklint, L.; Polfeldt, T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 2000, 108, 119–123. [Google Scholar] [CrossRef]
- Medlock, J.M.; Leach, S.A. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect. Dis. 2015, 15, 721–730. [Google Scholar] [CrossRef]
- Randolph, S.E. Evidence that climate change has caused ‘emergence’ of tick-borne diseases in Europe? Int. J. Med. Microbiol. 2004, 293 (Suppl. 37), 5–15. [Google Scholar] [CrossRef]
- Dobler, G.; Chitimia-Dobler, L.; High Tick Activity in Southern Germany (ProMED No. 20200627.7517303). Available online: https://promedmail.org (accessed on 27 June 2020).
- Wormser, G.P.; Jacobson, E.; Shanker, E.M. Negative impact of the COVID-19 pandemic on the timely diagnosis of tick-borne infections. Diagn. Microbiol. Infect. Dis. 2021, 99, 115226. [Google Scholar] [CrossRef] [PubMed]
- Federal Association for Statutory Health Insurance Physicians (KBV). More and More Practices are Using Cameras—Number of Video Consultations Has Risen to over One Million. Available online: https://www.kbv.de/html/1150_50419.php (accessed on 27 August 2021).
- Kuehn, B.M. CDC estimates 300,000 US cases of Lyme disease annually. JAMA 2013, 310, 1110. [Google Scholar] [CrossRef] [PubMed]



| Diagnosis | 2013 (Mar–Dec) | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2013–2020 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Lyme borreliosis (LB) | 5703 | 100 | 3162 | 100 | 2974 | 100 | 4596 | 100 | 3537 | 100 | 5014 | 100 | 4255 | 100 | 6217 | 100 | 35,458 | 100 |
| Erythema migrans (EM) | 5593 | 98.1 # | 2980 | 94.2 # | 2,825 | 95.0 # | 4395 | 95.6 # | 3393 | 95.9 # | 4869 | 97.1 # | 4120 | 96.8 # | 6108 | 98.2 # | 34,283 | 96.7 |
| Acute neuroborreliosis (NB) thereof | 64 | 1.1 # | 66 | 2.1 # | 71 | 2.4 # | 101 | 2.2# | 68 | 1.9 # | 81 | 1.6 # | 82 | 1.9 # | 82 | 1.3 # | 615 | 1.7 |
| Radiculoneuritis | 29 | 45.3 * | 18 | 27.3 * | 31 | 43.7 * | 37 | 36.6 * | 21 | 30.9 * | 43 | 53.1 * | 40 | 48.8 * | 27 | 32.9 * | 246 | 40.0 |
| Meningitis | 15 | 23.4 * | 16 | 24.2 * | 21 | 29.6 * | 31 | 30.7 * | 15 | 22.1 * | 15 | 18.5 * | 21 | 25.6 * | 30 | 36.6 * | 164 | 26.7 |
| Neuritis cranialis | 34 | 53.1 * | 45 | 68.2 * | 42 | 59.2 * | 58 | 57.4 * | 44 | 64.7 * | 50 | 61.7 * | 45 | 54.9 * | 49 | 59.8 * | 367 | 59.7 |
| Lyme arthritis (LA) | 50 | 0.9 # | 125 | 4.0 # | 87 | 2.9 # | 111 | 2.4 # | 84 | 2.4 # | 75 | 1.5 # | 69 | 1.6 # | 32 | 0.5 # | 633 | 1.8 |
| EM and NB + | 2 | - | 4 | - | 7 | - | 6 | - | 3 | - | 7 | - | 12 | - | 4 | - | 45 | - |
| EM and LA + | 2 | - | 5 | - | 2 | - | 5 | - | 5 | - | 4 | - | 4 | - | 1 | - | 28 | - |
| Lyme Arthritis OR (95% CI) | Radiculoneuritis OR (95% CI) | Meningitis OR (95% CI) | Neuritis Cranialis OR (95% CI) | |
|---|---|---|---|---|
| Sex | ||||
| Female | Ref. | Ref. | Ref. | Ref. |
| Male | 1.36 (1.16–1.60) ** | 1.84 (1.42–2.38) ** | 1.00 (0.73–1.37) | 2.07 (1.67–2.57) ** |
| Age group 1 | ||||
| All other age groups | Ref. | Ref. | Ref. | Ref. |
| 5–9 years | 0.83 (0.56–1.21) | 0.15 (0.04–0.58) ** | 3.40 (2.23–5.18) ** | 4.63 (3.58–6.01) ** |
| Age group 2 | ||||
| All other age groups | Ref. | Ref. | Ref. | Ref. |
| Age 60–69 years | 0.88 (0.72–1.08) | 1.62 (1.22–2.14) * | 0.71 (0.46–1.09) | 0.63 (0.47–0.85) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böhmer, M.M.; Ens, K.; Böhm, S.; Heinzinger, S.; Fingerle, V. Epidemiological Surveillance of Lyme Borreliosis in Bavaria, Germany, 2013–2020. Microorganisms 2021, 9, 1872. https://doi.org/10.3390/microorganisms9091872
Böhmer MM, Ens K, Böhm S, Heinzinger S, Fingerle V. Epidemiological Surveillance of Lyme Borreliosis in Bavaria, Germany, 2013–2020. Microorganisms. 2021; 9(9):1872. https://doi.org/10.3390/microorganisms9091872
Chicago/Turabian StyleBöhmer, Merle Margarete, Katharina Ens, Stefanie Böhm, Susanne Heinzinger, and Volker Fingerle. 2021. "Epidemiological Surveillance of Lyme Borreliosis in Bavaria, Germany, 2013–2020" Microorganisms 9, no. 9: 1872. https://doi.org/10.3390/microorganisms9091872
APA StyleBöhmer, M. M., Ens, K., Böhm, S., Heinzinger, S., & Fingerle, V. (2021). Epidemiological Surveillance of Lyme Borreliosis in Bavaria, Germany, 2013–2020. Microorganisms, 9(9), 1872. https://doi.org/10.3390/microorganisms9091872






