Neonatal Exposure to Agonists and Antagonists of Sex Steroid Receptors Affects AMH and FSH Plasma Level and Their Receptors Expression in the Adult Pig Ovary
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of Experiment and Sample Collection
2.2. Hormone Assays
2.3. Quantitative Real-Time PCR
2.4. Western Blotting
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
3.1. Plasma AMH and FSH Concentrations
3.2. Expression of mRNA and Protein for ACVR1, BMPR1A, AMHR2, AMH, and FSHR in Preantral Follicles
3.3. Expression of mRNA and Protein for ACVR1, BMPR1A, AMHR2, AMH, and FSHR in Small Antral Follicles
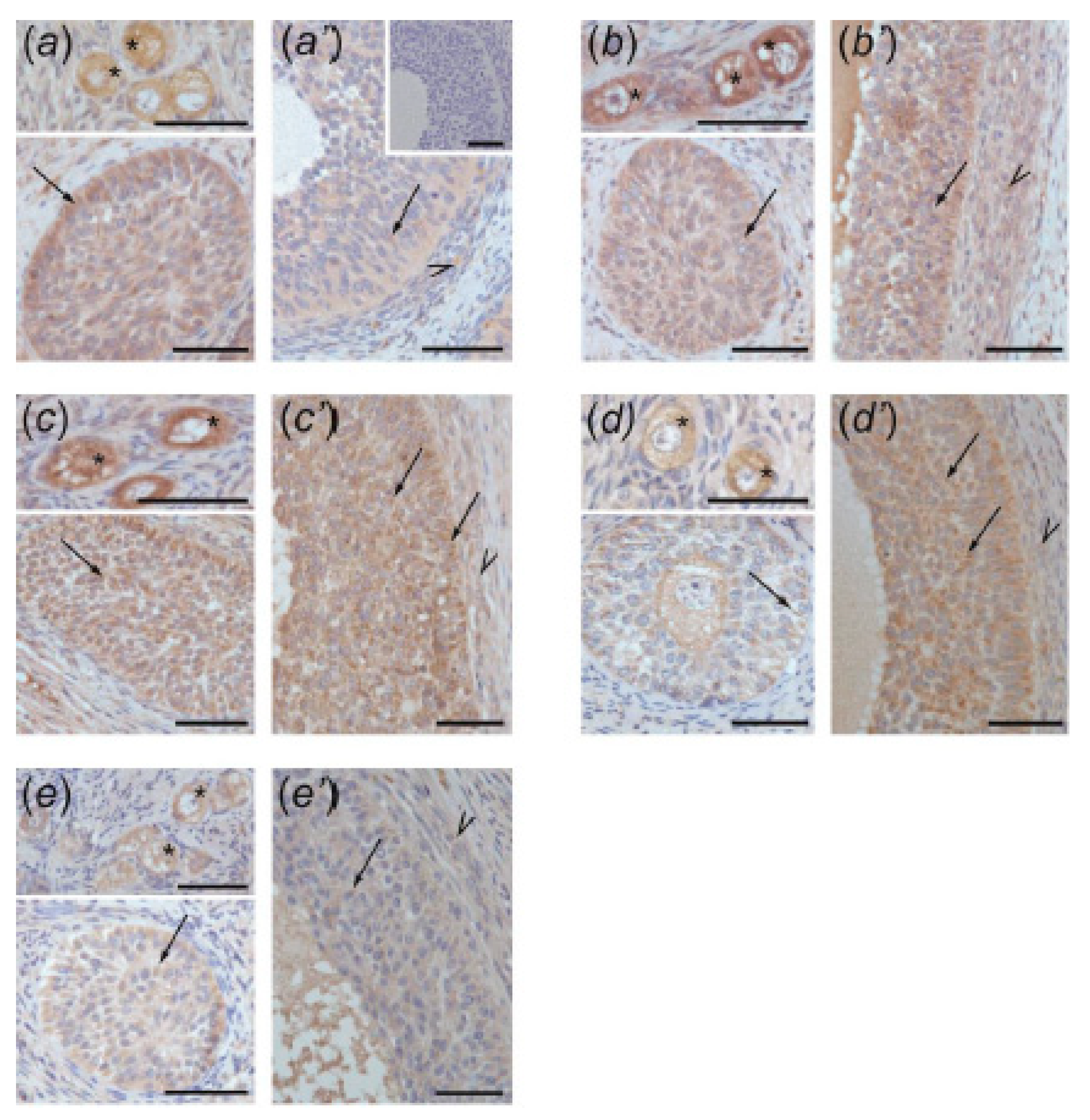
3.4. Immunolocalization of ACVR1, BMPR1A, AMHR2, and FSHR in Ovarian Follicles
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pepling, M.E. Follicular assembly: Mechanisms of action. Reproduction 2012, 143, 139–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, F.R.C.L.; Costermans, N.G.J.; Soede, N.M.; Bunschoten, A.; Keijer, J.; Kemp, B.; Teerds, K.J. Presence of anti-Müllerian hormone (AMH) during follicular development in the porcine ovary. PLoS ONE 2018, 13, e0197894. [Google Scholar] [CrossRef] [PubMed]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Ingraham, H.A.; Nachtigal, M.W.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002, 143, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Kumar, T.R.; Matzuk, M.M.; Rose, U.M.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001, 142, 4891–4899. [Google Scholar] [CrossRef] [PubMed]
- Di Clemente, N.; Josso, N.; Gouédard, L.; Belville, C. Components of the anti-Müllerian hormone signaling pathway in gonads. Mol. Cell. Endocrinol. 2003, 211, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Monniaux, D.; Clément, F.; Dalbiès-Tran, R.; Estienne, A.; Fabre, S.; Mansanet, C.; Monget, P. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: What is the link? Biol. Reprod. 2014, 90, 85. [Google Scholar] [CrossRef] [Green Version]
- Veiga-Lopez, A.; Luense, L.J.; Christenson, L.K.; Padmanabhan, V. Developmental programming: Gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology 2013, 154, 1873–1884. [Google Scholar] [CrossRef] [Green Version]
- Zama, A.M.; Uzumcu, M. Targeted genome-wide methylation and gene expression analyses reveal signalling pathways involved in ovarian dysfunction after developmental EDC exposure in rats. Biol. Reprod. 2013, 88, 52. [Google Scholar] [CrossRef] [Green Version]
- Uzumcu, M.; Zachow, R. Developmental exposure to environmental endocrine disruptors: Consequences within the ovary and on female reproductive function. Reprod. Toxicol. 2007, 23, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Knapczyk-Stwora, K.; Grzesiak, M.; Ciereszko, R.E.; Czaja, E.; Koziorowski, M.; Slomczynska, M. The impact of sex steroid agonists and antagonists on folliculogenesis in the neonatal porcine ovary via cell proliferation and apoptosis. Theriogenology 2018, 113, 19–26. [Google Scholar] [CrossRef]
- Knapczyk-Stwora, K.; Grzesiak, M.; Witek, P.; Duda, M.; Koziorowski, M.; Slomczynska, M. Neonatal exposure to agonists and antagonists of sex steroid receptors induces changes in the expression of oocyte-derived growth factors and their receptors in ovarian follicles in gilts. Theriogenology 2019, 134, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Durlej, M.; Knapczyk-Stwora, K.; Duda, M.; Galas, J.; Slomczynska, M. The expression of FSH receptor (FSHR) in the neonatal porcine ovary and its regulation by flutamide. Reprod. Domest. Anim. 2011, 46, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Aydoğan, M.; Barlas, N. Effects of maternal 4-tert-octylphenol exposure on the reproductive tract of male rats at adulthood. Reprod. Toxicol. 2006, 22, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Kummer, V.; Masková, J.; Zralý, Z.; Neca, J.; Simecková, P.; Vondrácek, J.; Machala, M. Estrogenic activity of environmental polycyclic aromatic hydrocarbons in uterus of immature Wistar rats. Toxicol. Lett. 2008, 180, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, V.; Broyde, M.; Sharpe, R.; Welsh, M.; Drake, A.J.; McNeilly, A.S. Effect of androgen treatment during foetal and/or neonatal life on ovarian function in prepubertal and adult rats. Reproduction 2012, 143, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.; McKinnell, C.; Saunders, P.T.; Walker, M.; Fisher, J.S.; Turner, K.J.; Atanassova, N.; Sharpe, M. Neonatal exposure to potent and environmental oestrogens and abnormalities of the male reproductive system in the rat: Evidence for importance of the androgen-oestrogen balance and assessment of the relevance to man. Hum. Reprod. Update 2001, 7, 236–247. [Google Scholar] [CrossRef]
- Zhao, S.; Fernald, R.D. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Grzesiak, M.; Knapczyk-Stwora, K.; Duda, M.; Slomczynska, M. Elevated level of 17β-estradiol is associated with over expression of FSHR, CYP19A1, and CTNNB1 genes in porcine ovarian follicles after prenatal and neonatal flutamide exposure. Theriogenology 2012, 78, 2050–2060. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Findlay, J.K.; Hutt, K.J.; Hickey, M.; Anderson, R.A. How is the number of primordial follicles in the ovarian reserve established? Biol. Reprod. 2015, 93, 111. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Kramer, P.; Karels, B.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology 1999, 140, 5789–5796. [Google Scholar] [CrossRef] [PubMed]
- Daneshian, Z.; Ramezani Tehrani, F.; Zarkesh, M.; Norooz Zadeh, M.; Mahdian, R.; Zadeh Vakili, A. Antimullerian hormone and its receptor gene expression in prenatally androgenized female rats. Int. J. Endocrinol. Metab. 2015, 13, e19511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGee, E.A.; Smith, R.; Spears, N.; Nachtigal, M.W.; Ingraham, H.; Hsueh, A.J. Müllerian inhibitory substance induces growth of rat preantral ovarian follicles. Biol. Reprod. 2001, 64, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, M.; Thomson, K.; Fenwick, M.; Mora, J.; Franks, S.; Hardy, K. Androgen stimulates growth of mouse preantral follicles in vitro: Interaction with follicle-stimulating hormone and with growth factors of the TGFβ superfamily. Endocrinology 2017, 158, 920–935. [Google Scholar] [CrossRef] [Green Version]
- Cruz, G.; Foster, W.; Paredes, A.; Yi, K.D.; Uzumcu, M. Long-term effects of early-life exposure to environmental oestrogens on ovarian function: Role of epigenetics. J. Neuroendocrinol. 2014, 26, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Gervásio, C.G.; Bernuci, M.P.; Silva-de-Sá, M.F.; Rosa-E-Silva, A.C. The role of androgen hormones in early follicular development. ISRN Obstet. Gynecol. 2014, 2014, 818010. [Google Scholar] [CrossRef] [Green Version]
- Knapczyk-Stwora, K.; Grzesiak, M.; Duda, M.; Koziorowski, M.; Galas, J.; Slomczynska, M. TGFβ (transforming growth factor β) superfamily members and their receptors in the fetal porcine ovaries: Effect of prenatal flutamide treatment. Folia Histochem. Cytobiol. 2014, 52, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Britt, K.L.; Findlay, J.K. Estrogen actions in the ovary revisited. J. Endocrinol. 2002, 175, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Abbott, D.H.; Padmanabhan, V.; Dumesic, D.A. Contributions of androgen and estrogen to fetal programming of ovarian dysfunction. Reprod. Biol. Endocrinol. 2006, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Cui, Y.Q.; Zhao, H.; Liu, H.B.; Zhao, S.D.; Gao, Y.; Mu, X.L.; Gao, F.; Chen, Z.J. High levels of testosterone inhibit ovarian follicle development by repressing the FSH signaling pathway. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015, 35, 723–729. [Google Scholar] [CrossRef]
- Quinn, R.L.; Shuttleworth, G.; Hunter, M.G. Immunohistochemical localization of the bone morphogenetic protein receptors in the porcine ovary. J. Anat. Physiol. 2004, 205, 15–23. [Google Scholar] [CrossRef] [PubMed]







| Antibody | Dilution Used for | Host | Type | Supplier | |
|---|---|---|---|---|---|
| WB | IHC | ||||
| Anti-ACVR1 | 1:1000 | 1:200 | Goat | Polyclonal | Acris Antibodies GmbH, Herford, Germany (AP22507PU-N) |
| Anti-BMPR1A | 1:1000 | 1:100 | Rabbit | Polyclonal | Kindly provided by Prof. C. H. Heldin (Ludwig Institute for Cancer Research Ltd., Uppsala, Sweden) |
| Anti-AMHR2 | 1:1000 | 1:100 | Rabbit | Polyclonal | LifeSpan BioSciences Inc., Seattle, WA, USA (LS-B11943) |
| Anti-AMH | 1:1000 | - | Rabbit | Polyclonal | Acris Antibodies GmbH, Herford, Germany (TA336233) |
| Anti-FSHR | 1:1000 | 1:100 | Rabbit | Polyclonal | Bioss Antibodies, Woburn, MA, USA (bs-0895R) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapczyk-Stwora, K.; Grzesiak, M.; Witek, P.; Duda, M.; Koziorowski, M.; Slomczynska, M. Neonatal Exposure to Agonists and Antagonists of Sex Steroid Receptors Affects AMH and FSH Plasma Level and Their Receptors Expression in the Adult Pig Ovary. Animals 2020, 10, 12. https://doi.org/10.3390/ani10010012
Knapczyk-Stwora K, Grzesiak M, Witek P, Duda M, Koziorowski M, Slomczynska M. Neonatal Exposure to Agonists and Antagonists of Sex Steroid Receptors Affects AMH and FSH Plasma Level and Their Receptors Expression in the Adult Pig Ovary. Animals. 2020; 10(1):12. https://doi.org/10.3390/ani10010012
Chicago/Turabian StyleKnapczyk-Stwora, Katarzyna, Malgorzata Grzesiak, Patrycja Witek, Malgorzata Duda, Marek Koziorowski, and Maria Slomczynska. 2020. "Neonatal Exposure to Agonists and Antagonists of Sex Steroid Receptors Affects AMH and FSH Plasma Level and Their Receptors Expression in the Adult Pig Ovary" Animals 10, no. 1: 12. https://doi.org/10.3390/ani10010012
APA StyleKnapczyk-Stwora, K., Grzesiak, M., Witek, P., Duda, M., Koziorowski, M., & Slomczynska, M. (2020). Neonatal Exposure to Agonists and Antagonists of Sex Steroid Receptors Affects AMH and FSH Plasma Level and Their Receptors Expression in the Adult Pig Ovary. Animals, 10(1), 12. https://doi.org/10.3390/ani10010012





