Ligilactobacillus salivarius PS2 Supplementation during Pregnancy and Lactation Prevents Mastitis: A Randomised Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Study Participants and Study Procedure
2.3. Intervention
2.4. Randomization
2.5. Data Collection
2.6. Study Outcomes
2.7. Determination of Sample Size
2.8. Statistical Analysis
3. Results
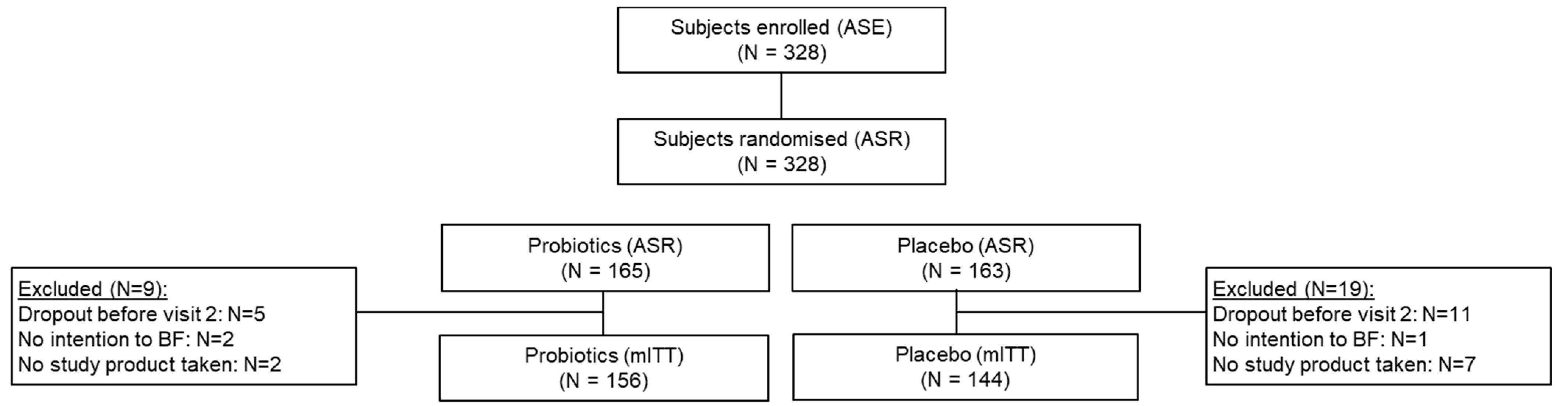
3.1. Study Population/Participants
3.2. Occurrence of Mastitis
3.3. Secondary and Exploratory Outcome
3.4. Safety and Tolerance Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Mastitis–Causes and Management. World Health Organization-Department of Child and Adolscent Health and Development. Available online: http://whqlibdoc.who.int/hq/2000/WHO_FCH_CAH_00.13.pdf (accessed on 30 June 2020).
- Abou-Dakn, M.; Louwen, F. S3-Leitlinie: Brustentzündungen in der Stillzeit–ein Thema für den Frauenarzt? Frauenarzt 2015, 8, 668–670. [Google Scholar]
- Dener, C.; Inan, A. Breast abscesses in lactating women. World J. Surg. 2003, 27, 130–133. [Google Scholar] [CrossRef]
- Gianni, M.L.; Bettinelli, M.E.; Manfra, P.; Sorrentino, G.; Bezze, E.; Plevani, L.; Cavallaro, G.; Raffaeli, G.; Crippa, B.L.; Colombo, L.; et al. Breastfeeding Difficulties and Risk for Early Breastfeeding Cessation. Nutrients 2019, 11, 2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, E.; Woodd, S.L.; Benova, L. Incidence of and Risk Factors for Lactational Mastitis: A Systematic Review. J. Hum. Lact. 2020, 36, 673–686. [Google Scholar] [CrossRef]
- Cusack, L.; Brennan, M. Lactational mastitis and breast abscess–diagnosis and management in general practice. Aust. Fam. Physician 2011, 40, 976–979. [Google Scholar]
- Contreras, G.A.; Rodríguez, J.M. Mastitis: Comparative etiology and epidemiology. J. Mammary Gland Biol. Neoplasia 2011, 16, 339–356. [Google Scholar] [CrossRef]
- Kvist, L.J. Toward a clarification of the concept of mastitis as used in empirical studies of breast inflammation during lactation. J. Hum. Lact. 2010, 26, 53–59. [Google Scholar] [CrossRef]
- de Oliveira, L.D.; Giugliani, E.R.; do Espírito Santo, L.C.; França, M.C.; Weigert, E.M.; Kohler, C.V.; de Lourenzi Bonilha, A.L. Effect of intervention to improve breastfeeding technique on the frequency of exclusive breastfeeding and lactation-related problems. J. Hum. Lact. 2006, 22, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Crepinsek, M.A.; Taylor, E.A.; Michener, K.; Stewart, F. Interventions for preventing mastitis after childbirth. Cochrane Database Syst. Rev. 2020, 9, Cd007239. [Google Scholar] [CrossRef] [PubMed]
- Amir, L.H.; Lumley, J.; Garland, S.M. A failed RCT to determine if antibiotics prevent mastitis: Cracked nipples colonized with Staphylococcus aureus: A randomized treatment trial [ISRCTN65289389]. BMC Pregnancy Childbirth 2004, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Jahanfar, S.; Ng, C.J.; Teng, C.L. Antibiotics for mastitis in breastfeeding women. Sao Paulo Med. J. 2016, 134, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirotta, M.V.; Garland, S.M. Genital Candida species detected in samples from women in Melbourne, Australia, before and after treatment with antibiotics. J. Clin. Microbiol. 2006, 44, 3213–3217. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.; Qi, C.; Zembower, T.; Noskin, G.A.; Bolon, M. Postpartum mastitis and community-acquired methicillin-resistant Staphylococcus aureus. Emerg. Infect. Dis. 2007, 13, 298–301. [Google Scholar] [CrossRef]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef]
- Arroyo, R.; Martin, V.; Maldonado, A.; Jimenez, E.; Fernandez, L.; Rodriguez, J.M. Treatment of infectious mastitis during lactation: Antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clin. Infect. Dis. 2010, 50, 1551–1558. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, E.; Fernández, L.; Maldonado, A.; Martín, R.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Oral administration of Lactobacillus strains isolated from breast milk as an alternative for the treatment of infectious mastitis during lactation. Appl. Environ. Microbiol. 2008, 74, 4650–4655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado-Lobón, J.A.; Díaz-López, M.A.; Carputo, R.; Duarte, P.; Díaz-Ropero, M.P.; Valero, A.D.; Sañudo, A.; Sempere, L.; Ruiz-López, M.D.; Bañuelos, Ó.; et al. Lactobacillus fermentum CECT 5716 Reduces Staphylococcus Load in the Breastmilk of Lactating Mothers Suffering Breast Pain: A Randomized Controlled Trial. Breastfeed. Med. 2015, 10, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Cárdenas, N.; Arroyo, R.; Manzano, S.; Jiménez, E.; Martín, V.; Rodríguez, J.M. Prevention of Infectious Mastitis by Oral Administration of Lactobacillus salivarius PS2 During Late Pregnancy. Clin. Infect. Dis. 2016, 62, 568–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurtado, J.A.; Maldonado-Lobón, J.A.; Díaz-Ropero, M.P.; Flores-Rojas, K.; Uberos, J.; Leante, J.L.; Affumicato, L.; Couce, M.L.; Garrido, J.M.; Olivares, M.; et al. Oral Administration to Nursing Women of Lactobacillus fermentum CECT5716 Prevents Lactational Mastitis Development: A Randomized Controlled Trial. Breastfeed. Med. 2017, 12, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Espinosa-Martos, I.; Jiménez, E.; de Andrés, J.; Rodríguez-Alcalá, L.M.; Tavárez, S.; Manzano, S.; Fernández, L.; Alonso, E.; Fontecha, J.; Rodríguez, J.M. Milk and blood biomarkers associated to the clinical efficacy of a probiotic for the treatment of infectious mastitis. Benef. Microbes 2016, 7, 305–318. [Google Scholar] [CrossRef] [Green Version]
- Kvist, L.J.; Larsson, B.W.; Hall-Lord, M.L.; Steen, A.; Schalén, C. The role of bacteria in lactational mastitis and some considerations of the use of antibiotic treatment. Int. Breastfeed. J. 2008, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.A.; Robertson, M.; Fitzpatrick, J.; Knight, C.; Mulholland, S. Occurrence of lactational mastitis and medical management: A prospective cohort study in Glasgow. Int. Breastfeed. J. 2008, 3, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mediano, P.; Fernández, L.; Rodríguez, J.M.; Marín, M. Case-control study of risk factors for infectious mastitis in Spanish breastfeeding women. BMC Pregnancy Childbirth 2014, 14, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-Fresno, R.; Llorach, R.; Marinic, J.; Tulipani, S.; Garcia-Aloy, M.; Espinosa-Martos, I.; Jiménez, E.; Rodríguez, J.M.; Andres-Lacueva, C. Urinary metabolomic fingerprinting after consumption of a probiotic strain in women with mastitis. Pharm. Res. 2014, 87, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Barker, M.; Peters, M.D.J.; Adelson, P.; Steen, M. Probiotics and human lactational mastitis: A scoping review protocol. JBI Database Syst. Rev. Implement. Rep. 2019, 18, 1341–1348. [Google Scholar] [CrossRef]
- Mitrano, J.A.; Spooner, L.M.; Belliveau, P. Excretion of antimicrobials used to treat methicillin-resistant Staphylococcus aureus infections during lactation: Safety in breastfeeding infants. Pharmacotherapy 2009, 29, 1103–1109. [Google Scholar] [CrossRef]
- Rowe, H.E.; Felkins, K.; Cooper, S.D.; Hale, T.W. Transfer of linezolid into breast milk. J. Hum. Lact. 2014, 30, 410–412. [Google Scholar] [CrossRef]
- Roberts, S.C.; Zembower, T.R. Global increases in antibiotic consumption: A concerning trend for WHO targets. Lancet Infect. Dis. 2021, 21, 10–11. [Google Scholar] [CrossRef]
- Salmanov, A.G.; Savchenko, S.E.; Chaika, K.; Vitiuk, A.D.; Ruban, I.; Dyndar, O.A.; Zhelezov, D.; Vorobey, L.; Semeniuk, L.M.; Hetsko, N.V.; et al. Postpartum mastitis in the breastfeeding women and antimicrobial resistance of responsible pathogens in ukraine: Results a multicenter study. Wiad. Lek. 2020, 73, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; de Andrés, J.; Manrique, M.; Pareja-Tobes, P.; Tobes, R.; Martínez-Blanch, J.F.; Codoñer, F.M.; Ramón, D.; Fernández, L.; Rodríguez, J.M. Metagenomic Analysis of Milk of Healthy and Mastitis-Suffering Women. J. Hum. Lact. 2015, 31, 406–415. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Field, D.; Ryan, C.A.; Stanton, C.; Hill, C.; Ross, R.P. The microbiology and treatment of human mastitis. Med. Microbiol. Immunol. 2018, 207, 83–94. [Google Scholar] [CrossRef]
- Mediano, P.; Fernández, L.; Jiménez, E.; Arroyo, R.; Espinosa-Martos, I.; Rodríguez, J.M.; Marín, M. Microbial Diversity in Milk of Women with Mastitis: Potential Role of Coagulase-Negative Staphylococci, Viridans Group Streptococci, and Corynebacteria. J. Hum. Lact. 2017, 33, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.H.; Vaidya, Y.H.; Patel, R.J.; Pandit, R.J.; Joshi, C.G.; Kunjadiya, A.P. Culture independent assessment of human milk microbial community in lactational mastitis. Sci. Rep. 2017, 7, 7804. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, L.; García-Carral, C.; Rodriguez, J.M. Unfolding the Human Milk Microbiome Landscape in the Omics Era. Front. Microbiol. 2019, 10, 1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Andrés, J.; Jiménez, E.; Chico-Calero, I.; Fresno, M.; Fernández, L.; Rodríguez, J.M. Physiological Translocation of Lactic Acid Bacteria during Pregnancy Contributes to the Composition of the Milk Microbiota in Mice. Nutrients 2017, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.M. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Sakwinska, O.; Bosco, N. Host Microbe Interactions in the Lactating Mammary Gland. Front. Microbiol. 2019, 10, 1863. [Google Scholar] [CrossRef]
- de Andrés, J.; Jiménez, E.; Espinosa-Martos, I.; Rodríguez, J.M.; García-Conesa, M.T. An Exploratory Search for Potential Molecular Targets Responsive to the Probiotic Lactobacillus salivarius PS2 in Women with Mastitis: Gene Expression Profiling vs. Interindividual Variability. Front. Microbiol. 2018, 9, 2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Placebo (N = 144) | Probiotics (N = 156) | |
|---|---|---|
| Age at inclusion (years) † | 33.0 (30.0–36.0) | 33.0 (30.0–36.0) |
| Pre-gravid BMI (kg/m2) † | 21.8 (20.4–24.0) | 22.3 (20.3–24.4) |
| Number of previous children, n (%) | ||
| 0 | 67 (46.5%) | 64 (41.0%) |
| 1 | 57 (39.6%) | 66 (42.3%) |
| 2 | 15 (10.4%) | 24 (15.4%) |
| ≥3 | 5 (3.5%) | 2 (1.3%) |
| Previous Mastitis, n (%) | ||
| No | 124 (86.1%) | 140 (89.7%) |
| Yes | 20 (13.9%) | 16 (10.3%) |
| Infant birth weight (g) † | 3385.0 (3095.0–3652.5) | 3367.5 (3144.5–3617.5) |
| Gestational age, weeks † | 40.4 (39.4–41.1) | 40.5 (39.9–41.1) |
| Type of birth | ||
| C-section, n (%) | 59 (41.0%) | 58 (37.2%) |
| Vaginal, n (%) | 85 (59.0%) | 98 (62.8%) |
| Sex of the infant | ||
| Female, n (%) | 70 (48.6%) | 73 (46.8%) |
| Male, n (%) | 74 (51.4%) | 83 (53.2%) |
| Placebo (N = 22) | Probiotic (N = 9) | |
|---|---|---|
| Fever due to mastitis | ||
| Absent n (%) | 13 (59.1%) | 4 (44.4%) |
| Present n (%) | 6 (27.3%) | 5 (55.6%) |
| Unspecified n (%) | 3 (13.6%) | 0 (0.0%) |
| Duration of mastitis episodes, days † | 15.0 (11.0, 22.0) | 16.0 (10.0–18.0) |
| Antibiotic use | ||
| Yes n (%) | 16 (72.7%) | 4 (44.4%) |
| No n (%) | 6 (27.3%) | 5 (55.6%) |
| Anti-inflammatory drugs | ||
| Yes, n (%) | 15 (68.2%) | 7 (77.8%) |
| No n (%) | 7 (31.8%) | 2 (22.2%) |
| Other | ||
| Yes n (%) | 2 (9.1%) | 2 (22.2%) |
| No n (%) | 20 (90.9%) | 7 (77.8%) |
| Placebo (N = 22) | Probiotic (N = 9) | ||
|---|---|---|---|
| Mastitis pain score | |||
| Visit A1 | n (n miss) | 19 (3) | 9 (0) |
| Mean (SD) | 5.8 (2.3) | 6.9 (1.4) | |
| Median (Q1, Q3) | 7.0 (3.0, 8.0) | 6.0 (6.0, 7.0) | |
| Visit A2 | n (n miss) | 18 (4) | 8 (1) |
| Mean (SD) | 2.2 (2.7) | 1.1 (2.4) | |
| Median (Q1, Q3) | 1.0 (0.0, 3.0) | 0.0 (0.0, 1.0) | |
| Change in mastitis pain score † | |||
| n (n miss) | 18 (4) | 8 (1) | |
| Mean (SD) | −3.7 (2.6) | −5.9 (2.5) | |
| Median (Q1, Q3) | −3.0 (−6.0, −2.0) | −6.0 (−7.0, −5.0) | |
| Mastitis severity index | |||
| Visit A1 | n (n miss) | 19 (3) | 9 (0) |
| Mean (SD) | 11.4 (3.3) | 12.1 (1.1) | |
| Median (Q1, Q3) | 12.0 (8.0, 15.0) | 12.0 (11.0, 13.0) | |
| Visit A2 | n (n miss) | 18 (4) | 8 (1) |
| Mean (SD) | 3.9 (5.2) | 2.3 (4.5) | |
| Median (Q1, Q3) | 1.0 (0.0, 6.0) | 0.5 (0.0, 2.0) | |
| Change in mastitis severity index † | |||
| n (n miss) | 18 (4) | 8 (1) | |
| Mean (SD) | −7.7 (4.8) | −10.0 (3.9) | |
| Median (Q1, Q3) | −8.0 (−11.0, −5.0) | −11.5 (−12.0, −9.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez, E.; Manzano, S.; Schlembach, D.; Arciszewski, K.; Martin, R.; Ben Amor, K.; Roelofs, M.; Knol, J.; Rodríguez, J.M.; Abou-Dakn, M.; et al. Ligilactobacillus salivarius PS2 Supplementation during Pregnancy and Lactation Prevents Mastitis: A Randomised Controlled Trial. Microorganisms 2021, 9, 1933. https://doi.org/10.3390/microorganisms9091933
Jiménez E, Manzano S, Schlembach D, Arciszewski K, Martin R, Ben Amor K, Roelofs M, Knol J, Rodríguez JM, Abou-Dakn M, et al. Ligilactobacillus salivarius PS2 Supplementation during Pregnancy and Lactation Prevents Mastitis: A Randomised Controlled Trial. Microorganisms. 2021; 9(9):1933. https://doi.org/10.3390/microorganisms9091933
Chicago/Turabian StyleJiménez, Esther, Susana Manzano, Dietmar Schlembach, Krzysztof Arciszewski, Rocio Martin, Kaouther Ben Amor, Mieke Roelofs, Jan Knol, Juan Miguel Rodríguez, Michael Abou-Dakn, and et al. 2021. "Ligilactobacillus salivarius PS2 Supplementation during Pregnancy and Lactation Prevents Mastitis: A Randomised Controlled Trial" Microorganisms 9, no. 9: 1933. https://doi.org/10.3390/microorganisms9091933
APA StyleJiménez, E., Manzano, S., Schlembach, D., Arciszewski, K., Martin, R., Ben Amor, K., Roelofs, M., Knol, J., Rodríguez, J. M., Abou-Dakn, M., & PREMIUM Study Group. (2021). Ligilactobacillus salivarius PS2 Supplementation during Pregnancy and Lactation Prevents Mastitis: A Randomised Controlled Trial. Microorganisms, 9(9), 1933. https://doi.org/10.3390/microorganisms9091933







