Interrelationship between the Microbial Communities of the Root Canals and Periodontal Pockets in Combined Endodontic-Periodontal Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection
2.3. Evaluation of the RC and PP Microbiomes
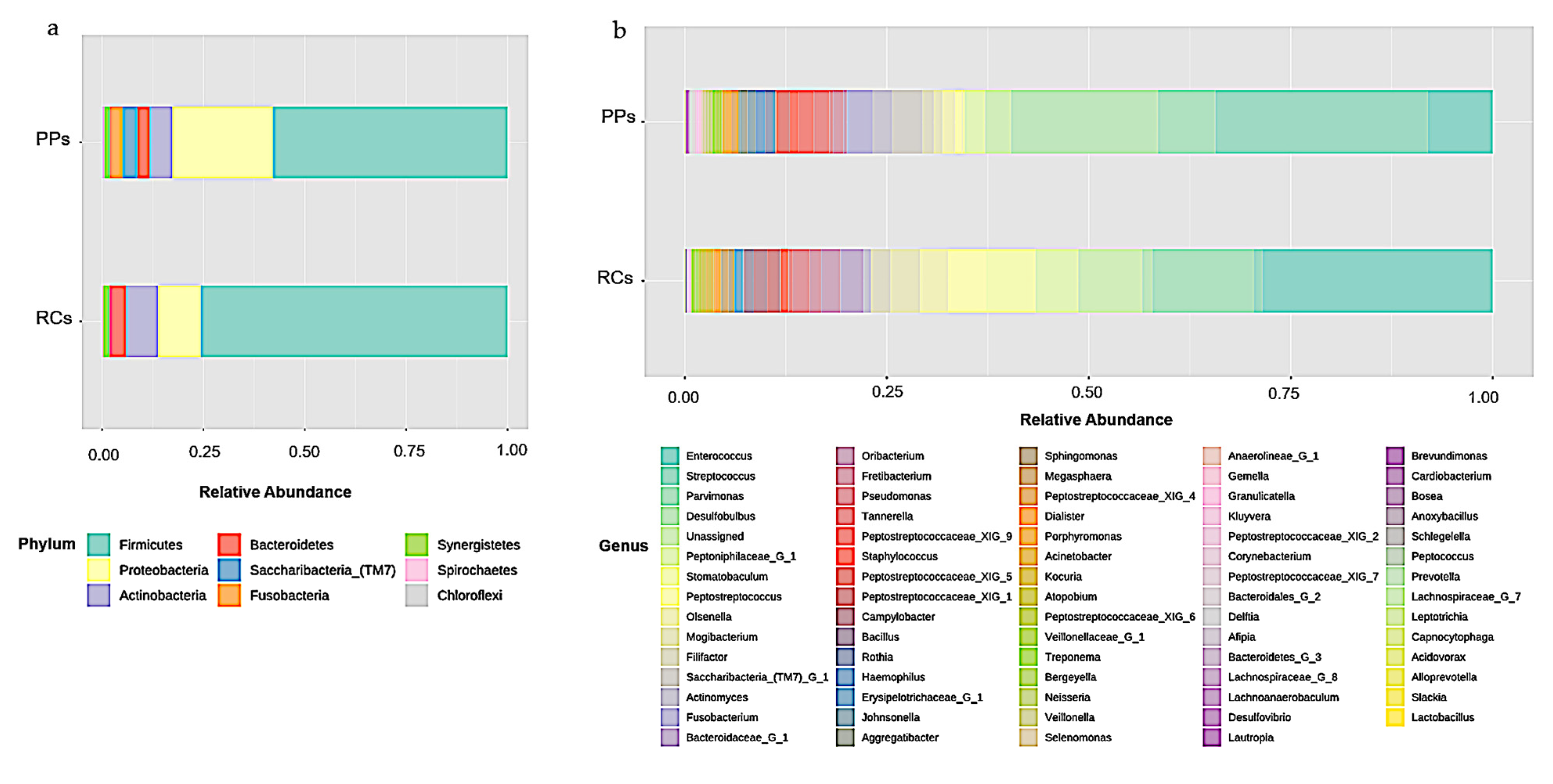
3. Results
3.1. Microbiome of the RCs and PPs
3.2. Comparative Studies of RC and PPs Microbiomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rotstein, I. Interaction between Endodontics and Periodontics. Periodontol 2000 2017, 74, 11–39. [Google Scholar] [CrossRef]
- Gomes, B.P.F.A.; Berber, V.B.; Kokaras, A.S.; Chen, T.; Paster, B.J. Microbiomes of Endodontic-Periodontal Lesions before and after Chemomechanical Preparation. J. Endod. 2015, 41, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.F.A.; Rodrigues, H.H.; Tancredo, N. The Use of a Modelling Technique to Investigate the Root Canal Morphology of Mandibular Incisors. Int. Endod. J. 1996, 29, 29–36. [Google Scholar] [CrossRef]
- Langeland, K.; Rodrigues, H.; Dowden, W. Periodontal Disease, Bacteria, and Pulpal Histopathology. Oral Surg. Oral Med. Oral Pathol. 1974, 37, 257–270. [Google Scholar] [CrossRef]
- Oktawati, S.; Siswanto, H.; Mardiana, A.; Supiaty; Neormansyah, I.; Basir, I. Endodontic–Periodontic Lesion Management: A Systematic Review. Med. Clínica Práctica 2020, 3, 100098. [Google Scholar] [CrossRef]
- Narayanan, L.L.; Vaishnavi, C. Endodontic Microbiology. J. Conserv. Dent. 2010, 13, 233. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Marsh, P.D. Microbial Ecology of Dental Plaque and Its Significance in Health and Disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef]
- Costerton, J.W. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Ray, J.M.; Triplett, R.G. What Is the Role of Biofilms in Severe Head and Neck Infections? Oral Maxillofac. Surg. Clin. N. Am. 2011, 23, 497–505. [Google Scholar] [CrossRef]
- Waltimo, T.M.T.; Sirén, E.K.; Torkko, H.L.K.; Olsen, I.; Haapasalo, M.P.P. Fungi in Therapy-Resistant Apical Periodontitis. Int. Endod. J. 1997, 30, 96–101. [Google Scholar] [CrossRef] [PubMed]
- de Beer, D.; Stoodley, P.; Lewandowski, Z. Liquid Flow in Heterogeneous Biofilms. Biotechnol. Bioeng. 1994, 44, 636–641. [Google Scholar] [CrossRef]
- Costerton, W.; Veeh, R.; Shirtliff, M.; Pasmore, M.; Post, C.; Ehrlich, G. The Application of Biofilm Science to the Study and Control of Chronic Bacterial Infections. J. Clin. Investig. 2003, 112, 1466–1477. [Google Scholar] [CrossRef] [Green Version]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival Strategies of Infectious Biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.E. OrCGDB: A Database of Genes Involved in Oral Cancer. Nucleic Acids Res. 2001, 29, 300–302. [Google Scholar] [CrossRef] [Green Version]
- Novick, R.P. Autoinduction and Signal Transduction in the Regulation of Staphylococcal Virulence: Regulation of Staphylococcus Virulence. Mol. Microbiol. 2003, 48, 1429–1449. [Google Scholar] [CrossRef] [PubMed]
- Mougeot, J.-L.C.; Stevens, C.B.; Cotton, S.L.; Morton, D.S.; Krishnan, K.; Brennan, M.T.; Lockhart, P.B.; Paster, B.J.; Bahrani Mougeot, F.K. Concordance of HOMIM and HOMI NGS Technologies in the Microbiome Analysis of Clinical Samples. J. Oral Microbiol. 2016, 8, 30379. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Yu, W.-H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome Database: A Web Accessible Resource for Investigating Oral Microbe Taxonomic and Genomic Information. Database 2010, 2010, baq013. [Google Scholar] [CrossRef]
- Cole, J.R. The Ribosomal Database Project (RDP-II): Sequences and Tools for High-Throughput RRNA Analysis. Nucleic Acids Res. 2004, 33, D294–D296. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S RRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S RRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the Human Oral Microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Tagami, A.; Okuda, K. Isolation and Classification of Anaerobic Bacteria from Pulp Cavities of Nonvital Teeth in Man. Bull. Tokyo Dent. Coll. 1991, 32, 95–98. [Google Scholar]
- Didilescu, A.C.; Rusu, D.; Anghel, A.; Nica, L.; Iliescu, A.; Greabu, M.; Bancescu, G.; Stratul, S.I. Investigation of Six Selected Bacterial Species in Endo-Periodontal Lesions: Microorganisms in Endo-Periodontal Lesions. Int. Endod. J. 2012, 45, 282–293. [Google Scholar] [CrossRef]
- Kipioti, A.; Nakou, M.; Legakis, N.; Mitsis, F. Microbiological Findings of Infected Root Canals and Adjacent Periodontal Pockets in Teeth with Advanced Periodontitis. Oral Surg. Oral Med. Oral Pathol. 1984, 58, 213–220. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hayashi, A.; Yoshikawa, R.; Ookuda, K.; Hara, K. The Microbial Flora from Root Canals and Periodontal Pockets of Non-Vital Teeth Associated with Advanced Periodontitis. Int. Endod. J. 1990, 23, 100–106. [Google Scholar] [CrossRef]
- Pereira, C.V.; Stipp, R.N.; Fonseca, D.C.; Pereira, L.J.; Höfling, J.F. Detection and Clonal Analysis of Anaerobic Bacteria Associated to Endodontic-Periodontal Lesions. J. Periodontol. 2011, 82, 1767–1775. [Google Scholar] [CrossRef]
- Rupf, S.; Kannengießer, S.; Merte, K.; Pfister, W.; Sigusch, B.; Eschrich, K. Comparison of Profiles of Key Periodontal Pathogens in Periodontium and Endodontium. Dent. Traumatol. 2000, 16, 269–275. [Google Scholar] [CrossRef]
- Xia, M.; Qi, Q. Bacterial Analysis of Combined Periodontal-Endodontic Lesions by Polymerase Chain Reactiondenaturing Gradient Gel Electrophoresis. J. Oral. Sci. 2013, 55, 287–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, Z.; Mewis, K.; Liu, F.; Morgan-Lang, C.; Scofield, M.; Durno, E.; Chen, H.M.; Mehr, K.; Withers, S.G.; Hallam, S.J. Metagenomics Reveals Functional Synergy and Novel Polysaccharide Utilization Loci in the Castor Canadensis Fecal Microbiome. ISME J. 2018, 12, 2757–2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besser, J.; Carleton, H.A.; Gerner-Smidt, P.; Lindsey, R.L.; Trees, E. Next-Generation Sequencing Technologies and Their Application to the Study and Control of Bacterial Infections. Clin. Microbiol. Infect. 2018, 24, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Buermans, H.P.J.; den Dunnen, J.T. Next Generation Sequencing Technology: Advances and Applications. Biochim. Biophys. Acta-Mol. Basis Dis. 2014, 1842, 1932–1941. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, S.; McPherson, J.D.; McCombie, W.R. Coming of Age: Ten Years of next-Generation Sequencing Technologies. Nat. Rev. Genet. 2016, 17, 333–351. [Google Scholar] [CrossRef]
- Brailsford, S.R.; Shah, B.; Simons, D.; Gilbert, S.; Clark, D.; Ines, I.; Adams, S.E.; Allison, C.; Beighton, D. The Predominant Aciduric Microflora of Root-Caries Lesions. J. Dent. Res. 2001, 80, 1828–1833. [Google Scholar] [CrossRef]
- Foster, K.R.; Bell, T. Competition, Not Cooperation, Dominates Interactions among Culturable Microbial Species. Curr. Biol. 2012, 22, 1845–1850. [Google Scholar] [CrossRef] [Green Version]
- Kwang, S.; Abbott, P. The Presence and Distribution of Bacteria in Dentinal Tubules of Root Filled Teeth. Int. Endod. J. 2014, 47, 600–610. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricucci, D.; Siqueira, J.F.; Loghin, S.; Berman, L.H. The Cracked Tooth: Histopathologic and Histobacteriologic Aspects. J. Endod. 2015, 41, 343–352. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Rôças, I.N.; Alves, F.R.F.; Silva, M.G. Bacteria in the Apical Root Canal of Teeth with Primary Apical Periodontitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, 721–726. [Google Scholar] [CrossRef]
- Sundqvist, G. Ecology of the Root Canal Flora. J. Endod. 1992, 18, 427–430. [Google Scholar] [CrossRef]
- Dioguardi, M.; Di Gioia, G.; Illuzzi, G.; Arena, C.; Caponio, V.C.A.; Caloro, G.A.; Zhurakivska, K.; Adipietro, I.; Troiano, G.; Lo Muzio, L. Inspection of the Microbiota in Endodontic Lesions. Dent. J. 2019, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Lupatini, M.; Suleiman, A.K.A.; Jacques, R.J.S.; Antoniolli, Z.I.; de Siqueira Ferreira, A.; Kuramae, E.E.; Roesch, L.F.W. Network Topology Reveals High Connectance Levels and Few Key Microbial Genera within Soils. Front. Environ. Sci. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Grenier, D.; Mayrand, D. Nutritional Relationships between Oral Bacteria. Infect. Immun. 1986, 53, 616–620. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, H.; Kobayashi, Y.; Francisco, I.; Isoshima, O.; Nagai, A.; Murayama, Y. A Microbiological and Immunological Study of Endodontic-Periodontic Lesions. J. Endod. 1995, 21, 617–621. [Google Scholar] [CrossRef]
- Zehnder, M.; Belibasakis, G.N. On the Dynamics of Root Canal Infections—What We Understand and What We Don’t. Virulence 2015, 6, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16S RRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Cintra, L.T.A.; Estrela, C.; Azuma, M.M.; de Azevedo Queiroz, Í.O.; Kawai, T.; Gomes-Filho, J.E. Endodontic Medicine: Interrelationships among Apical Periodontitis, Systemic Disorders, and Tissue Responses of Dental Materials. Braz. Oral Res. 2018, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, H.; Hirai, K.; Martins, C.M.; Furusho, H.; Battaglino, R.; Hashimoto, K. Interrelationship Between Periapical Lesion and Systemic Metabolic Disorders. Curr. Pharm. Des. 2016, 22, 2204–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Li, B.; Jiang, X.; Yang, Y.; Wells, G.F.; Zhang, T.; Li, X. Antibiotic Resistome in a Large-Scale Healthy Human Gut Microbiota Deciphered by Metagenomic and Network Analyses: Antibiotic Resistome. Environ. Microbiol. 2018, 20, 355–368. [Google Scholar] [CrossRef]
- Fitzpatrick, D.; Walsh, F. Antibiotic Resistance Genes across a Wide Variety of Metagenomes. FEMS Microbiol. Ecol. 2016, 92, fiv168. [Google Scholar] [CrossRef]
- Diaz-Torres, M.L.; Villedieu, A.; Hunt, N.; McNab, R.; Spratt, D.A.; Allan, E.; Mullany, P.; Wilson, M. Determining the Antibiotic Resistance Potential of the Indigenous Oral Microbiota of Humans Using a Metagenomic Approach. FEMS Microbiol. Lett. 2006, 258, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Forslund, K.; Sunagawa, S.; Kultima, J.R.; Mende, D.R.; Arumugam, M.; Typas, A.; Bork, P. Country-Specific Antibiotic Use Practices Impact the Human Gut Resistome. Genome Res. 2013, 23, 1163–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, E.M.; Passini, M.R.Z.; Kishi, L.T.; Chen, T.; Paster, B.J.; Gomes, B.P.F.A. Interrelationship between the Microbial Communities of the Root Canals and Periodontal Pockets in Combined Endodontic-Periodontal Diseases. Microorganisms 2021, 9, 1925. https://doi.org/10.3390/microorganisms9091925
Lopes EM, Passini MRZ, Kishi LT, Chen T, Paster BJ, Gomes BPFA. Interrelationship between the Microbial Communities of the Root Canals and Periodontal Pockets in Combined Endodontic-Periodontal Diseases. Microorganisms. 2021; 9(9):1925. https://doi.org/10.3390/microorganisms9091925
Chicago/Turabian StyleLopes, Erica M., Maicon R. Z. Passini, Luciano T. Kishi, Tsute Chen, Bruce J. Paster, and Brenda P. F. A. Gomes. 2021. "Interrelationship between the Microbial Communities of the Root Canals and Periodontal Pockets in Combined Endodontic-Periodontal Diseases" Microorganisms 9, no. 9: 1925. https://doi.org/10.3390/microorganisms9091925
APA StyleLopes, E. M., Passini, M. R. Z., Kishi, L. T., Chen, T., Paster, B. J., & Gomes, B. P. F. A. (2021). Interrelationship between the Microbial Communities of the Root Canals and Periodontal Pockets in Combined Endodontic-Periodontal Diseases. Microorganisms, 9(9), 1925. https://doi.org/10.3390/microorganisms9091925






