Comparison between the Effects of Adding Vitamins, Trace Elements, and Nanoparticles to SHOTOR Extender on the Cryopreservation of Dromedary Camel Epididymal Spermatozoa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Nano-Sized Elements (Particle Size, Zeta Potential, and Ultramorphology)
2.2. Epididymal Camel Spermatozoa Collection
2.3. Experimental Design
2.4. Epididymal Spermatozoa Evaluation
2.4.1. Sperm Progressive Motility
2.4.2. Sperm Vitality and Abnormalities
2.4.3. Sperm Plasma Membrane Integrity
2.4.4. Antioxidants Assay
2.4.5. Assessment of Sperm Apoptosis and Necrosis through Flowcytometric Analysis
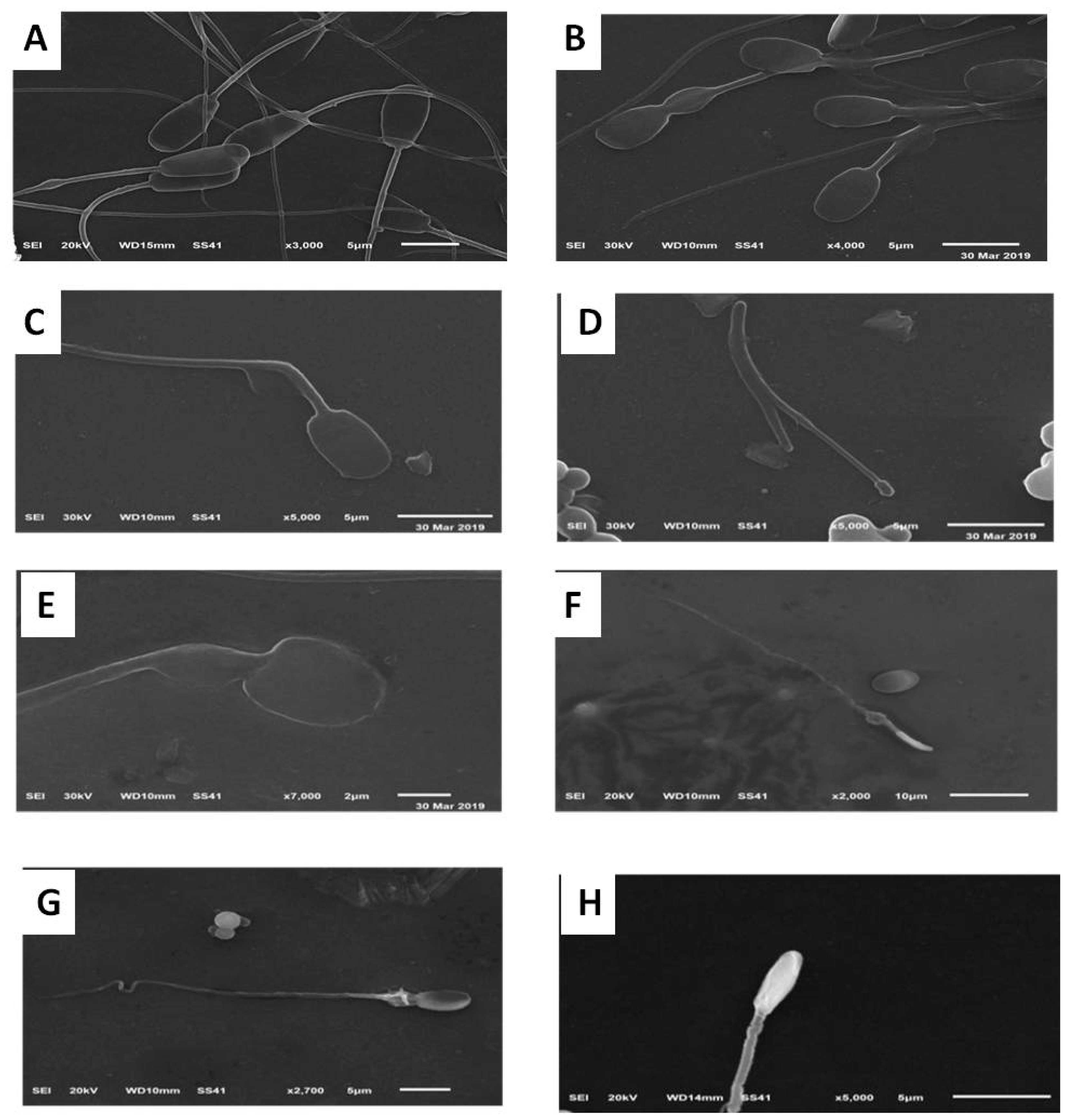
2.4.6. Assessment of Sperm Morphology Using Scanning Electron Microscope (SEM)
2.4.7. Assessment of Sperm Ultrastructure Using Transmission Electron Microscope (TEM)
2.5. Statistical Analysis
3. Results
3.1. Effects on Sperm Quality After Cooling (5 °C for 2 h) and Pre-Freezing
3.2. Effects on Post-Thawing Sperm Quality
3.3. Effects on Sperm Apoptosis and Necrosis (Annexin V/PI Assay) Post-Thawing
3.4. Effects on Oxidative Stress of the Extender Post-Thawing
3.5. Effects on Sperm Ultra-Morphological Characters of Plasma Membrane (PM) and Acrosome Post-Thawing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skidmore, J. Reproduction in dromedary camels: An update. Anim. Reprod. Sci. 2005, 2, 161–171. [Google Scholar]
- Spencer, P.; Wilson, K.; Tinson, A. Parentage testing of racing camels (Camelus dromedarius) using microsatellite DNA typing. Anim. Genet. 2010, 41, 662–665. [Google Scholar] [CrossRef]
- Swelum, A.A.; Ismael, A.B.; Khalaf, A.F.; Abouheif, M.A. Clinical and laboratory findings associated with naturally occurring babesiosis in dromedary camels. Bull. Vet. Inst. Pulawy 2014, 58, 229–233. [Google Scholar] [CrossRef] [Green Version]
- El-Hassanein, E.-S. Prospects of Improving Semen Collection and Preservation from Elite Dromedary Camel Breeds. World Vet. J. 2017, 7, 47–64. [Google Scholar] [CrossRef]
- Derar, D.R.; Hussein, H.A.; Ali, A. Reference values for the genitalia of male dromedary before and after puberty using caliper and ultrasonography in subtropics. Theriogenology 2012, 77, 459–465. [Google Scholar] [CrossRef]
- Swelum, A.A.; Saadeldin, I.M.; Ba-Awadh, H.; Alowaimer, A.N. Shortened daily photoperiod during the non-breeding season can improve the reproductive performance of camel bulls (Camelus dromedarius). Anim. Reprod. Sci. 2018, 195, 334–344. [Google Scholar] [CrossRef]
- Swelum, A.A.; Saadeldin, I.M.; Ba-Awadh, H.; Alowaimer, A.N. Effects of melatonin implants on the reproductive performance and endocrine function of camel (Camelus dromedarius) bulls during the non-breeding and subsequent breeding seasons. Theriogenology 2018, 119, 18–27. [Google Scholar] [CrossRef]
- Swelum, A.A.; Saadeldin, I.M.; Ba-Awadh, H.; Al-Mutary, M.G.; Alowaimer, A.N. Effect of short artificial lighting and low temperature in housing rooms during non-rutting season on reproductive parameters of male dromedary camels. Theriogenology 2019, 131, 133–139. [Google Scholar] [CrossRef]
- Wani, N.A.; Morton, K.; Billah, M.; Skidmore, J. Biophysical and biochemical characteristics of ejaculated semen of dromedary camel (Camelus dromedarius) and Llama (Llama glama). J. Camel. Pract. Res. 2011, 18, 97–102. [Google Scholar]
- Swelum, A.A.; Saadeldin, I.; Ba-Awadh, H.G.; Al-Mutary, M.F.; Moumen, A.N.; Alowaimer, A.; Abdalla, H. Efficiency of Commercial Egg Yolk-Free and Egg Yolk-Supplemented Tris-Based Extenders for Dromedary Camel Semen Cryopreservation. Animals 2019, 9, 999. [Google Scholar] [CrossRef] [Green Version]
- Waheed, M.; Al-Eknah, M.; El-Bahr, S. Some biochemical characteristics and preservation of epididymal camel spermatozoa (Camelus dromedarius). Theriogenology 2011, 76, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, J.A.; Malo, C.M.; Crichton, E.G.; Morrell, J.M.; Pukazhenthi, B.S. An update on semen collection, preservation and artificial insemination in the dromedary camel (Camelus dromedarius). Anim. Reprod. Sci. 2018, 194, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Rodenburg, S.E.; Huang, C.; Vandevoort, C.A. Cryopreservation of rhesus monkey (Macaca mulatta) epididymal spermatozoa before and after refrigerated storage. J. Androl. 2008, 29, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, A.; Ansari, M.; Garg, A.; Kataria, M. Effect of oviductal proteins on sperm functions and lipid peroxidation levels during cryopreservation in buffaloes. Animl. Reprod. Sci. 2006, 93, 246–257. [Google Scholar] [CrossRef]
- Bucak, M.N.; Ateşşahin, A.; Yüce, A. Effect of anti-oxidants and oxidative stress parameters on ram semen after the freeze-thawing process. Small Rum. Res. 2008, 75, 128–134. [Google Scholar] [CrossRef]
- Sardoy, M.; Carretero, M.; Neild, D. Evaluation of stallion sperm DNA alterations during cryopreservation using toluidine blue. Anim. Reprod. Sci. 2008, 3, 349–350. [Google Scholar] [CrossRef]
- El-Badry, D.; Azab, A.; Karima Gh, M.; Scholkamy, T. The effect of extender type on freezability of one-humped camel spermatozoa with special reference to their fine structure. In Proceedings of the 25th Annual Conf, Taba, Egypt, 14–17 April 2016. [Google Scholar]
- El-Harairy, M.A.; El-Razek, I.M.A.; Shamiah, S.M.; Zaghloul, H.; Khalil, W. Effect of antioxidants on the stored dromedary camel epididymal sperm characteristics. Asian J. Anim. Sci. 2016, 10, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Kotdawala, A.P.; Kumar, S.; Salian, S.R.; Thankachan, P.; Govindraj, K.; Kumar, P.; Kalthur, G.; Adiga, S.K. Addition of zinc to human ejaculate prior to cryopreservation prevents freeze-thaw-induced DNA damage and preserves sperm function. J. Assist. Reprod. Genet. 2012, 29, 1447–1453. [Google Scholar] [CrossRef] [Green Version]
- Barkhordari, A.; Hekmatimoghaddam, S.; Jebali, A.; Khalili, M.A.; Talebi, A.; Noorani, M. Effect of zinc oxide nanoparticles on viability of human spermatozoa. Iran. J. Reprod. Med. 2013, 11, 767. [Google Scholar]
- Dawson, E.B.; Harris, W.A.; Teter, M.C.; Powell, L.C. Effect of ascorbic acid supplementation on the sperm quality of smokers. Fertil. Steril. 1992, 58, 1034–1039. [Google Scholar] [CrossRef]
- Abdelazim, A.M.; Saadeldin, I.M.; Swelum, A.A.; Afifi, M.M.; Alkaladi, A. Oxidative Stress in the Muscles of the Fish Nile Tilapia Caused by Zinc Oxide Nanoparticles and Its Modulation by Vitamins C and E. Oxid. Med. Cell. Longev. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chinoy, N.; Mehta, R.; Seethalakshmi, L.; Sharma, J.; Chinoy, M. Effects of vitamin C deficiency on physiology of male reproductive organs of guinea pigs. Int. J. Fertil. 1986, 31, 232–239. [Google Scholar] [PubMed]
- Benhenia, K.; Lamara, A.; Fatmi, S.; Iguer-Ouada, M. Effect of cyclodextrins, cholesterol and vitamin E and their complexation on cryopreserved epididymal ram semen. Small Rum. Res. 2016, 141, 29–35. [Google Scholar] [CrossRef]
- El-Sawy, H.B.I.; Soliman, M.M.; El-Shazly, S.A.; Ali, H.A.-M. Protective effects of camel milk and vitamin E against monosodium glutamate induced biochemical and testicular dysfunctions. Prog. Nutr. 2018, 20, 76–85. [Google Scholar]
- Hu, J.-H.; Zhao, X.-L.; Tian, W.-Q.; Zan, L.-S.; Li, Q.-W. Effects of vitamin E supplementation in the extender on frozen-thawed bovine semen preservation. Animal 2011, 5, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Yousef, M.; Abdallah, G.; Kamel, K. Effect of ascorbic acid and vitamin E supplementation on semen quality and biochemical parameters of male rabbits. Anim. Reprod. Sci. 2003, 76, 99–111. [Google Scholar] [CrossRef]
- Safa, S.; Moghaddam, G.; Jozani, R.J.; Kia, H.D.; Janmohammadi, H. Effect of vitamin E and selenium nanoparticles on post-thaw variables and oxidative status of rooster semen. Anim. Reprod. Sci. 2016, 174, 100–106. [Google Scholar] [CrossRef]
- Luo, H.; Jia, Z.; Zhu, S.; Ding, J. Effect of vitamin E on the qualities of fresh and frozen-thawed Ram Semen. China Herbiv. Sci. 2004, 5, 4–16. [Google Scholar]
- Zago, M.P.; Oteiza, P.I. The antioxidant properties of zinc: Interactions with iron and antioxidants. Free Radical. Biol. Med. 2001, 31, 266–274. [Google Scholar] [CrossRef]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an essential element for male fertility: A review of zn roles in men’s health, germination, sperm quality, and fertilization. J. Reprod. Infertil. 2018, 19, 69. [Google Scholar]
- Dorostkar, K.; Alavi-Shoushtari, S.M.; Mokarizadeh, A. Effects of in vitro selenium addition to the semen extender on the spermatozoa characteristics before and after freezing in water buffaloes (Bubalus bubalis). Vet. Res. Forum 2012, 3, 263–268. [Google Scholar] [PubMed]
- Ghallab, A.M.; Shahat, A.M.; Fadl, A.M.; Ayoub, M.M.; Moawad, A.R. Impact of supplementation of semen extender with antioxidants on the quality of chilled or cryopreserved Arabian stallion spermatozoa. Cryobiology 2017, 79, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Heim, S.; Kiess, M.; Maiorino, M.; Roveri, A.; Wissing, J.; Flohe, L. Dual function of the selenoprotein PHGPx during sperm maturation. Science 1999, 285, 1393–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marai, I.F.M.; El-Darawany, A.-H.; Ismail, E.; Abdel-Hafez, M.A.M. Reproductive and physiological traits of Egyptian Suffolk rams as affected by selenium dietary supplementation and housing heat radiation effects during winter of the sub-tropical environment of Egypt. Archiv. Anim. Breed. 2009, 52, 402–409. [Google Scholar] [CrossRef]
- Shi, L.-G.; Yang, R.-J.; Yue, W.-B.; Xun, W.-J.; Zhang, C.-X.; Ren, Y.-S.; Shi, L.; Lei, F.-L. Effect of elemental nano-selenium on semen quality, glutathione peroxidase activity, and testis ultrastructure in male Boer goats. Anim. Reprod. Sci. 2010, 118, 248–254. [Google Scholar] [CrossRef]
- Peters, K.; Unger, R.E.; Kirkpatrick, C.J.; Gatti, A.M.; Monari, E. Effects of nano-scaled particles on endothelial cell function in vitro: Studies on viability, proliferation and inflammation. J. Mat. Sci. Mater. Med. 2004, 15, 321–325. [Google Scholar] [CrossRef]
- Afifi, M.; Almaghrabi, O.A.; Kadasa, N.M. Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. BioMed Res. Int. 2015, 2015, 153573. [Google Scholar] [CrossRef]
- Hidiroglou, M.; Knipfel, J. Zinc in mammalian sperm: A review. J. Dairy Sci. 1984, 67, 1147–1156. [Google Scholar] [CrossRef]
- Cheah, Y.; Yang, W. Functions of essential nutrition for high quality spermatogenesis. Adv. Biosci. Biotechnol. 2011, 2, 182. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, D.; Wijesinghe, P.; Ratnasooriya, W.; Wimalasena, S. Relationship between seminal plasma zinc and semen quality in a subfertile population. J. Hum. Reprod. Sci. 2010, 3, 124. [Google Scholar] [CrossRef]
- Baumber, J.; Ball, B.A.; Gravance, C.G.; Medina, V.; Davies-morel, M.C. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 2000, 21, 895–902. [Google Scholar] [PubMed]
- Roy, B.; Baghel, R.; Mohanty, T.; Mondal, G. Zinc and male reproduction in domestic animals: A Review. Indian J. Anim. Nutr. 2013, 30, 339–350. [Google Scholar]
- Rangari, K.; Shrivastav, T. A correlation study between steroid hormone levels and anti-sperm antibodies in serum and seminal plasma of men with or without reduced sperm motility. J. Endocrinol. Reprod. 2007, 11, 31–35. [Google Scholar]
- Balázs, C.; Rácz, K. The role of selenium in endocrine system diseases. Orvosi Hetilap 2013, 154, 1628–1635. [Google Scholar] [CrossRef] [Green Version]
- Tareq, K.; Akter, Q.S.; Takagi, Y.; Hamano, K.-I.; Sawada, T.; Tsujii, H. Effect of selenium and vitamin E on acrosome reaction in porcine spermatozoa. Reprod. Med. Biol. 2010, 9, 73–81. [Google Scholar] [CrossRef]
- Khalil, W.A.; El-Harairy, M.A.; Zeidan, A.E.; Hassan, M.A. Impact of selenium nano-particles in semen extender on bull sperm quality after cryopreservation. Theriogenology 2019, 126, 121–127. [Google Scholar] [CrossRef]
- Rezvanfar, M.A.; Rezvanfar, M.A.; Shahverdi, A.R.; Ahmadi, A.; Baeeri, M.; Mohammadirad, A.; Abdollahi, M. Protection of cisplatin-induced spermatotoxicity, DNA damage and chromatin abnormality by selenium nano-particles. Toxicol. Appl. Pharmacol. 2013, 266, 356–365. [Google Scholar] [CrossRef]
- Deen, A.; Vyas, S.; Sahani, M. Semen collection, cryopreservation and artificial insemination in the dromedary camel. Anim. Reprod. Sci. 2003, 77, 223–233. [Google Scholar] [CrossRef]
- Hammadi, M.; Zarrouk, O.; Barmat, A.; Trimeche, A.; Khorchani, T.; Khaldi, G. Characterization and conservation of Maghrebi camel semen. In Proceedings of the WBC/ICAR Satellite Meeting on Camelid Reproduction, Budapest, Hungary, 12–13 July 2008. [Google Scholar]
- Niasari-Naslaji, A.; Mosaferi, S.; Bahmani, N.; Gharahdaghi, A.; Abarghani, A.; Ghanbari, A.; Gerami, A. Effectiveness of a tris-based extender (SHOTOR diluent) for the preservation of Bactrian camel (Camelus bactrianus) semen. Cryobiology 2006, 53, 12–21. [Google Scholar] [CrossRef]
- Marin-Guzman, J.; Mahan, D.; Whitmoyer, R. Effect of dietary selenium and vitamin E on the ultrastructure and ATP concentration of boar spermatozoa, and the efficacy of added sodium selenite in extended semen on sperm motility. J. Anim. Sci. 2000, 78, 1544–1550. [Google Scholar] [CrossRef] [Green Version]
- Heath, E.; Mostafa, M.; Karasek, S. Ultrastructure of Camel (Camelus dromedarius) Spermatozoa. Anat. Histol. Embryol. 1986, 15, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Tingari, M. Studies on camel semen. III. Ultrastructure of the spermatozoon. Anim. Reprod. Sci. 1991, 26, 333–344. [Google Scholar] [CrossRef]
- Taylor, K.; Roberts, P.; Sanders, K.; Burton, P. Effect of antioxidant supplementation of cryopreservation medium on post-thaw integrity of human spermatozoa. Reprod. Biomed. Online 2009, 18, 184–189. [Google Scholar] [CrossRef]
- Salisbury, G.W.; VanDemark, N.L.; Lodge, J.R. Physiology of Reproduction and Artificial Insemination of Cattle; WH Freeman and Company: Stuttgart, Germany, 1978. [Google Scholar]
- Moskovtsev, S.I.; Librach, C.L. Methods of sperm vitality assessment. Method Mol. Biol. 2013, 927, 13–19. [Google Scholar] [CrossRef]
- Menon, A.G.; Thundathil, J.C.; Wilde, R.; Kastelic, J.P.; Barkema, H.W. Validating the assessment of bull sperm morphology by veterinary practitioners. Canad. Vet. J. 2011, 52, 407–408. [Google Scholar]
- Caycho, K.; Santolaria, P.; Soler, C.; Yániz, J. Effect of hypoosmotic swelling test and water test on the distribution of sperm subpopulations in bull. Anim. Reprod. Sci. 2016, 169, 101. [Google Scholar] [CrossRef]
- Ochseedorf, F.R.; Buhl, R.; Bästlein, A.; Beschmann, H. Glutathione le spermatozoa and seminal plasma of infertile men. Hum. Reprod. 1998, 13, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Koziorowska-Gilun, M.; Koziorowski, M.; Fraser, L.; Strzeżek, J. Antioxidant defence system of boar cauda epididymidal spermatozoa and reproductive tract fluids. Reprod. Domest. Anim. 2011, 46, 527–533. [Google Scholar] [CrossRef]
- Tuncer, P.B.; Bucak, M.N.; Sarıözkan, S.; Sakin, F.; Yeni, D.; Çiğerci, İ.H.; Ateşşahin, A.; Avdatek, F.; Gündoğan, M.; Büyükleblebici, O. The effect of raffinose and methionine on frozen/thawed Angora buck (Capra hircus ancryrensis) semen quality, lipid peroxidation and antioxidant enzyme activities. Cryobiology 2010, 61, 89–93. [Google Scholar] [CrossRef]
- Chaveiro, A.; Santos, P.; Da Silva, F. Assessment of Sperm Apoptosis in Cryopreserved Bull Semen After Swim-up Treatment: A Flow Cytometric Study. Reprod. Domest. Anim. 2007, 42, 17–21. [Google Scholar] [CrossRef]
- Masters, A.; Harrison, P. Platelet counting with the BD AccuriTM C6 flow cytometer. Platelets 2014, 25, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; Johannisson, A.; Wallgren, M. Assessment of fresh and frozen–thawed boar semen using an Annexin-V assay: A new method of evaluating sperm membrane integrity. Theriogenology 2003, 60, 677–689. [Google Scholar] [CrossRef]
- Graham, R.C., Jr.; Lundholm, U.; Karnovsky, M.J. Cytochemical demonstration of peroxidase activity with 3-amino-9-ethylcarbazole. J. Histochem. Cytochem. 1965, 13, 150–152. [Google Scholar] [CrossRef] [PubMed]
- SAS. Statistical Analysis System. Stat-User’s Guid. Release 9.1.3; SAS Institute: Cary, NC, USA, 2007. [Google Scholar]
- Duncan, D.B. Multiple Range and Multiple “F” Test. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef]
- Wang, A.W.; Zhang, H.; Ikemoto, I.; Anderson, D.J.; Loughlin, K.R. Reactive oxygen species generation by seminal cells during cryopreservation. Urology 1997, 49, 921–925. [Google Scholar] [CrossRef]
- Tatone, C.; Di Emidio, G.; Vento, M.; Ciriminna, R.; Artini, P.G. Cryopreservation and oxidative stress in reproductive cells. Gynecol. Endocrinol. 2010, 26, 563–567. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Method Enzymol 1990, 186, 407–421. [Google Scholar] [CrossRef]
- Simões, R.; Feitosa, W.B.; Siqueira, A.F.P.; Nichi, M.; Paula-Lopes, F.F.; Marques, M.G.; Peres, M.A.; Barnabe, V.H.; Visintin, J.A.; Assumpção, M.E.O. Influence of bovine sperm DNA fragmentation and oxidative stress on early embryo in vitro development outcome. Reproduction 2013, 146, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Bucak, M.N.; Sarıözkan, S.; Tuncer, P.B.; Sakin, F.; Ateşşahin, A.; Kulaksız, R.; Çevik, M. The effect of antioxidants on post-thawed Angora goat (Capra hircus ancryrensis) sperm parameters, lipid peroxidation and antioxidant activities. Small Rum. Res. 2010, 89, 24–30. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell. Endocrinol. 2006, 250, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Sarıözkan, S.; Bucak, M.N.; Tuncer, P.B.; Ulutaş, P.A.; Bilgen, A. The influence of cysteine and taurine on microscopic–oxidative stress parameters and fertilizing ability of bull semen following cryopreservation. Cryobiology 2009, 58, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Fadel, A.M.; Hamada, M.M.Z.; El-Badry, A.A. Effects of exposure to 50 Hz, 1 Gauss magnetic field on reproductive traits in male albino rats. Acta. Vet. Brno. 2011, 80, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Pelyhe, C.; Mézes, M. Myths and facts about the effects of nano selenium in farm animals–mini-review. Eur. Chem. Bull. 2013, 2, 1049–1052. [Google Scholar]
- Isaac, A.V.; Kumari, S.; Nair, R.; Urs, D.R.; Salian, S.R.; Kalthur, G.; Adiga, S.K.; Manikkath, J.; Mutalik, S.; Sachdev, D.; et al. Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem. Biophys. Res. Commun. 2017, 494, 656–662. [Google Scholar] [CrossRef]
- Heidari, G.; Seifdavati, J.; Mohebodini, H.; Seyed Sharifi, R.; Abdi Benemar, H. Effect of Nano Zinc Oxide on Post-Thaw Variables and Oxidative Status of Moghani Ram Semen. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 2018, 25, 71–76. [Google Scholar] [CrossRef]
- Falchi, L.; Khalil, W.A.; Hassan, M.; Marei, W.F.A. Perspectives of nanotechnology in male fertility and sperm function. Int. J. Vet. Sci. Med. 2019, 6, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Handy, R.D.; Owen, R.; Valsami-Jones, E. The ecotoxicology of nanoparticles and nanomaterials: Current status, knowledge gaps, challenges, and future needs. Ecotoxicology 2008, 17, 315–325. [Google Scholar] [CrossRef]
- Hussein, M.M.A.; Ali, H.A.; Saadeldin, I.M.; Ahmed, M.M. Querectin Alleviates Zinc Oxide Nanoreprotoxicity in Male Albino Rats. J. Bioch. Mol. Toxicol. 2016, 30, 489–496. [Google Scholar] [CrossRef]
- Abbasalipourkabir, R.; Moradi, H.; Zarei, S.; Asadi, S.; Salehzadeh, A.; Ghafourikhosroshahi, A.; Mortazavi, M.; Ziamajidi, N. Toxicity of zinc oxide nanoparticles on adult male Wistar rats. Food Chem. Toxicol. 2015, 84, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Celino, F.T.; Yamaguchi, S.; Miura, C.; Ohta, T.; Tozawa, Y.; Iwai, T.; Miura, T. Tolerance of Spermatogonia to Oxidative Stress Is Due to High Levels of Zn and Cu/Zn Superoxide Dismutase. PLoS ONE 2011, 6, e16938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Maddawy, Z.; Abd El Naby, W.S.H. Protective effects of zinc oxide nanoparticles against doxorubicin induced testicular toxicity and DNA damage in male rats. Toxicol. Res. 2019, 8, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Asri-Rezaei, S.; Nourian, A.; Shalizar-Jalali, A.; Najafi, G.; Nazarizadeh, A.; Koohestani, M.; Karimi, A. Selenium supplementation in the form of selenium nanoparticles and selenite sodium improves mature male mice reproductive performances. Iran. J. Basic Med. Sci. 2018, 21, 577–585. [Google Scholar] [PubMed]
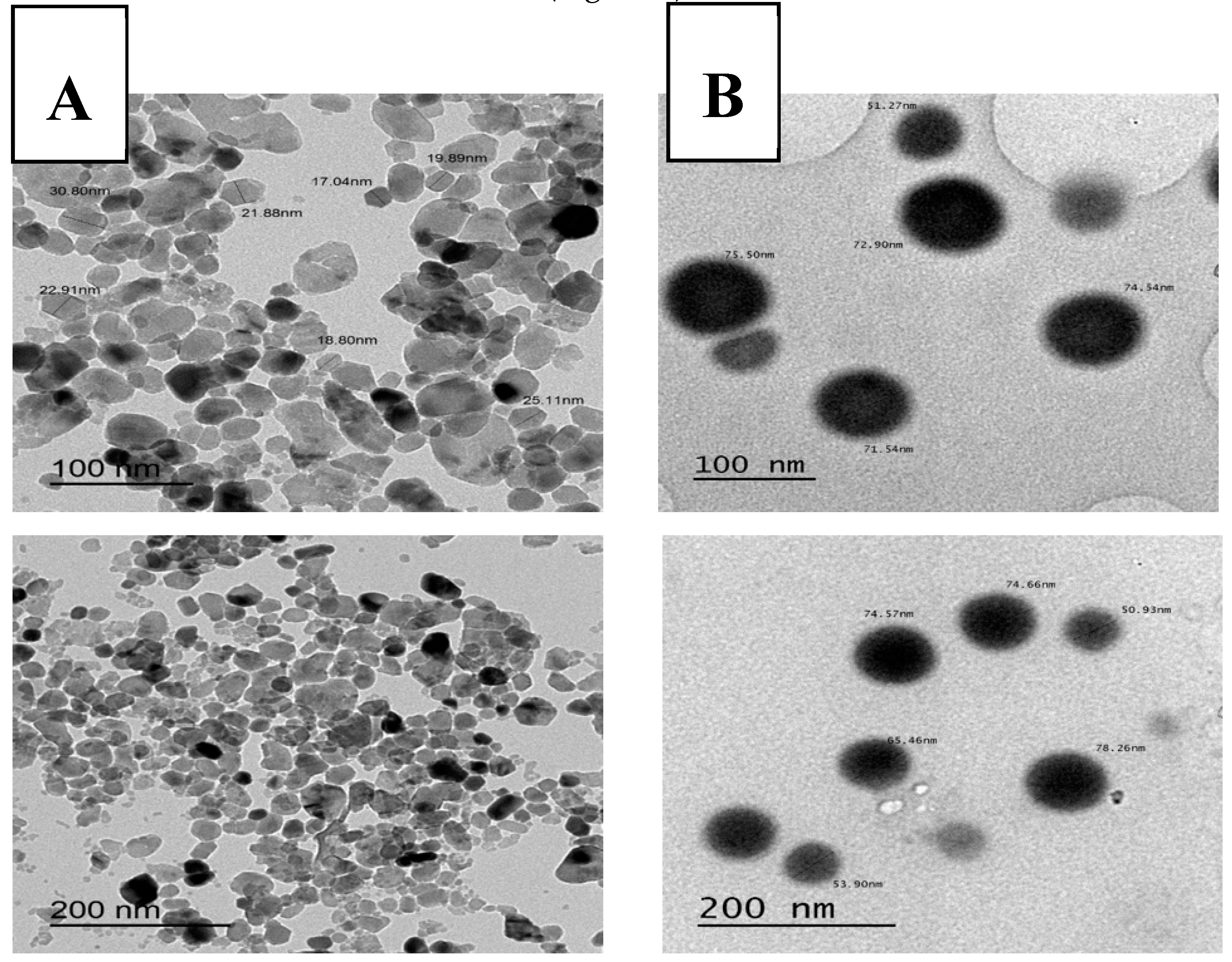

| Treatment | Progressive Motility | Vitality | Plasma Membrane Integrity | Abnormality | Cytoplasmic Droplet |
|---|---|---|---|---|---|
| Control | 60.8 ± 2.01 c | 63.2 ± 2.91 b | 60.2 ± 2.04 c | 12.3 ± 1.87 | 36.2 ± 1.51 |
| Vitamin C | 67.5 ± 2.14 ab | 70.2 ± 3.39 ab | 68.5 ± 1.61 ab | 11.0 ± 1.29 | 36.7 ± 1.69 |
| Vitamin E | 63.3 ± 1.67 bc | 65.5 ± 1.45 ab | 65.8 ± 0.31 b | 14.2 ± 1.68 | 34.5 ± 0.62 |
| SeNPs | 72.5 ± 1.44 a | 73.3 ± 0.85 a | 73.0 ± 1.83 a | 15.5 ± 1.50 | 35.3 ± 1.11 |
| Na2SeO3 | 68.0 ± 1.22 ab | 69.8 ± 1.77 ab | 69.2 ± 2.48 ab | 11.6 ± 1.69 | 33.8 ± 1.71 |
| ZnONPs | 70.8 ± 2.01 a | 73.3 ± 2.14 a | 71.3 ± 1.87 ab | 12.5 ± 0.92 | 34.5 ± 1.48 |
| ZnSO4 | 68.8 ± 2.39 ab | 72.0 ± 2.35 a | 70.5 ± 2.84 ab | 15.8 ± 2.53 | 33.8 ± 0.75 |
| Treatment | Progressive Motility | Vitality | Plasma Membrane Integrity | Abnormality | Cytoplasmic Droplet |
|---|---|---|---|---|---|
| Control | 27.0 ± 1.22 c | 28.8 ± 1.32 c | 28.4 ± 1.12 c | 26.2 ± 1.02 a | 21.6 ± 2.84 |
| Vitamin C | 30.0 ± 1.58 c | 32.2 ± 2.11 c | 30.4 ± 2.36 c | 18.6 ± 0.75 b | 21.0 ± 2.61 |
| Vitamin E | 31.0 ± 1.00 c | 33.8 ± 0.86 c | 31.0 ± 0.89 c | 24.8 ± 0.86 a | 21.2 ± 2.71 |
| SeNPs | 48.3 ± 1.67 a | 50.7 ± 2.33 a | 50.0 ± 1.15 a | 18.7 ± 0.88 b | 20.7 ± 2.40 |
| Na2SeO3 | 40.0 ± 2.04 b | 42.3 ± 2.14 b | 41.5 ± 2.87 b | 19.5 ± 0.65 b | 21.3 ± 2.46 |
| ZnONPs | 49.0 ± 1.87 a | 52.2 ± 2.15 a | 51.0 ± 2.19 a | 18.8 ± 0.58 b | 19.4 ± 1.81 |
| ZnSO4 | 36.7 ± 1.67 b | 39.7 ± 1.76 b | 38.3 ± 0.33 b | 18.7 ± 1.20 b | 22.3 ± 2.91 |
| Treatment | Viable (%) (A−/PI−) | Early Apoptosis (%) (A+/PI−) | Late Apoptosis (%) (A+/PI+) | Necrosis (%) (A−/PI+) |
|---|---|---|---|---|
| Control | 26.4 ± 1.10 g | 38.4 ± 0.26 a | 31.0 ± 1.30 a | 4.3 ± 0.07 |
| Vitamin C | 57.4 ± 0.92 c | 24.0 ± 0.55 c | 15.7 ± 1.30 c | 2.9 ± 0.18 |
| Vitamin E | 52.4 ± 0.58 d | 24.2 ± 0.61 c | 15.9 ± 0.18 c | 7.5 ± 0.20 |
| SeNPs | 78.1 ± 0.58 a | 12.0 ± 0.43 d | 4.3 ± 0.17 e | 5.6 ± 0.03 |
| Na2SeO3 | 47.0 ± 0.09 e | 38.2 ± 0.32 a | 12.8 ± 0.30 d | 2.0 ± 0.12 |
| ZnONPs | 69.6 ± 0.26 b | 4.5 ± 0.35 e | 20.0 ± 0.35 b | 5.9 ± 0.26 |
| ZnSO4 | 42.5 ± 0.64 f | 35.0 ± 0.23 b | 21.4 ± 0.43 b | 1.2 ± 0.03 |
| Treatment | GSH (mg/dL) | SOD (U/mL) | MDA (nmol/mL) |
|---|---|---|---|
| Control | 0.45 ± 0.03 e | 48.3 ± 5.93 f | 30.1 ± 2.15 a |
| Vitamin C | 0.59 ± 0.03 dc | 113.4 ± 5.66 dc | 14.8 ± 0.26 d |
| Vitamin E | 0.64 ± 0.02 bc | 124.3 ± 3.94 c | 14.8 ± 0.17 d |
| SeNPs | 0.77 ± 0.04 a | 168.0 ± 6.67 a | 12.7 ± 0.70 d |
| Na2SeO3 | 0.55 ± 0.01 d | 106.8 ± 1.70 d | 18.4 ± 1.07 c |
| ZnONPs | 0.70 ± 0.00 ab | 148.2 ± 2.33 b | 13.6 ± 0.48 d |
| ZnSO4 | 0.51 ± 0.03 de | 67.2 ± 6.28 e | 22.6 ± 1.32 b |
| Treatment | Intact PM | Slightly Swollen PM | Swollen PM | Lost PM |
|---|---|---|---|---|
| Control | 38 ± 4.88 b | 12 ± 3.27 ab | 37 ± 4.85 a | 13 ± 3.38 |
| Vitamin C | 58 ± 4.96 ab | 7 ± 2.56 b | 23 ± 4.23 b | 12 ± 3.27 |
| Vitamin E | 55 ± 5.00 ab | 8 ± 2.73 b | 26 ± 4.41 ab | 11 ± 3.14 |
| SeNPs | 68 ± 4.69 a | 9 ± 2.88a b | 15 ± 3.59 b | 8 ± 2.73 |
| Na2SeO3 | 53 ± 5.02 ab | 18 ± 3.86 a | 18 ± 3.86 b | 11 ± 3.14 |
| ZnONPs | 65 ± 4.79 ab | 7 ± 2.56 b | 17 ± 3.78 b | 11 ± 3.14 |
| ZnSO4 | 51 ± 5.02 bc | 16 ± 3.68 ab | 21 ± 4.09 b | 12 ± 3.27 |
| Treatment | Intact Acrosome | Typical AR | Atypical AR | Lost Acrosome |
|---|---|---|---|---|
| Control | 61 ± 4.90 | 24 ± 4.23 | 10 ± 2.88 | 5 ± 2.19 |
| Vitamin C | 68 ± 4.69 | 16 ± 3.68 | 12 ± 3.27 | 4 ± 1.97 |
| Vitamin E | 67 ± 4.73 | 18 ± 3.86 | 10 ± 3.02 | 5 ± 2.19 |
| SeNPs | 78 ± 4.16 | 10 ± 3.02 | 8 ± 2.73 | 4 ± 1.97 |
| Na2SeO3 | 70 ± 4.61 | 16 ± 3.68 | 9 ± 2.88 | 5 ± 2.19 |
| ZnONPs | 74 ± 4.41 | 13 ± 3.38 | 10 ± 3.02 | 3 ± 1.71 |
| ZnSO4 | 72 ± 4.51 | 12 ± 3.27 | 10 ± 3.02 | 6 ± 2.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahin, M.A.; Khalil, W.A.; Saadeldin, I.M.; Swelum, A.A.-A.; El-Harairy, M.A. Comparison between the Effects of Adding Vitamins, Trace Elements, and Nanoparticles to SHOTOR Extender on the Cryopreservation of Dromedary Camel Epididymal Spermatozoa. Animals 2020, 10, 78. https://doi.org/10.3390/ani10010078
Shahin MA, Khalil WA, Saadeldin IM, Swelum AA-A, El-Harairy MA. Comparison between the Effects of Adding Vitamins, Trace Elements, and Nanoparticles to SHOTOR Extender on the Cryopreservation of Dromedary Camel Epididymal Spermatozoa. Animals. 2020; 10(1):78. https://doi.org/10.3390/ani10010078
Chicago/Turabian StyleShahin, Mohamed A., Wael A. Khalil, Islam M. Saadeldin, Ayman Abdel-Aziz Swelum, and Mostafa A. El-Harairy. 2020. "Comparison between the Effects of Adding Vitamins, Trace Elements, and Nanoparticles to SHOTOR Extender on the Cryopreservation of Dromedary Camel Epididymal Spermatozoa" Animals 10, no. 1: 78. https://doi.org/10.3390/ani10010078
APA StyleShahin, M. A., Khalil, W. A., Saadeldin, I. M., Swelum, A. A.-A., & El-Harairy, M. A. (2020). Comparison between the Effects of Adding Vitamins, Trace Elements, and Nanoparticles to SHOTOR Extender on the Cryopreservation of Dromedary Camel Epididymal Spermatozoa. Animals, 10(1), 78. https://doi.org/10.3390/ani10010078







