Crimean-Congo Hemorrhagic Fever Virus in Asia, Africa and Europe
Abstract
:1. Introduction
2. Etiological Agent and Biology
3. CCHFV Genome
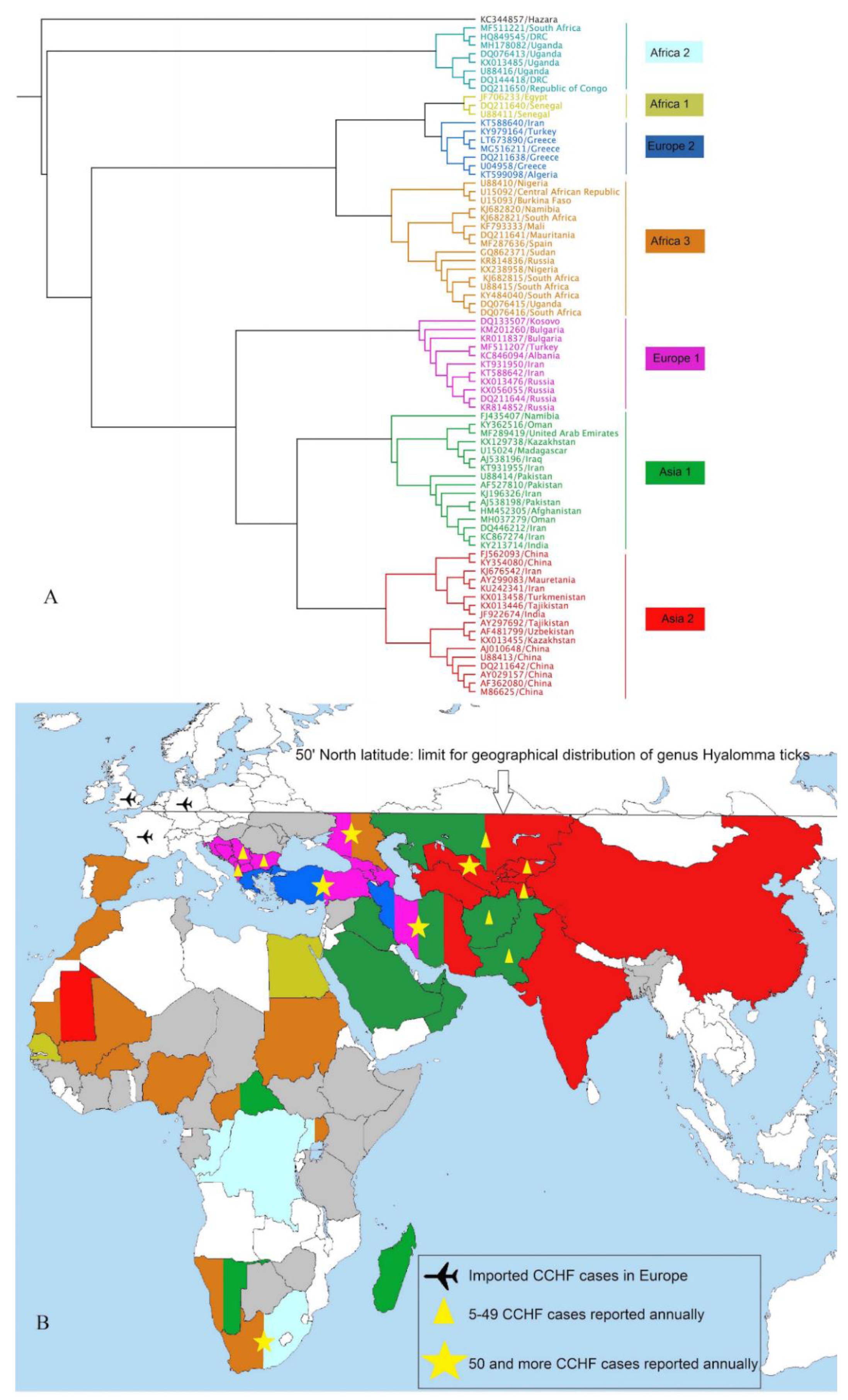
4. Worldwide Burden and Phylogenetics of CCHFV
4.1. Trends in Asia
4.1.1. Iran
4.1.2. Afghanistan
4.1.3. Pakistan
4.1.4. Iraq
4.1.5. United Arab Emirates
4.1.6. Kuwait
4.1.7. Oman
4.1.8. Saudi Arabia
4.1.9. China
4.1.10. Tajikistan
4.1.11. India
4.1.12. Turkey
4.1.13. Georgia
4.1.14. Russia
4.2. Trends in Africa
4.2.1. South Africa
4.2.2. Egypt
4.2.3. Senegal
4.2.4. Mauritania
4.2.5. Kenya
4.2.6. Sudan
4.2.7. Madagascar
4.2.8. Niger
4.2.9. Nigeria
4.2.10. Ghana
4.2.11. Uganda
4.3. Trends in Europe
4.3.1. Albania
4.3.2. Bulgaria
4.3.3. Greece
4.3.4. Kosovo
4.3.5. Spain
4.3.6. Imported Cases to Europe
5. Zoonotic Maintenance of CCHFV
5.1. Vectors
5.2. Vertebrate Hosts
5.3. Transmission Modes and Environmental Amplification
6. Factors Affecting the Range Expansion and Introduction of CCHFV
7. Prevention and Control
8. Outbreak Control and Response
9. Vaccine Option
10. Future Forecast
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elevli, M.; Ozkul, A.A.; Civilibal, M.; Midilli, K.; Gargili, A.; Duru, N.S. A newly identified Crimean-Congo hemorrhagic fever virus strain in Turkey. Int. J. Infect. Dis. 2010, 14, e213–e216. [Google Scholar] [CrossRef] [Green Version]
- Tahmasebi, F.; Ghiasi, S.; Mostafavi, E.; Moradi, M.; Piazak, N.; Mozafari, A.; Haeri, A.; Fooks, A.; Chinikar, S. Molecular epidemiology of Crimean-Congo hemorrhagic fever virus genome isolated from ticks of Hamadan province of Iran. J. Vector Borne Dis. 2010, 47, 211–216. [Google Scholar]
- Chinikar, S.; Shayesteh, M.; Khakifirouz, S.; Jalali, T.; Varaie, F.S.R.; Rafigh, M.; Mostafavi, E.; Shahhosseini, N. Nosocomial infection of Crimean–Congo haemorrhagic fever in eastern Iran: Case report. Travel Med. Infect. Dis. 2013, 11, 252–255. [Google Scholar] [CrossRef]
- Whitehouse, C.A. Crimean–Congo hemorrhagic fever. Antivir. Res. 2004, 64, 145–160. [Google Scholar] [CrossRef]
- Baron, M.; Holzer, B. Nairobi sheep disease virus/Ganjam virus. Rev. Sci. Tech.-Off. Int. Des Épizooties 2015, 34, 411–417. [Google Scholar] [CrossRef]
- Marriott, A.C.; Nuttall, P.A. Comparison of the S RNA segments and nucleoprotein sequences of Crimean-Congo hemorrhagic fever, Hazara, and Dugbe viruses. Virology 1992, 189, 795–799. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Wang, X.; Dong, H.; Ma, C.; Wang, J.; Liu, B.; Mao, Y.; Wang, Y.; Li, T. Structural and functional diversity of nairovirus-encoded nucleoproteins. J. Virol. 2015, 89, 11740. [Google Scholar] [CrossRef] [Green Version]
- Walker, P.J.; Widen, S.G.; Wood, T.G.; Guzman, H.; Tesh, R.B.; Vasilakis, N. A global genomic characterization of nairoviruses identifies nine discrete genogroups with distinctive structural characteristics and host-vector associations. Am. J. Trop. Med. Hyg. 2016, 94, 1107. [Google Scholar] [CrossRef] [Green Version]
- Bertolotti-Ciarlet, A.; Smith, J.; Strecker, K.; Paragas, J.; Altamura, L.A.; McFalls, J.M.; Frias-Stäheli, N.; García-Sastre, A.; Schmaljohn, C.S.; Doms, R.W. Cellular localization and antigenic characterization of Crimean-Congo hemorrhagic fever virus glycoproteins. J. Virol. 2005, 79, 6152–6161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, B.R.; Deyde, V.; Sanchez, A.J.; Vincent, M.J.; Nichol, S.T. N-linked glycosylation of Gn (but not Gc) is important for Crimean Congo hemorrhagic fever virus glycoprotein localization and transport. Virology 2007, 361, 348–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, R. Emerging viruses: The Bunyaviridae. Mol. Med. 1997, 3, 572. [Google Scholar] [CrossRef]
- Hewson, R.; Gmyl, A.; Gmyl, L.; Smirnova, S.E.; Karganova, G.; Jamil, B.; Hasan, R.; Chamberlain, J.; Clegg, C. Evidence of segment reassortment in Crimean-Congo haemorrhagic fever virus. J. Gen. Virol. 2004, 85, 3059–3070. [Google Scholar] [CrossRef]
- Lukashev, A.N. Evidence for recombination in Crimean-Congo hemorrhagic fever virus. J. Gen. Virol. 2005, 86, 2333–2338. [Google Scholar] [CrossRef]
- Chinikar, S.; Shahhosseini, N.; Bouzari, S.; Shokrgozar, M.A.; Mostafavi, E.; Jalali, T.; Khakifirouz, S.; Groschup, M.H.; Niedrig, M. Assessment of recombination in the S-segment genome of Crimean-Congo hemorrhagic fever virus in Iran. J. Arthropod-Borne Dis. 2016, 10, 12. [Google Scholar]
- Schmaljohn, C.; Nichol, S. Bunyaviridae. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 1741–1789. [Google Scholar]
- Tezer, H.; Polat, M. Diagnosis of Crimean-Congo hemorrhagic fever. Expert Rev. Anti-Infect. Ther. 2015, 13, 555–566. [Google Scholar] [CrossRef]
- Dai, S.; Wu, Q.; Wu, X.; Peng, C.; Liu, J.; Tang, S.; Zhang, T.; Deng, F.; Shen, S. Differential Cell Line Susceptibility to Crimean-Congo hemorrhagic fever virus. Front. Cell. Infect. Microbiol. 2021, 11, 236. [Google Scholar] [CrossRef]
- Shepherd, A.; Swanepoel, R.; Leman, P.; Shepherd, S. Comparison of methods for isolation and titration of Crimean-Congo hemorrhagic fever virus. J. Clin. Microbiol. 1986, 24, 654–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogstraal, H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 1979, 15, 307–417. [Google Scholar] [CrossRef]
- Al-Abri, S.S.; Al Abaidani, I.; Fazlalipour, M.; Mostafavi, E.; Leblebicioglu, H.; Pshenichnaya, N.; Memish, Z.A.; Hewson, R.; Petersen, E.; Mala, P. Current status of Crimean-Congo haemorrhagic fever in the World Health Organization Eastern Mediterranean Region: Issues, challenges, and future directions. Int. J. Infect. Dis. 2017, 58, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temur, A.I.; Kuhn, J.H.; Pecor, D.B.; Apanaskevich, D.A.; Keshtkar-Jahromi, M. Epidemiology of Crimean-Congo hemorrhagic fever (CCHF) in Africa-Underestimated for decades. Am. J. Trop. Med. Hyg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.; Andonova, L.; Andraghetti, R.; Bouloy, M.; Ergonul, O.; Jongejan, F.; Kalvatchev, N.; Nichol, S.; Niedrig, M.; Platonov, A. Crimean-Congo hemorrhagic fever in Europe: Current situation calls for preparedness. Eurosurveillance 2010, 15, 19504. [Google Scholar] [CrossRef]
- Bente, D.A.; Forrester, N.L.; Watts, D.M.; McAuley, A.J.; Whitehouse, C.A.; Bray, M. Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir. Res. 2013, 100, 159–189. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Beck, A.S.; Gargili, A.; Forrester, N.; Barrett, A.D.; Bente, D.A. Transstadial transmission and long-term association of Crimean-Congo hemorrhagic fever virus in ticks shapes genome plasticity. Sci. Rep. 2016, 6, 35819. [Google Scholar] [CrossRef] [Green Version]
- Hua, B.L.; Scholte, F.E.; Ohlendorf, V.; Kopp, A.; Marklewitz, M.; Drosten, C.; Nichol, S.T.; Spiropoulou, C.; Junglen, S.; Bergeron, É. A single mutation in Crimean-Congo hemorrhagic fever virus discovered in ticks impairs infectivity in human cells. Elife 2020, 9, e50999. [Google Scholar] [CrossRef]
- Biglari, P.; Chinikar, S.; Belqeiszadeh, H.; Telmadarraiy, Z.; Mostafavi, E.; Ghaffari, M.; Javaherizadeh, S.; Nowotny, N.; Fooks, A.R.; Shahhosseini, N. Phylogeny of tick-derived Crimean-Congo hemorrhagic fever virus strains in Iran. Ticks Tick-Borne Dis. 2016, 7, 1216–1221. [Google Scholar] [CrossRef]
- Chinikar, S.; Ghiasi, S.; Hewson, R.; Moradi, M.; Haeri, A. Crimean-Congo hemorrhagic fever in Iran and neighboring countries. J. Clin. Virol. 2010, 47, 110–114. [Google Scholar] [CrossRef]
- Chinikar, S.; Ghiasi, S.M.; Naddaf, S.; Piazak, N.; Moradi, M.; Razavi, M.R.; Afzali, N.; Haeri, A.; Mostafavizadeh, K.; Ataei, B. Serological evaluation of Crimean-Congo hemorrhagic fever in humans with high-risk professions living in enzootic regions of Isfahan Province of Iran and genetic analysis of circulating strains. Vector Borne Zoonotic Dis. 2012, 12, 733–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinikar, S.; Shahhosseini, N.; Khakifirouz, S.; Varaie, F.; Rafigh, M.; Jalali, T.; Hasanzehi, A. Crimean Congo haemorrhagic fever as an infectious virus in Iran, an epidemiology approach. Int. J. Med. Microbiol. 2012, 302, 85. [Google Scholar]
- Chinikar, S.; Moghadam, A.H.; Parizadeh, S.J.; Moradi, M.; Bayat, N.; Zeinali, M.; Mostafavi, E. Seroepidemiology of Crimean Congo hemorrhagic fever in slaughterhouse workers in north eastern Iran. Iran. J. Public Health 2012, 41, 72. [Google Scholar] [PubMed]
- Chinikar, S.; Shahhosseini, N.; Khakifirouz, S.; Rafigh, M.; Hasanzehi, A. Serological and molecular evaluation of Crimean-Congo haemorrhagic fever in Iranian probable patients. Int. J. Infect. Dis. 2012, 16, e250. [Google Scholar] [CrossRef] [Green Version]
- Chinikar, S.; Shahhosseini, N.; Bouzari, S.; Jalali, T.; Shokrgozar, M.A.; Mostafavi, E. New circulating genomic variant of Crimean-Congo hemorrhagic fever virus in Iran. Arch. Virol. 2013, 158, 1085–1088. [Google Scholar] [CrossRef]
- Kayedi, M.H.; Chinikar, S.; Mostafavi, E.; Khakifirouz, S.; Jalali, T.; Hosseini-Chegeni, A.; Naghizadeh, A.; Niedrig, M.; Fooks, A.R.; Shahhosseini, N. Crimean–Congo hemorrhagic fever virus clade iv (Asia 1) in ticks of western Iran. J. Med. Entomol. 2015, 52, 1144–1149. [Google Scholar] [CrossRef]
- Chinikar, S.; Bouzari, S.; Shokrgozar, M.A.; Mostafavi, E.; Jalali, T.; Khakifirouz, S.; Nowotny, N.; Fooks, A.R.; Shahhosseini, N. Genetic diversity of Crimean Congo hemorrhagic fever virus strains from Iran. J. Arthropod-Borne Dis. 2016, 10, 127. [Google Scholar]
- Shahhosseini, N.; Chinikar, S.; Shams, E.; Nowotny, N.; Fooks, A.R. Crimean-Congo hemorrhagic fever cases in the North of Iran have three distinct origins. Virusdisease 2017, 28, 50–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahhosseini, N.; Jafarbekloo, A.; Telmadarraiy, Z.; Chinikar, S.; Haeri, A.; Nowotny, N.; Groschup, M.H.; Fooks, A.R.; Faghihi, F. Co-circulation of Crimean-Congo hemorrhagic fever virus strains Asia 1 and 2 between the border of Iran and Pakistan. Heliyon 2017, 3, e00439. [Google Scholar] [CrossRef] [PubMed]
- Chinikar, S.; Shahhosseini, N. Phylogenetic analysis on emerging Arboviruses in Iran. Int. J. Infect. Dis. 2016, 53, 160. [Google Scholar] [CrossRef] [Green Version]
- Mofleh, J.; Ahmad, A. Crimean-Congo haemorrhagic fever outbreak investigation in the western region of Afghanistan in 2008. EMHJ-East. Mediterr. Health J. 2012, 18, 522–526. [Google Scholar] [CrossRef]
- Mustafa, M.; Leslie, T.; Mohareb, E.; Pinzon, J.; Zayed, A.; Ayazid, E.; Barthel, R.; Tucker, C.; Witt, C. A Monitoring System for Crimean Congo Hemorrhagic Fever Epidemiology Studies in Afghanistan. 2011. Available online: https://core.ac.uk/reader/195381660 (accessed on 7 September 2021).
- Mustafa, M.L.; Ayazi, E.; Mohareb, E.; Yingst, S.; Zayed, A.; Rossi, C.A.; Schoepp, R.J.; Mofleh, J.; Fiekert, K.; Akhbarian, Z. Crimean-Congo hemorrhagic fever, Afghanistan. Emerg. Infecioust Dis. 2009, 17, 1940–1941. [Google Scholar] [CrossRef]
- Qaderi, S.; Mardani, M.; Shah, A.; Shah, J.; Bazgir, N.; Sayad, J.; Ghandchi, E.; Samsami, M.; Bagherpour, J.Z. Crimean-Congo hemorrhagic fever (CCHF) in Afghanistan: A retrospective single center study. Int. J. Infect. Dis. 2021, 103, 323–328. [Google Scholar] [CrossRef]
- Jamil, B.; Hasan, R.S.; Sarwari, A.R.; Burton, J.; Hewson, R.; Clegg, C. Crimean-Congo hemorrhagic fever: Experience at a tertiary care hospital in Karachi, Pakistan. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 577–584. [Google Scholar] [CrossRef]
- Athar, M.N.; Baqai, H.Z.; Ahmad, M.; Khalid, M.A.; Bashir, N.; Ahmad, A.M.; Balouch, A.H.; Bashir, K. Short report: Crimean-Congo hemorrhagic fever outbreak in Rawalpindi, Pakistan, February 2002. Am. J. Trop. Med. Hyg. 2003, 69, 284–287. [Google Scholar] [CrossRef]
- Kasi, K.K.; Sas, M.A.; Sauter-Louis, C.; von Arnim, F.; Gethmann, J.M.; Schulz, A.; Wernike, K.; Groschup, M.H.; Conraths, F.J. Epidemiological investigations of Crimean-Congo haemorrhagic fever virus infection in sheep and goats in Balochistan, Pakistan. Ticks Tick-Borne Dis. 2020, 11, 101324. [Google Scholar] [CrossRef] [PubMed]
- Pirkani, G.S.; Jogezai, E.K.; Ilyas, M. Crimean-Congo haemorrhagic fever (CCHF) in Balochistan. Prof. Med. J. 2006, 13, 464–467. [Google Scholar]
- Saleem, J.; Usman, M.; Nadeem, A.; Sethi, S.A.; Salman, M. Crimean-Congo hemorrhagic fever: A first case from Abbottabad, Pakistan. Int. J. Infect. Dis. 2009, 13, e121–e123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.A.; Khanani, M.R.; Warraich, H.J.; Hayat, A.; Ali, S.H. Crimean-Congo hemorrhagic fever in Pakistan. J. Med. Virol. 2008, 80, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Zohaib, A.; Saqib, M.; Athar, M.A.; Hussain, M.H.; Sial, A.-u.-R.; Tayyab, M.H.; Batool, M.; Sadia, H.; Taj, Z.; Tahir, U. Crimean-Congo hemorrhagic fever virus in humans and livestock, Pakistan, 2015–2017. Emerg. Infect. Dis. 2020, 26, 773. [Google Scholar] [CrossRef] [Green Version]
- Al-Tikriti, S.; Al-Ani, F.; Jurji, F.; Tantawi, H.; Al-Moslih, M.; Al-Janabi, N.; Mahmud, M.; Al-Bana, A.; Habib, H.; Al-Munthri, H. Congo/Crimean haemorrhagic fever in Iraq. Bull. World Health Organ. 1981, 59, 85. [Google Scholar]
- Tantawi, H.; Shony, M.; Al-Tikriti, S. Antibodies to Crimean-Congo haemorrhagic fever virus in domestic animals in Iraq: A seroepidemiological survey. Int. J. Zoonoses 1981, 8, 115–120. [Google Scholar]
- Majeed, B.; Dicker, R.; Nawar, A.; Badri, S.; Noah, A.; Muslem, H. Morbidity and mortality of Crimean-Congo hemorrhagic fever in Iraq: Cases reported to the National Surveillance System, 1990–2010. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 480–483. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Ibrahim, K.S.; Mohammed, M.O.; Al-Shaikhani, M.A.; Barzanji, A.A.; Saeed, S.J.; Muhiaden, S.; Bhnam, M.N. Crimean Congo hemorrhagic fever management in Erbil during 2010–2011. Eur. Sci. J. 2014, 10, 219–229. [Google Scholar]
- Schwarz, T.F.; Nsanze, H.; Longson, M.; Nitschko, H.; Gilch, S.; Shurie, H.; Ameen, A.; Zahir, A.R.M.; Acharya, U.G.; Jager, G. Polymerase chain reaction for diagnosis and identification of distinct variants of Crimean-Congo hemorrhagic fever virus in the United Arab Emirates. Am. J. Trop. Med. Hyg. 1996, 55, 190–196. [Google Scholar] [CrossRef]
- Rodriguez, L.L.; Maupin, G.O.; Ksiazek, T.G.; Rollin, P.E.; Khan, A.S.; Schwarz, T.F.; Lofts, R.S.; Smith, J.F.; Noor, A.M.; Peters, C.J. Molecular investigation of a multisource outbreak of Crimean-Congo hemorrhagic fever in the United Arab Emirates. Am. J. Trop. Med. Hyg. 1997, 57, 512–518. [Google Scholar] [CrossRef]
- Khan, A.S.; Maupin, G.O.; Rollin, P.E.; Noor, A.M.; Shurie, H.H.M.; Shalabi, A.G.A.; Wasef, S.; Haddad, Y.M.A.; Sadek, R.; Ijaz, K. An outbreak of Crimean-Congo hemorrhagic fever in the United Arab Emirates, 1994–1995. Am. J. Trop. Med. Hyg. 1997, 57, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, A.I.; Li, Y.; Uehara, A.; Hussein, N.A.; Zhang, J.; Tao, Y.; Bergeron, E.; Ibrahim, I.H.; Al Hosani, M.A.; Yusof, M.F. Identification of a novel lineage of Crimean–Congo haemorrhagic fever virus in dromedary camels, United Arab Emirates. J. Gen. Virol. 2021, 102, 001473. [Google Scholar] [CrossRef]
- Camp, J.V.; Kannan, D.O.; Osman, B.M.; Shah, M.S.; Howarth, B.; Khafaga, T.; Weidinger, P.; Karuvantevida, N.; Kolodziejek, J.; Mazrooei, H. Crimean-Congo hemorrhagic fever virus endemicity in United Arab Emirates, 2019. Emerg. Infect. Dis. 2020, 26, 1019–1021. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.V.; Weidinger, P.; Ramaswamy, S.; Kannan, D.O.; Osman, B.M.; Kolodziejek, J.; Karuvantevida, N.; Abou Tayoun, A.; Loney, T.; Nowotny, N. Association of dromedary camels and camel ticks with reassortant Crimean-Congo hemorrhagic fever virus, United Arab Emirates. Emerg. Infect. Dis. 2021, 27, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Al-Nakib, W.; Lloyd, G.; El-Mekki, A.; Platt, G.; Beeson, A.; Southee, T. Preliminary report on arbovirus-antibody prevalence among patients in Kuwait: Evidence of Congo/Crimean virus infection. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 474–476. [Google Scholar] [CrossRef]
- Williams, R.; Al-Busaidy, S.; Mehta, F.; Maupin, G.; Wagoner, K.; Al-Awaidy, S.; Suleiman, A.; Khan, A.; Peters, C.; Ksiazek, T. Crimean-Congo haemorrhagic fever: A seroepidemiological and tick survey in the Sultanate of Oman. Trop. Med. Int. Health 2000, 5, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Body, M.H.H.; Abdulmajeed, H.A.; Hammad, M.H.; Mohamed, S.A.; Saif, S.A.; Salim, A.-M.; Al-Maewaly, M.; Rajamony, S. Cross-sectional survey of Crimean-Congo hemorrhagic fever virus in the sultanate of Oman. J. Vet. Med. Anim. Health 2016, 8, 44–49. [Google Scholar]
- Al-Abri, S.S.; Hewson, R.; Al-Kindi, H.; Al-Abaidani, I.; Al-Jardani, A.; Al-Maani, A.; Almahrouqi, S.; Atkinson, B.; Al-Wahaibi, A.; Al-Rawahi, B. Clinical and molecular epidemiology of Crimean-Congo hemorrhagic fever in Oman. PLoS Negl. Trop. Dis. 2019, 13, e0007100. [Google Scholar] [CrossRef]
- El-Azazy, O.; Scrimgeour, E. Crimean-Congo haemorrhagic fever virus infection in the western province of Saudi Arabia. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 275–278. [Google Scholar] [CrossRef]
- Memish, Z.A. Infection control in Saudi Arabia: Meeting the challenge. Am. J. Infect. Control 2002, 30, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Ma, B.; Kouidou, S.; Tang, Q.; Hang, C.; Antoniadis, A. Genetic characterization of the M RNA segment of Crimean Congo hemorrhagic fever virus strains, China. Emerg. Infect. Dis. 2002, 8, 50. [Google Scholar] [CrossRef]
- Morikawa, S.; Qing, T.; Xinqin, Z.; Saijo, M.; Kurane, I. Genetic diversity of the M RNA segment among Crimean-Congo hemorrhagic fever virus isolates in China. Virology 2002, 296, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Tang, Q.; Zhao, X.; Saijo, M.; Tao, X. Serologic studies of Xinjiang hemorrhagic fever in Bachu county, 2001. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi 2002, 23, 179–181. [Google Scholar]
- Qing, T.; Prehaud, C.; Bouloy, M. Sequencing and analysis of S gene segment of XHFV. Chin. J. Microbiol. Immunol.-Bejing 1999, 19, 461–465. [Google Scholar]
- Gao, X.; Nasci, R.; Liang, G. The neglected arboviral infections in mainland China. PLoS Negl. Trop. Dis. 2010, 4, e624. [Google Scholar] [CrossRef]
- Tishkova, F.H.; Belobrova, E.A.; Valikhodzhaeva, M.; Atkinson, B.; Hewson, R.; Mullojonova, M. Crimean-Congo hemorrhagic fever in Tajikistan. Vector-Borne Zoonotic Dis. 2012, 12, 722–726. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, D.S.; Patel, K.M.; Patel, R.K.; Sen, D.J.; Patel, J.S.; Garg, C.S. Crimean-Congo hemorrhagic fever from tick-borne viral disease. Int. J. Compr. Pharm. 2011, 2, 0976–8157. [Google Scholar]
- Yadav, P.D.; Cherian, S.S.; Zawar, D.; Kokate, P.; Gunjikar, R.; Jadhav, S.; Mishra, A.C.; Mourya, D.T. Genetic characterization and molecular clock analyses of the Crimean-Congo hemorrhagic fever virus from human and ticks in India, 2010–2011. Infect. Genet. Evol. 2013, 14, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.D.; Gurav, Y.K.; Mistry, M.; Shete, A.M.; Sarkale, P.; Deoshatwar, A.R.; Unadkat, V.B.; Kokate, P.; Patil, D.Y.; Raval, D.K. Emergence of Crimean-Congo hemorrhagic fever in Amreli district of Gujarat state, India, June to July 2013. Int. J. Infect. Dis. 2014, 18, 97–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatansever, Z.; Uzun, R.; Estrada-Pena, A.; Ergonul, O. Crimean-Congo hemorrhagic fever in Turkey. In Crimean-Congo Hemorrhagic Fever; Springer: Berlin/Heidelberg, Germany, 2007; pp. 59–74. [Google Scholar]
- Yilmaz, R.; Ozcetin, M.; Erkorkmaz, U.; Ozer, S.; Ekici, F. Public knowledge and attitude toward Crimean Congo hemorrhagic fever in Tokat Turkey. Iran. J. Arthropod-Borne Dis. 2009, 3, 12. [Google Scholar]
- Estrada-Pena, A.; Zatansever, Z.; Gargili, A.; Aktas, M.; Uzun, R.; Ergonul, O.; Jongejan, F. Modeling the spatial distribution of Crimean-Congo hemorrhagic fever outbreaks in Turkey. Vector-Borne Zoonotic Dis. 2007, 7, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Karti, S.S.; Odabasi, Z.; Korten, V.; Yilmaz, M.; Sonmez, M.; Caylan, R.; Akdogan, E.; Eren, N.; Koksal, I.; Ovali, E. Crimean-Congo hemorrhagic fever in Turkey. Emerg. Infect. Dis. 2004, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Midilli, K.; Gargılı, A.; Ergonul, O.; Elevli, M.; Ergin, S.; Turan, N.; Şengöz, G.; Bakar, M. The first clinical case due to AP92 like strain of Crimean-Congo hemorrhagic fever virus and a field survey. BMC Infect. Dis. 2009, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakhashvili, K.; Tsertsvadze, N.; Chikviladze, T.; Jghenti, E.; Bekaia, M.; Kuchuloria, T.; Hepburn, M.J.; Imnadze, P.; Nanuashvili, A. Crimean-Congo hemorrhagic fever in man, Republic of Georgia, 2009. Emerg. Infect. Dis. 2010, 16, 1326. [Google Scholar] [CrossRef]
- Greiner, A.L.; Mamuchishvili, N.; Kakutia, N.; Stauffer, K.; Geleishvili, M.; Chitadze, N.; Chikviladze, T.; Zakhashvili, K.; Morgan, J.; Salyer, S.J. Crimean-Congo hemorrhagic fever knowledge, attitudes, practices, risk factors, and Seroprevalence in rural Georgian villages with known transmission in 2014. PLoS ONE 2016, 11, e0158049. [Google Scholar] [CrossRef]
- Yilmaz, G.R.; Buzgan, T.; Irmak, H.; Safran, A.; Uzun, R.; Cevik, M.A.; Torunoglu, M.A. The epidemiology of Crimean-Congo hemorrhagic fever in Turkey, 2002–2007. Int. J. Infect. Dis. 2009, 13, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Butenko, A.; Karganova, G. Crimean-Congo hemorrhagic fever in Russia and other countries of the former Soviet Union. In Crimean-Congo Hemorrhagic Fever; Springer: Berlin/Heidelberg, Germany, 2007; pp. 99–114. [Google Scholar] [CrossRef]
- Onishchenko, G.; Efremenko, V. Crimean-Congo haemorrhagic fever in southern Russia. Zhurnal Mikrobiol. Epidemiol. I Immunobiol. 2004, 4, 86. [Google Scholar]
- Lukashev, A.; Deviatkin, A. Phylodynamics of Crimean Congo hemorrhagic fever virus in South Russia. Infect. Genet. Evol. 2018, 59, 23–27. [Google Scholar] [CrossRef]
- Swanepoel, R.; Shepherd, A.; Leman, P.; Shepherd, S.; McGillivray, G.; Erasmus, M.; Searle, L.; Gill, D. Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in southern Africa. Am. J. Trop. Med. Hyg. 1987, 36, 120. [Google Scholar] [CrossRef]
- Burt, F.J.; Janusz, T.P.; Swanepoel, R. Crimean-congo hemorrhagic fever in South africa. In Crimean-Congo Hemorrhagic Fever; Springer: Berlin/Heidelberg, Germany, 2007; pp. 131–141. [Google Scholar]
- Mohamed, M.; Said, A.R.; Murad, A.; Graham, R. A serological survey of Crimean-Congo haemorrhagic fever in animals in the Sharkia Governorate of Egypt. Vet. Ital. 2008, 44, 513–517. [Google Scholar]
- Vorou, R.; Pierroutsakos, I.N.; Maltezou, H.C. Crimean-Congo hemorrhagic fever. Curr. Opin. Infect. Dis. 2007, 20, 495. [Google Scholar] [CrossRef]
- Chapman, L.E.; Wilson, M.L.; Hall, D.B.; LeGuenno, B.; Dykstra, E.A.; Ba, K.; Fisher-Hoch, S.P. Risk factors for Crimean-Congo hemorrhagic fever in rural northern Senegal. J. Infect. Dis. 1991, 164, 686–692. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; LeGuenno, B.; Guillaud, M.; Wilson, M.L. A fatal case of Crimean-Congo haemorrhagic fever in Mauritania: Virological and serological evidence suggesting epidemic transmission. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 573–576. [Google Scholar] [CrossRef]
- Nabeth, P.; Cheikh, D.O.; Lo, B.; Faye, O.; Vall, I.; Niang, M.; Wague, B.; Diop, D.; Diallo, M.; Diallo, B. Crimean-Congo hemorrhagic fever, Mauritania. Emerg. Infect. Dis. 2004, 10, 2143–2149. [Google Scholar] [CrossRef]
- Sang, R.; Lutomiah, J.; Koka, H.; Makio, A.; Chepkorir, E.; Ochieng, C.; Yalwala, S.; Mutisya, J.; Musila, L.; Richardson, J.H. Crimean-Congo hemorrhagic fever virus in Hyalommid ticks, northeastern Kenya. Emerg. Infect. Dis. 2011, 17, 1502. [Google Scholar] [CrossRef]
- Rahden, P.; Adam, A.; Mika, A.; Jassoy, C. Elevated Human Crimean–Congo hemorrhagic fever virus seroprevalence in Khashm el Girba, Eastern Sudan. Am. J. Trop. Med. Hyg. 2019, 100, 1549–1551. [Google Scholar] [CrossRef]
- Aradaib, I.E.; Erickson, B.R.; Mustafa, M.E.; Khristova, M.L.; Saeed, N.S.; Elageb, R.M.; Nichol, S.T. Nosocomial outbreak of Crimean-Congo hemorrhagic fever, Sudan. Emerg. Infect. Dis. 2010, 16, 837. [Google Scholar] [CrossRef]
- Aradaib, I.E.; Erickson, B.R.; Karsany, M.S.; Khristova, M.L.; Elageb, R.M.; Mohamed, M.E.; Nichol, S.T. Multiple Crimean-Congo hemorrhagic fever virus strains are associated with disease outbreaks in Sudan, 2008–2009. PLoS Negl. Trop. Dis. 2011, 5, e1159. [Google Scholar] [CrossRef] [Green Version]
- Mathiot, C.; Fontenille, D.; Digoutte, J.; Coulanges, P. First isolation of Congo-Crimean haemorrhagic fever virus in Madagascar. In Annales de l’Institut Pasteur/Virologie; Elsevier: Masson, Paris, 1988; pp. 239–241. [Google Scholar]
- Andriamandimby, S.F.; Marianneau, P.; Rafisandratantsoa, J.T.; Rollin, P.E.; Heraud, J.M.; Tordo, N.; Reynes, J.M. Crimean-Congo hemorrhagic fever serosurvey in at-risk professionals, Madagascar, 2008 and 2009. J. Clin. Virol. 2011, 52, 370–372. [Google Scholar] [CrossRef]
- Mariner, J.C.; Morrill, J.; Ksiazek, T. Antibodies to hemorrhagic fever viruses in domestic livestock in Niger: Rift Valley fever and Crimean-Congo hemorrhagic fever. Am. J. Trop. Med. Hyg. 1995, 53, 217–221. [Google Scholar] [CrossRef]
- Oluwayelu, D.; Afrough, B.; Adebiyi, A.; Varghese, A.; Eun-Sil, P.; Fukushi, S.; Yoshikawa, T.; Saijo, M.; Neumann, E.; Morikawa, S. Prevalence of Antibodies to Crimean-Congo hemorrhagic fever virus in ruminants, Nigeria, 2015. Emerg. Infect. Dis. 2020, 26, 744. [Google Scholar] [CrossRef] [PubMed]
- Bukbuk, D.N.; Dowall, S.D.; Lewandowski, K.; Bosworth, A.; Baba, S.S.; Varghese, A.; Watson, R.J.; Bell, A.; Atkinson, B.; Hewson, R. Serological and virological evidence of Crimean-Congo haemorrhagic fever virus circulation in the human population of Borno State, northeastern Nigeria. PLoS Negl. Trop. Dis. 2016, 10, e0005126. [Google Scholar] [CrossRef] [Green Version]
- Akuffo, R.; Brandful, J.; Zayed, A.; Adjei, A.; Watany, N.; Fahmy, N.; Hughes, R.; Doman, B.; Voegborlo, S.; Aziati, D. Crimean-Congo hemorrhagic fever virus in livestock ticks and animal handler seroprevalence at an abattoir in Ghana. BMC Infect. Dis. 2016, 16, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balinandi, S.; von Brömssen, C.; Tumusiime, A.; Kyondo, J.; Kwon, H.; Monteil, V.M.; Mirazimi, A.; Lutwama, J.; Mugisha, L.; Malmberg, M. Serological and molecular study of Crimean-Congo hemorrhagic fever virus in cattle from selected districts in Uganda. J. Virol. Methods 2021, 290, 114075. [Google Scholar] [CrossRef] [PubMed]
- Mirembe, B.B.; Musewa, A.; Kadobera, D.; Kisaakye, E.; Birungi, D.; Eurien, D.; Nyakarahuka, L.; Balinandi, S.; Tumusiime, A.; Kyondo, J. Sporadic outbreaks of crimean-congo haemorrhagic fever in Uganda, July 2018–January 2019. PLoS Negl. Trop. Dis. 2021, 15, e0009213. [Google Scholar] [CrossRef]
- Papa, A.; Bino, S.; Llagami, A.; Brahimaj, B.; Papadimitriou, E.; Pavlidou, V.; Velo, E.; Cahani, G.; Hajdini, M.; Pilaca, A. Crimean-Congo hemorrhagic fever in Albania, 2001. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Christova, I.; Papadimitriou, E.; Antoniadis, A. Crimean-Congo hemorrhagic fever in Bulgaria. Emerg. Infect. Dis. 2004, 10, 1465–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltezou, H.C.; Papa, A.; Tsiodras, S.; Dalla, V.; Maltezos, E.; Antoniadis, A. Crimean-Congo hemorrhagic fever in Greece: A public health perspective. Int. J. Infect. Dis. 2009, 13, 713–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniadis, A.; Alexiou-Daniel, S.; Malissiovas, N.; Doutsos, J.; Polyzoni, T.; LeDuc, J.; Peters, C.; Saviolakis, G.; Calisher, C. Seroepidemiological survey for antibodies to arboviruses in Greece. In Hemorrhagic Fever with Renal Syndrome, Tick-and Mosquito-Borne Viruses; Springer: Vienna, Austria, 1990; pp. 277–285. [Google Scholar]
- Papa, A.; Tzala, E.; Maltezou, H.C. Crimean-Congo hemorrhagic fever virus, northeastern Greece. Emerg. Infect. Dis. 2011, 17, 141. [Google Scholar] [CrossRef]
- Ahmeti, S.; Raka, L. Crimean-Congo haemorrhagic fever in Kosova: A fatal case report. Virol. J. 2006, 3, 2002–2007. [Google Scholar] [CrossRef] [Green Version]
- Humolli, I.; Dedushaj, I.; Zupanac, T.A.; Muçaj, S. Epidemiological, serological and herd immunity of Crimean-Congo haemorrhagic fever in Kosovo. Med. Arh. 2010, 64, 91–93. [Google Scholar]
- Emmerich, P.; Jakupi, X.; von Possel, R.; Berisha, L.; Halili, B.; Günther, S.; Cadar, D.; Ahmeti, S.; Schmidt-Chanasit, J. Viral metagenomics, genetic and evolutionary characteristics of Crimean-Congo hemorrhagic fever orthonairovirus in humans, Kosovo. Infect. Genet. Evol. 2018, 65, 6–11. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Palomar, A.M.; Santibáñez, P.; Sánchez, N.; Habela, M.A.; Portillo, A.; Romero, L.; Oteo, J.A. Crimean-Congo hemorrhagic fever virus in ticks, Southwestern Europe, 2010. Emerg. Infect. Dis. 2012, 18, 179. [Google Scholar] [CrossRef]
- De Arellano, E.R.; Hernández, L.; Goyanes, M.J.; Arsuaga, M.; Cruz, A.F.; Negredo, A.; Sánchez-Seco, M.P. Phylogenetic characterization of Crimean-Congo hemorrhagic fever virus, Spain. Emerg. Infect. Dis. 2017, 23, 2078. [Google Scholar] [CrossRef] [Green Version]
- Negredo, A.; Sánchez-Ledesma, M.; Llorente, F.; Pérez-Olmeda, M.; Belhassen-García, M.; González-Calle, D.; Sánchez-Seco, M.P.; Jiménez-Clavero, M.Á. Retrospective Identification of Early Autochthonous Case of Crimean-Congo Hemorrhagic Fever, Spain, 2013. Emerg. Infect. Dis. 2021, 27, 1754. [Google Scholar] [CrossRef]
- Portillo, A.; Palomar, A.M.; Santibáñez, P.; Oteo, J.A. Epidemiological Aspects of Crimean-Congo Hemorrhagic Fever in Western Europe: What about the Future? Microorganisms 2021, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Negredo, A.; Sánchez-Arroyo, R.; Díez-Fuertes, F.; de Ory, F.; Budiño, M.A.; Vázquez, A.; Garcinuño, Á.; Hernández, L.; De la Hoz González, C.; Gutiérrez-Arroyo, A. Fatal Case of Crimean-Congo Hemorrhagic Fever Caused by Reassortant Virus, Spain, 2018. Emerg. Infect. Dis. 2021, 27, 1211. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.; Latham, J.; Chamberlain, J.; Logue, C.; O’Donoghue, L.; Osborne, J.; Carson, G.; Brooks, T.; Carroll, M.; Jacobs, M. Sequencing and phylogenetic characterisation of a fatal Crimean–Congo haemorrhagic fever case imported into the United Kingdom, October 2012. Eurosurveillance 2012, 17, 20327. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.; Atkinson, B.; Dowall, S.; Pitman, J.; Staplehurst, S.; Busuttil, J.; Simpson, A.; Aarons, E.; Petridou, C.; Nijjar, M. Non-fatal case of Crimean-Congo haemorrhagic fever imported into the United Kingdom (ex Bulgaria), June 2014. Eurosurveillance 2014, 19, 20864. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, J.; Atkinson, B.; Logue, C.H.; Latham, J.; Newman, E.N.; Hewson, R. Genome sequence of ex-Afghanistan Crimean-Congo hemorrhagic fever virus SCT strain, from an imported United Kingdom case in October 2012. Genome Announc. 2013, 1, e00161-13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarantola, A.; Nabeth, P.; Tattevin, P.; Michelet, C.; Zeller, H. Lookback exercise with imported Crimean-Congo hemorrhagic fever, Senegal and France. Emerg. Infect. Dis. 2006, 12, 1424. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.; Sall, A.; Faye, O.; Diatta, B.; Sylla, R.; Faye, J.; Faye, P.; Ly, A.; Sarr, F.; Diab, H. Two cases of Crimean-Congo haemorrhagic fever (CCHF) in two tourists in Senegal in 2004. Bull. Soc. Pathol. Exot. (1990) 2009, 102, 159–161. [Google Scholar]
- Ölschläger, S.; Gabriel, M.; Schmidt-Chanasit, J.; Meyer, M.; Osborn, E.; Conger, N.G.; Allan, P.F.; Günther, S. Complete sequence and phylogenetic characterisation of Crimean–Congo hemorrhagic fever virus from Afghanistan. J. Clin. Virol. 2011, 50, 90–92. [Google Scholar] [CrossRef]
- Conger, N.G.; Paolino, K.M.; Osborn, E.C.; Rusnak, J.M.; Günther, S.; Pool, J.; Rollin, P.E.; Allan, P.F.; Schmidt-Chanasit, J.; Rieger, T. Health care response to CCHF in US soldier and nosocomial transmission to health care providers, Germany, 2009. Emerg. Infect. Dis. 2015, 21, 23. [Google Scholar] [CrossRef]
- Mehravaran, A.; Moradi, M.; Telmadarraiy, Z.; Mostafavi, E.; Moradi, A.R.; Khakifirouz, S.; Shahhosseini, N.; Varaie, F.S.R.; Jalali, T.; Hekmat, S. Molecular detection of Crimean-Congo haemorrhagic fever (CCHF) virus in ticks from southeastern Iran. Ticks Tick-Borne Dis. 2013, 4, 35–38. [Google Scholar] [CrossRef]
- Punyua, D.K. A review of the development and survival of ticks in tropical Africa. Int. J. Trop. Insect Sci. 1992, 13, 537–544. [Google Scholar] [CrossRef]
- Telmadarraiy, Z.; Vatandoost, H.; Chinikar, S.; Oshaghi, M.; Moradi, M.; Ardakan, E.M.; Hekmat, S.; Nasiri, A. Hard ticks on domestic ruminants and their seasonal population dynamics in Yazd Province, Iran. Iran. J. Arthropod-Borne Dis. 2010, 4, 66. [Google Scholar]
- Palomar, A.M.; Portillo, A.; Santibáñez, P.; Mazuelas, D.; Arizaga, J.; Crespo, A.; Gutiérrez, Ó.; Cuadrado, J.F.; Oteo, J.A. Crimean-Congo hemorrhagic fever virus in ticks from migratory birds, Morocco. Emerg. Infect. Dis. 2013, 19, 260. [Google Scholar] [CrossRef]
- Gordon, S.W.; Linthicum, K.J.; Moulton, J. Transmission of Crimean-Congo hemorrhagic fever virus in two species of Hyalomma ticks from infected adults to cofeeding immature forms. Am. J. Trop. Med. Hyg. 1993, 48, 576–580. [Google Scholar] [CrossRef]
- Mohammadian, M.; Chinikar, S.; Telmadarraiy, Z.; Vatandoost, H.; Oshaghi, M.A.; Hanafi-Bojd, A.A.; Sedaghat, M.M.; Noroozi, M.; Faghihi, F.; Jalali, T. Molecular assay on Crimean Congo hemorrhagic fever virus in ticks (Ixodidae) collected from Kermanshah Province, western Iran. J. Arthropod-Borne Dis. 2016, 10, 381. [Google Scholar]
- Farhadpour, F.; Telmadarraiy, Z.; Chinikar, S.; Akbarzadeh, K.; Moemenbellah-Fard, M.; Faghihi, F.; Fakoorziba, M.; Jalali, T.; Mostafavi, E.; Shahhosseini, N. Molecular detection of Crimean–Congo haemorrhagic fever virus in ticks collected from infested livestock populations in a New Endemic Area, South of Iran. Trop. Med. Int. Health 2016, 21, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Telmadarraiy, Z.; Chinikar, S.; Vatandoost, H.; Faghihi, F.; Hosseini-Chegeni, A. Vectors of Crimean Congo hemorrhagic fever virus in Iran. J. Arthropod-Borne Dis. 2015, 9, 137. [Google Scholar]
- Swain, K.; Gupta, S.; Jwalagatti, V.K.; Panigrahi, S.; Routray, A.; Sahoo, S.; Ganguly, S. Crimean Congo hemorrhagic fever (CCHF): A chronicle of human, tick and animal. J. Entomol. Zool Stud. 2017, 5, 956–961. [Google Scholar]
- Cajimat, M.N.; Rodriguez, S.E.; Schuster, I.U.; Swetnam, D.M.; Ksiazek, T.G.; Habela, M.A.; Negredo, A.I.; Estrada-Peña, A.; Barrett, A.D.; Bente, D.A. Genomic characterization of crimean–congo hemorrhagic fever virus in hyalomma tick from Spain, 2014. Vector-Borne Zoonotic Dis. 2017, 17, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; Ruiz-Arrondo, I.; Oteo, J.A. Arthropods as vectors of transmissible diseases in Spain. Med. Clínica Engl. Ed. 2018, 151, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, M.M.; Sarani, M.; Chinikar, S.; Telmadarraiy, Z.; Moghaddam, A.S.; Azam, K.; Nowotny, N.; Fooks, A.R.; Shahhosseini, N. Vector prevalence and detection of Crimean-Congo haemorrhagic fever virus in Golestan Province, Iran. J. Vector Borne Dis. 2017, 54, 353. [Google Scholar] [PubMed]
- Faghihi, F.; Telmadarraiy, Z.; Chinikar, S.; Nowotny, N.; Fooks, A.R.; Shahhosseini, N. Spatial and phylodynamic survey on Crimean-Congo hemorrhagic fever virus strains in northeast of Iran. Jundishapur J. Microbiol. 2018, 11, e59412. [Google Scholar] [CrossRef] [Green Version]
- Champour, M.; Chinikar, S.; Mohammadi, G.; Razmi, G.; Shahhosseini, N.; Khakifirouz, S.; Mostafavi, E.; Jalali, T. Molecular epidemiology of Crimean–Congo hemorrhagic fever virus detected from ticks of one humped camels (Camelus dromedarius) population in northeastern Iran. J. Parasit. Dis. 2016, 40, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Camicas, J.-L.; Wilson, M.; Cornet, J.-P.; Digoutte, J.-P.; Calvo, M.-A.; Adam, F.; Gonzalez, J.-P. Ecology of ticks as potential vectors of Crimean-Congo hemorrhagic fever virus in Senegal: Epidemiological implications. In Hemorrhagic Fever with Renal Syndrome, Tick-and Mosquito-Borne Viruses; Springer: Berlin/Heidelberg, Germany, 1990; pp. 303–322. [Google Scholar]
- Schulz, A.; Karger, A.; Bettin, B.; Eisenbarth, A.; Sas, M.; Silaghi, C.; Groschup, M. Molecular discrimination of Hyalomma tick species serving as reservoirs and vectors for Crimean-Congo hemorrhagic fever virus in sub-Saharan Africa. Ticks Tick-Borne Dis. 2020, 11, 101382. [Google Scholar] [CrossRef] [PubMed]
- Mourya, D.T.; Yadav, P.D.; Patil, D.Y. Highly infectious tick-borne viral diseases: Kyasanur forest disease and Crimean–Congo haemorrhagic fever in India. WHO South-East Asia J. Public Health 2014, 3, 8–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, E.; Toma, L.; Polci, A.; d’Alessio, S.G.; Di Luca, M.; Orsini, M.; Di Domenico, M.; Marcacci, M.; Mancini, G.; Spina, F. Crimean-Congo hemorrhagic fever virus genome in tick from migratory bird, Italy. Emerg. Infect. Dis. 2019, 25, 1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, M.F.; Yaqub, T.; Ali, M.; Ul-Rahman, A.; Bente, D.A. Prevalence and phylogenetic analysis of Crimean-Congo hemorrhagic fever virus in ticks collected from Punjab province of Pakistan. Acta Trop. 2021, 218, 105892. [Google Scholar] [CrossRef] [PubMed]
- Tekin, S.; Bursali, A.; Mutluay, N.; Keskin, A.; Dundar, E. Crimean-Congo hemorrhagic fever virus in various ixodid tick species from a highly endemic area. Vet. Parasitol. 2012, 186, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Burt, F.; Paweska, J.; Ashkettle, B.; Swanepoel, R. Genetic relationship in southern African Crimean-Congo haemorrhagic fever virus isolates: Evidence for occurrence of reassortment. Epidemiol. Infect. 2009, 137, 1302–1308. [Google Scholar] [CrossRef] [Green Version]
- Gunes, T.; Poyraz, O.; Vatansever, Z. Crimean-Congo hemorrhagic fever virus in ticks collected from humans, livestock, and picnic sites in the hyperendemic region of Turkey. Vector-Borne Zoonotic Dis. 2011, 11, 1411–1416. [Google Scholar] [CrossRef]
- Orkun, Ö.; Karaer, Z.; Çakmak, A.; Nalbantoğlu, S. Crimean-Congo hemorrhagic fever virus in ticks in Turkey: A broad range tick surveillance study. Infect. Genet. Evol. 2017, 52, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.A.E.H.; Mohamed, N.; Aleanizy, F.S.; Alqahtani, F.Y.; Al Khalaf, A.; Al-Keridis, L.A. Investigation of hemorrhagic fever viruses inside wild populations of ticks: One of the pioneer studies in Saudi Arabia. Asian Pac. J. Trop. Dis. 2017, 7, 299–303. [Google Scholar] [CrossRef]
- Adjogoua, E.V.; Coulibaly-Guindo, N.; Diaha-Kouame, C.A.; Diane, M.K.; Kouassi, R.M.C.K.A.; Coulibaly, J.T.; Dosso, M. Geographical distribution of ticks Ixodidae in Côte d’Ivoire: Potential reservoir of the Crimean-Congo hemorrhagic fever virus. Vector-Borne Zoonotic Dis. 2021. [Google Scholar] [CrossRef]
- Zivcec, M.; Scholte, F.E.; Spiropoulou, C.F.; Spengler, J.R.; Bergeron, É. Molecular insights into Crimean-Congo hemorrhagic fever virus. Viruses 2016, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Gevorgyan, H.; Grigoryan, G.G.; Atoyan, H.A.; Rukhkyan, M.; Hakobyan, A.; Zakaryan, H.; Aghayan, S.A. Evidence of Crimean-Congo haemorrhagic fever virus occurrence in Ixodidae ticks of Armenia. J. Arthropod-Borne Dis. 2019, 13, 9. [Google Scholar] [PubMed]
- Zeller, H.G.; Cornet, J.-P.; Diop, A.; Camicas, J.-L. Crimean—Congo hemorrhagic fever in ticks (Acari: Ixodidae) and ruminants: Field observations of an epizootic in Bandia, Senegal (1989–1992). J. Med. Entomol. 1997, 34, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Wampande, E.M.; Waiswa, P.; Allen, D.J.; Hewson, R.; Frost, S.D.; Stubbs, S.C. Phylogenetic Characterization of Crimean-Congo hemorrhagic fever virus detected in African blue ticks feeding on cattle in a Ugandan abattoir. Microorganisms 2021, 9, 438. [Google Scholar] [CrossRef]
- Fakoorziba, M.R.; Naddaf-Sani, A.A.; Moemenbellah-Fard, M.D.; Azizi, K.; Ahmadnia, S.; Chinikar, S. First phylogenetic analysis of a Crimean-Congo hemorrhagic fever virus genome in naturally infected Rhipicephalus appendiculatus ticks (Acari: Ixodidae). Arch. Virol. 2015, 160, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- MIu, S.; Kolobukhina, L.; Moskvina, T.; Aushev, I.; Kartoev, A.; Kelli, E.; Merkulova, L.; Grenkova, E.; Samokhvalov, E.; Petriaev, V. Detection of the circulation of Crimean-Congo hemorrhagic fever virus in the piedmont steppes of the North Caucasus. Vopr. Virusol. 2005, 50, 9–15. [Google Scholar]
- Sherifi, K.; Cadar, D.; Muji, S.; Robaj, A.; Ahmeti, S.; Jakupi, X.; Emmerich, P.; Krüger, A. Crimean-Congo hemorrhagic fever virus clades V and VI (Europe 1 and 2) in ticks in Kosovo, 2012. PLoS Negl. Trop. Dis. 2014, 8, e3168. [Google Scholar] [CrossRef]
- Gergova, I.; Kunchev, M.; Kamarinchev, B. Crimean-congo hemorrhagic fever virus–tick survey in endemic areas in Bulgaria. J. Med. Virol. 2012, 84, 608–614. [Google Scholar] [CrossRef]
- Spengler, J.R.; Bergeron, É.; Rollin, P.E. Seroepidemiological studies of Crimean-Congo hemorrhagic fever virus in domestic and wild animals. PLoS Negl. Trop. Dis. 2016, 10, e0004210. [Google Scholar] [CrossRef] [Green Version]
- Champour, M.; Mohammadi, G.; Chinikar, S.; Razmi, G.; Shahhosseini, N.; Khakifirouz, S.; Mostafavi, E.; Jalali, T. Seroepidemiology of Crimean-Congo hemorrhagic fever virus in one-humped camels (Camelus dromedarius) population in northeast of Iran. J. Vector Borne Dis. 2014, 51, 62. [Google Scholar]
- Champour, M.; Chinikar, S.; Mohammadi, G.; Razmi, G.; Mostafavi, E.; Shahhosseini, N.; Khakifirouz, S.; Jalali, T. Crimean-Congo hemorrhagic fever in the one-humped camel (Camelus dromedarius) in East and Northeast of Iran. J. Arthropod-Borne Dis. 2016, 10, 168. [Google Scholar]
- Cooper, R.G.; Horbanczuk, J.O.; Fujihara, N. Viral diseases of the Ostrich (Struthio camelus var. domesticus). Anim. Sci. J. 2004, 75, 89–95. [Google Scholar] [CrossRef]
- Sonenshine, D.E.; Mather, T.N. Ecological Dynamics of Tick-Borne Zoonoses; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Saijo, M.; Tang, Q.; Shimayi, B.; Han, L.; Zhang, Y.; Asiguma, M.; Tianshu, D.; Maeda, A.; Kurane, I.; Morikawa, S. Possible horizontal transmission of Crimean-Congo hemorrhagic fever virus from a mother to her child. Jpn. J. Infect. Dis. 2004, 57, 55–57. [Google Scholar] [PubMed]
- Pshenichnaya, N.Y.; Leblebicioglu, H.; Bozkurt, I.; Sannikova, I.V.; Abuova, G.N.; Zhuravlev, A.S.; Barut, S.; Shermetova, M.B.; Fletcher, T.E. Crimean-Congo hemorrhagic fever in pregnancy: A systematic review and case series from Russia, Kazakhstan and Turkey. Int. J. Infect. Dis. 2017, 58, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Swanepoel, R.; Shepherd, A.; Leman, P.; Shepherd, S. Investigations following initial recognition of Crimean-Congo haemorrhagic fever in South Africa and the diagnosis of 2 further cases. S. Afr. Med. J. 1985, 68, 638–641. [Google Scholar] [PubMed]
- Burt, F.J.; Swanepoel, R. Crimean-Congo Hemorrhagic Fever. Tick-Borne Dis. Hum. 2005, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Swanepoel, R.; Shepherd, A.; Leman, P.; Shepherd, S.; Miller, G. A common-source outbreak of Crimean-Congo haemorrhagic fever on a dairy farm. S. Afr. Med. J. 1985, 68, 635–637. [Google Scholar]
- Deubel, V.; Murgue, B.; Service, M. Dengue. Service MW. The Encyclopedia of Arthropod-Transmitted Infections; CAB International: Wallingford, UK, 2001; pp. 133–143. [Google Scholar]
- Bodur, H.; Akinci, E.; Ascioglu, S.; Öngürü, P.; Uyar, Y. Subclinical infections with Crimean-Congo hemorrhagic fever virus, Turkey. Emerg. Infect. Dis. 2012, 18, 640. [Google Scholar] [CrossRef]
- Khurshid, A.; Hassan, M.; Alam, M.M.; Aamir, U.B.; Rehman, L.; Sharif, S.; Shaukat, S.; Rana, M.S.; Angez, M.; Zaidi, S.S.Z. CCHF virus variants in Pakistan and Afghanistan: Emerging diversity and epidemiology. J. Clin. Virol. 2015, 67, 25–30. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Roberts, F.H.; Kohls, G.M.; Tipton, V.J. Review of haemaphysalis (kaiseriana) longicornis neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Northeastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae). J. Parasitol. 1968, 54, 1197–1213. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, L.; Sun, Y.; Cui, X.-M.; Tang, F.; Hu, J.-G.; Wang, L.-Y.; Cui, N.; Yang, Z.-D.; Huang, D.-D.; Zhang, X.-A. Transmission of severe fever with thrombocytopenia syndrome virus by Haemaphysalis longicornis ticks, China. Emerg. Infect. Dis. 2018, 24, 868. [Google Scholar] [CrossRef] [Green Version]
- Nation, J.L. Non-Native and Invasive Ticks. Threats to Human and animal health in the United States. Fla. Entomol. 2011, 94, 1097. [Google Scholar] [CrossRef]
- Estrada-Pena, A.; de la Fuente, J.; Latapia, T.; Ortega, C. The impact of climate trends on a tick affecting public health: A retrospective modeling approach for Hyalomma marginatum (Ixodidae). PLoS ONE 2015, 10, e0125760. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Pena, A.; D’Amico, G.; Fernandez-Ruiz, N. Modelling the potential spread of Hyalomma marginatum ticks in Europe by migratory birds. Int. J. Parasitol. 2021, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Grandi, G.; Chitimia-Dobler, L.; Choklikitumnuey, P.; Strube, C.; Springer, A.; Albihn, A.; Jaenson, T.G.T.; Omazic, A. First records of adult Hyalomma marginatum and H. rufipes ticks (Acari: Ixodidae) in Sweden. Ticks Tick Borne Dis 2020, 11, 101403. [Google Scholar] [CrossRef]
- Duscher, G.G.; Hodzic, A.; Hufnagl, P.; Wille-Piazzai, W.; Schotta, A.M.; Markowicz, M.A.; Estrada-Pena, A.; Stanek, G.; Allerberger, F. Adult Hyalomma marginatum tick positive for Rickettsia aeschlimannii in Austria, October 2018. Eurosurveillance 2018, 23, 1800595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vial, L.; Stachurski, F.; Leblond, A.; Huber, K.; Vourc’h, G.; Rene-Martellet, M.; Desjardins, I.; Balanca, G.; Grosbois, V.; Pradier, S.; et al. Strong evidence for the presence of the tick Hyalomma marginatum Koch, 1844 in southern continental France. Ticks Tick Borne Dis 2016, 7, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Vial, L.; Appelgren, A.; Huber, K.; Calloix, C.; Andary, C.; Grosbois, V.; Lancelot, R.; Stachurski, F. Update in the geographical distribution of the invasive tick Hyalomma marginatum in South of France: First attempts to identify factors favoring its establishment. In Proceedings of the E-SOVE (European Society for Vector Ecology) Meeting, Palermo, Italy, 22–26 October 2018; p. 99. [Google Scholar]
- Hubalek, Z.; Sedlacek, P.; Estrada-Pena, A.; Vojtisek, J.; Rudolf, I. First record of Hyalomma rufipes in the Czech Republic, with a review of relevant cases in other parts of Europe. Ticks Tick Borne Dis. 2020, 11, 101421. [Google Scholar] [CrossRef] [PubMed]
- Negredo, A.; de la Calle-Prieto, F.; Palencia-Herrejón, E.; Mora-Rillo, M.; Astray-Mochales, J.; Sánchez-Seco, M.P.; Bermejo Lopez, E.; Menárguez, J.; Fernández-Cruz, A.; Sánchez-Artola, B. Autochthonous Crimean–Congo hemorrhagic fever in Spain. N. Engl. J. Med. 2017, 377, 154–161. [Google Scholar] [CrossRef]
- Keirans, J.E.; Durden, L.A. Invasion: Exotic ticks (Acari: Argasidae, Ixodidae) imported into the United States. A review and new records. J. Med. Entomol. 2001, 38, 850–861. [Google Scholar] [CrossRef]
- Kwak, M.L. The introduction and subsequent extinction of the camel tick Hyalomma (Euhyalomma) dromedarii (Acari, Ixodidae) in Australia, with a review of the introduction of foreign ticks to Australia. Exp. Appl. Acarol. 2018, 74, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Zehender, G.; Ebranati, E.; Shkjezi, R.; Papa, A.; Luzzago, C.; Gabanelli, E.; Presti, A.L.; Lai, A.; Rezza, G.; Galli, M. Bayesian phylogeography of Crimean-Congo hemorrhagic fever virus in Europe. PLoS ONE 2013, 8, e79663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukashev, A.N.; Klimentov, A.S.; Smirnova, S.E.; Dzagurova, T.K.; Drexler, J.F.; Gmyl, A.P. Phylogeography of Crimean Congo hemorrhagic fever virus. PLoS ONE 2016, 11, e0166744. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, C. Risk groups and control measures for Crimean-Congo hemorrhagic fever. In Crimean-Congo Hemorrhagic Fever; Springer: Dordrecht, The Netherlands, 2007; pp. 273–280. [Google Scholar]
- Weidmann, M.; Avsic-Zupanc, T.; Bino, S.; Bouloy, M.; Burt, F.; Chinikar, S.; Christova, I.; Dedushaj, I.; El-Sanousi, A.; Elaldi, N. Biosafety standards for working with Crimean-Congo hemorrhagic fever virus. J. Gen. Virol. 2016, 97, 2799–2808. [Google Scholar] [CrossRef] [Green Version]
- Hamill, F.A. National biosafety standards differ. Nature 2017, 543, 623. [Google Scholar] [CrossRef] [Green Version]
- Pourahmad, M.; Raoofi, R.; Chinikar, S.; Ghiasi, S.M.; Ghalyanchi-Langeroudi, A. Nosocomial transmission of Crimean-Congo hemorrhagic fever in a health care worker, Fars Province, Iran. Iran. J. Clin. Infect. Dis. 2011, 6, 47–50. [Google Scholar]
- Izadi, S.; Salehi, M.; Holakouie-Naieni, K.; Chinikar, S. The risk of transmission of Crimean-Congo hemorrhagic fever virus from human cases to first-degree relatives. Jpn. J. Infect. Dis. 2008, 61, 494–496. [Google Scholar] [PubMed]
- Shahhosseini, N.; Azari-Garmjan, G.-A.; Rezaiyan, M.K.; Haeri, A.; Nowotny, N.; Fooks, A.R.; Chinikar, S.; Youssefi, M. Factors Affecting Transmission of Crimean-Congo hemorrhagic fever among slaughterhouse employees: A serosurvey in Mashhad, Iran. Jundishapur J. Microbiol. 2018, 11, e57980. [Google Scholar] [CrossRef] [Green Version]
- Mostafavi, E.; Pourhossein, B.; Esmaeili, S.; Amiri, F.B.; Khakifirouz, S.; Shahhosseini, N.; Tabatabaei, S.M. Seroepidemiology and risk factors of Crimean-Congo hemorrhagic fever among butchers and slaughterhouse workers in southeastern Iran. Int. J. Infect. Dis. 2017, 64, 85–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltezou, H.C.; Papa, A. Crimean-Congo hemorrhagic fever: Epidemiological trends and controversies in treatment. BMC Med. 2011, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowall, S.D.; Carroll, M.W.; Hewson, R. Development of vaccines against Crimean-Congo haemorrhagic fever virus. Vaccine 2017, 35, 6015–6023. [Google Scholar] [CrossRef] [PubMed]
- Scholte, F.E.; Spengler, J.R.; Welch, S.R.; Harmon, J.R.; Coleman-McCray, J.D.; Freitas, B.T.; Kainulainen, M.H.; Pegan, S.D.; Nichol, S.T.; Bergeron, É. Single-dose replicon particle vaccine provides complete protection against Crimean-Congo hemorrhagic fever virus in mice. Emerg. Microbes Infect. 2019, 8, 575–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Bailey-Elkin, B.A.; Knaap, R.C.; Khare, B.; Dalebout, T.J.; Johnson, G.G.; van Kasteren, P.B.; McLeish, N.J.; Gu, J.; He, W. Potent and selective inhibition of pathogenic viruses by engineered ubiquitin variants. PLoS Pathog. 2017, 13, e1006372. [Google Scholar] [CrossRef] [PubMed]

| Family | Genus | Species | Geographical Distribution | References |
|---|---|---|---|---|
| Ixodidae | Hyalomma | Hy. marginatum | Middle East, Northern Africa, Southern Europe | [130,131,132] |
| Hy. dromedarii | Middle East, Northern Africa | [131,135,137] | ||
| Hy. rufipes | Middle East, Africa | [92,137,138,139,140,141,142] | ||
| Hy. turanicum | Asia, Africa | [140,143] | ||
| Hy. nitidum | Central Africa | [139,144] | ||
| Hy. anatolicum | Asia | [129,130,131,132,135] | ||
| Hy. asiaticum | Asia | [129,131,132] | ||
| Hy. detritum | Middle East, Africa | [131,143] | ||
| Hy. excavatum | Africa, Middle East | [55,144,145,146] | ||
| Hy. truncatum | Africa | [137,138,139,144] | ||
| Hy. schulzei | Arabia peninsula | [131,147] | ||
| Hy. impeltatum | Northern Africa, Arabian Peninsula | [55,138] | ||
| Hy. lusitanicum | Africa, Spain | [133] | ||
| Hy. isaaci | Africa | [140] | ||
| Hy. impressum | Africa, Pakistan | [142,148,149] | ||
| Rhipicephalus | Rh. sanguineus | Asia | [129,130,131,136] | |
| Rh. bursa | Southeastern Europe, Middle East | [131,132,146] | ||
| Rh. annulatus | Middle East, Central parts of Africa | [145,150] | ||
| Rh. turanicus | Southern Europe, Asia | [142,143,146] | ||
| Rh. rossicus | Caucasia, southern Russia | [19] | ||
| Rh. evertsi | Sub-Saharan Africa | [91,151] | ||
| Rh. decoloratus | Uganda | [152] | ||
| Rh. appendiculatus | Iran | [153] | ||
| Rh. microplus | Africa, Pakistan | [132,142,148] | ||
| Rh. guilhoni | Senegal | [138,151] | ||
| Haemaphysalis | Ha. punctata | Some parts of Asia, South-East of Europe | [131] | |
| Ha. inermis | Iran | [124,131] | ||
| Ha. concinna | Turkey | [143] | ||
| Ha. sulcata | Iran | [26] | ||
| Ha. parva | Turkey, North Caucasus | [145,154] | ||
| Dermacentor | De. marginatus | Southern Europe, Middle East, Mediterranean | [19,131,146] | |
| De. niveus | Tajikistan | [132] | ||
| Ixodes | Ix. ricinus | Europe, Mediterranean, Northern Africa | [155,156] | |
| Amblyomma | Am. variegatum | Sub-Saharan Africa | [138,151] | |
| Argasidae | Ornithodoros | Or. lahorensis | Iran | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahhosseini, N.; Wong, G.; Babuadze, G.; Camp, J.V.; Ergonul, O.; Kobinger, G.P.; Chinikar, S.; Nowotny, N. Crimean-Congo Hemorrhagic Fever Virus in Asia, Africa and Europe. Microorganisms 2021, 9, 1907. https://doi.org/10.3390/microorganisms9091907
Shahhosseini N, Wong G, Babuadze G, Camp JV, Ergonul O, Kobinger GP, Chinikar S, Nowotny N. Crimean-Congo Hemorrhagic Fever Virus in Asia, Africa and Europe. Microorganisms. 2021; 9(9):1907. https://doi.org/10.3390/microorganisms9091907
Chicago/Turabian StyleShahhosseini, Nariman, Gary Wong, George Babuadze, Jeremy V. Camp, Onder Ergonul, Gary P. Kobinger, Sadegh Chinikar, and Norbert Nowotny. 2021. "Crimean-Congo Hemorrhagic Fever Virus in Asia, Africa and Europe" Microorganisms 9, no. 9: 1907. https://doi.org/10.3390/microorganisms9091907
APA StyleShahhosseini, N., Wong, G., Babuadze, G., Camp, J. V., Ergonul, O., Kobinger, G. P., Chinikar, S., & Nowotny, N. (2021). Crimean-Congo Hemorrhagic Fever Virus in Asia, Africa and Europe. Microorganisms, 9(9), 1907. https://doi.org/10.3390/microorganisms9091907









