Function of Cryopreserved Cat Ovarian Tissue after Autotransplantation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
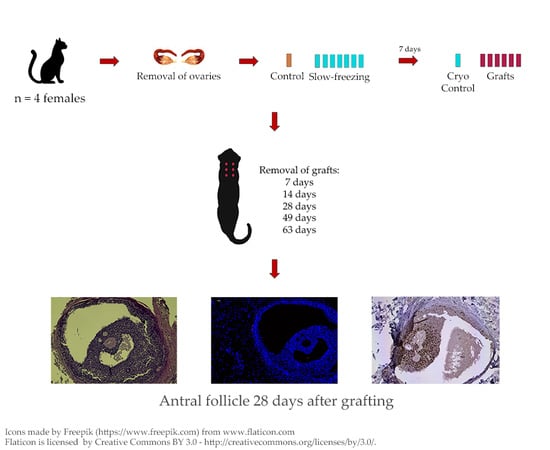
2.1. Animals
2.2. Ovariohysterectomy
2.3. Slow-Freezing and Thawing of Fragments
2.4. Autografting
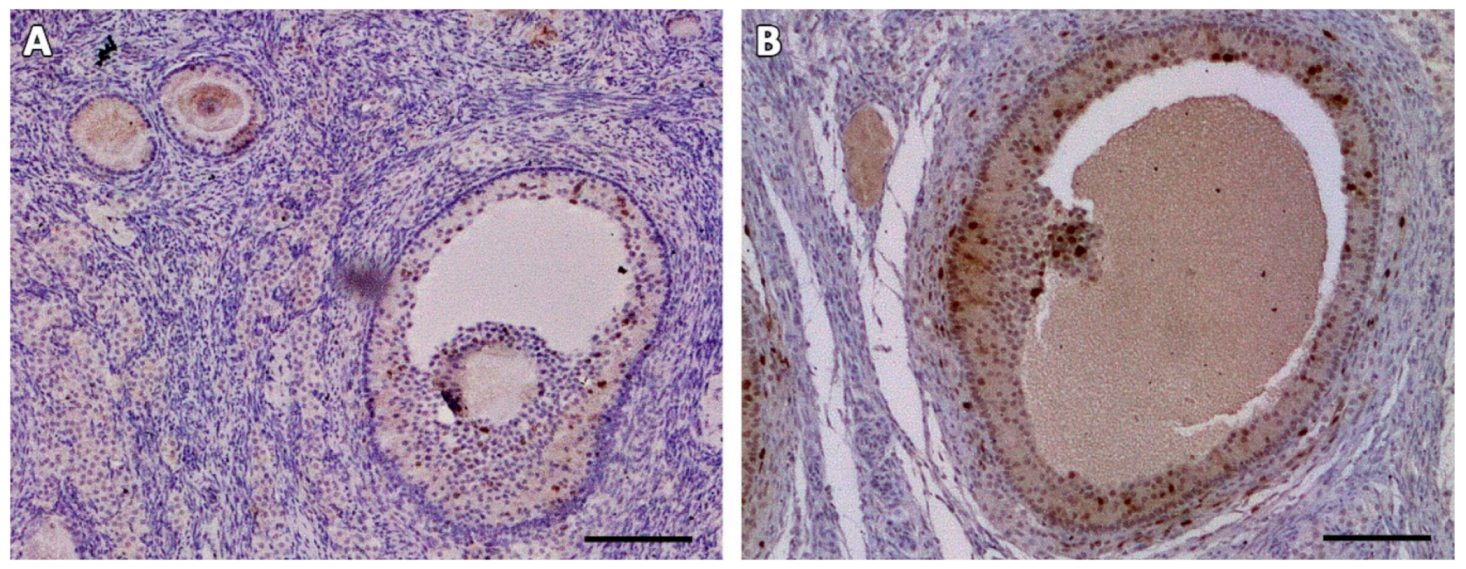
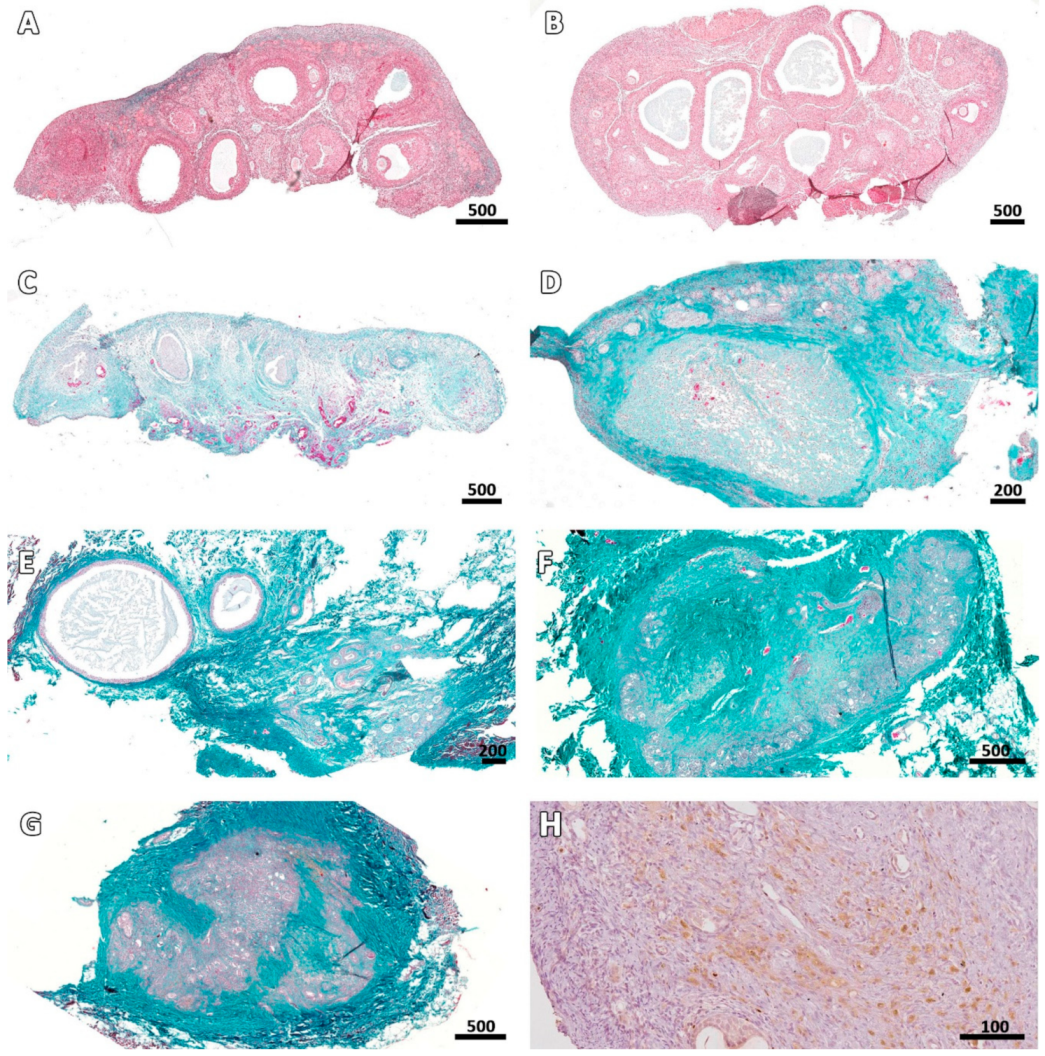
2.5. Histological, Immunohistochemical and Histochemical Analyses
2.6. Statistical Analyses
3. Results
3.1. Graft Retrieval
3.2. Microscopic Aspect of the Grafts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santos, R.R.; Amorim, C.; Cecconi, S.; Fassbender, M.; Imhof, M.; Lornage, J.; Paris, M.; Schoenfeldt, V.; Martinez-Madrid, B. Cryopreservation of ovarian tissue: An emerging technology for female germline preservation of endangered species and breeds. Anim. Reprod. Sci. 2010, 122, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Jewgenow, K.; Wiedemann, C.; Bertelsen, M.F.; Ringleb, J. Cryopreservation of mammalian ovaries and oocytes. Int. Zoo Yearb. 2011, 45, 124–132. [Google Scholar] [CrossRef]
- Ambrosini, G.; Andrisani, A.; Porcu, E.; Rebellato, E.; Revelli, A.; Caserta, D.; Cosmi, E.; Marci, R.; Moscarini, M. Oocytes cryopreservation: State of art. Reprod. Toxicol. 2006, 22, 250–262. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Transplantation of ovarian tissue. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.K.; Kristensen, S.G.; Andersen, C.Y. Chapter 7—Ovarian Tissue Cryopreservation for Fertility Preservation. In Cancer Treatment and the Ovary; Anderson, R.A., Spears, N., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 93–107. [Google Scholar] [CrossRef]
- Wang, X.; Catt, S.; Pangestu, M.; Temple-Smith, P. Live offspring from vitrified blastocysts derived from fresh and cryopreserved ovarian tissue grafts of adult mice. Reproduction 2009, 138, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Torre, A.; Vertu-Ciolino, D.; Mazoyer, C.; Selva, J.; Lornage, J.; Salle, B. Safeguarding Fertility With Whole Ovary Cryopreservation and Microvascular Transplantation: Higher Follicular Survival With Vitrification Than With Slow Freezing in a Ewe Model. Transplantation 2016, 100, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Aubard, Y.; Piver, P.; Cogni, Y.; Fermeaux, V.; Poulin, N.; Driancourt, M.A. Orthotopic and heterotopic autografts of frozen-thawed ovarian cortex in sheep. Hum. Reprod. 1999, 14, 2149–2154. [Google Scholar] [CrossRef]
- Kagawa, N.; Silber, S.; Kuwayama, M. Successful vitrification of bovine and human ovarian tissue. Reprod. Biomed. Online 2009, 18, 568–577. [Google Scholar] [CrossRef]
- Santos, R.R.; Knijn, H.M.; Vos, P.L.; Oei, C.H.; van Loon, T.; Colenbrander, B.; Gadella, B.M.; van den Hurk, R.; Roelen, B.A. Complete follicular development and recovery of ovarian function of frozen-thawed, autotransplanted caprine ovarian cortex. Fertil. Steril. 2009, 91, 1455–1458. [Google Scholar] [CrossRef]
- Chao, L.; Deng, X.; Wang, X.; Fu, Q.; Xu, A.; Hao, C.; Yu, H.; Yu, X. Normal developmental competence to the blastocyst stage is preserved in rabbit ovarian tissue following cryopreservation and autografting to the mesometrium. Reprod. Fertil. Dev. 2008, 20, 466–473. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Fertility Preservation in Women. N. Engl. J. Med. 2017, 377, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Bosch, P.; Hernandez-Fonseca, H.J.; Miller, D.M.; Wininger, J.D.; Massey, J.B.; Lamb, S.V.; Brackett, B.G. Development of antral follicles in cryopreserved cat ovarian tissue transplanted to immunodeficient mice. Theriogenology 2004, 61, 581–594. [Google Scholar] [CrossRef]
- Lima, A.K.; Silva, A.R.; Santos, R.R.; Sales, D.M.; Evangelista, A.F.; Figueiredo, J.R.; Silva, L.D. Cryopreservation of preantral ovarian follicles in situ from domestic cats (Felis catus) using different cryoprotective agents. Theriogenology 2006, 66, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Luvoni, G.C.; Tessaro, I.; Apparicio, M.; Ruggeri, E.; Luciano, A.M.; Modina, S.C. Effect of vitrification of feline ovarian cortex on follicular and oocyte quality and competence. Reprod. Domest. Anim. 2012, 47, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Tanpradit, N.; Comizzoli, P.; Srisuwatanasagul, S.; Chatdarong, K. Positive impact of sucrose supplementation during slow freezing of cat ovarian tissues on cellular viability, follicle morphology, and DNA integrity. Theriogenology 2015, 83, 1553–1561. [Google Scholar] [CrossRef]
- Brito, D.C.; Domingues, S.F.; Silva, J.K.; Wu, X.; Santos, R.R.; Pieczarka, J.C. Detrimental Effect of Phenol Red on the Vitrification of Cat (Felis catus) Ovarian Tissue. Biopreserv. Biobank 2016, 14, 17–22. [Google Scholar] [CrossRef]
- Mouttham, L.; Comizzoli, P. The preservation of vital functions in cat ovarian tissues during vitrification depends more on the temperature of the cryoprotectant exposure than on the sucrose supplementation. Cryobiology 2016, 73, 187–195. [Google Scholar] [CrossRef]
- Mouttham, L.; Comizzoli, P. Presence of sucrose in the vitrification solution and exposure for longer periods of time improve post-warming follicle integrity in cat ovarian tissues. Reprod. Domest. Anim. 2017, 52, 224–229. [Google Scholar] [CrossRef]
- Gorricho, C.M.; Tavares, M.R.; Apparicio, M.; Fonseca-Alves, C.E.; Macente, B.I.; Mansano, C.F.M.; Toniollo, G.H. Vitrification of cat ovarian tissue: Does fragment size matters? Reprod. Domest. Anim. 2018, 53 (Suppl. 3), 125–132. [Google Scholar] [CrossRef]
- Leonel, E.C.R.; Vilela, J.M.V.; Carrilho, D.d.J.; Lucci, C.M. Cat ovarian follicle ultrastructure after cryopreservation with ethylene glycol and dimethyl sulfoxide. Cryobiology 2018, 83, 9–14. [Google Scholar] [CrossRef]
- Vilela, J.M.V.; Leonel, E.C.R.; D‘Oliveira, L.; Paiva, R.E.G.; Miranda-Vilela, A.L.; Amorim, C.A.; Pic-Taylor, A.; Lucci, C.M. Culture of domestic cat ovarian tissue in vitro and in the chick embryo chorioallantoic membrane. Theriogenology 2016, 86, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Leonel, E.C.R.; Vilela, J.M.V.; Paiva, R.E.G.; Jivago, J.L.P.R.; Amaral, R.S.; Lucci, C.M. Restoration of fresh cat ovarian tissue function by autografting to subcutaneous tissue: A pilot study. Theriogenology 2018, 105, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Michel, C. Induction of oestrus in cats by photoperiodic manipulations and social stimuli. Lab. Anim. 1993, 27, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.M.C.C.P.; Cortopassi, S.R.G.; Machado, A. Transoperative analgesia induced by morphine or meperidine in cats submitted to osteosynthesis. Ciência Rural 2002, 32, 67–72. [Google Scholar] [CrossRef]
- Schiochet, F.; Beck, C.A.C.; Pinto, V.; Stedile, R.; Contesini, E.; Alievi, M.M.; Yamazaki, P.H.; Jurinitz, D.F.; Bernardes, S.B.L. Laparoscopic ovaryhysterectomy in a female cat with mumified fetus—Case report. Revista Portuguesa de Ciências Veterinárias 2007, 102, 361–364. [Google Scholar]
- Fossum, T.W. Cirurgia dos Sistemas Reprodutivo e Genital. In Cirurgia de Pequenos Animais, 3rd ed.; Elsevier: São Paulo, Brazil, 2007; pp. 729–745. [Google Scholar]
- Nugent, D.; Newton, H.; Gallivan, L.; Gosden, R.G. Protective effect of vitamin E on ischaemia-reperfusion injury in ovarian grafts. J. Reprod. Fertil. 1998, 114, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Mohd Mutalip, S.S.; Ab-Rahim, S.; Rajikin, M.H. Vitamin E as an Antioxidant in Female Reproductive Health. Antioxidants 2018, 7, 22. [Google Scholar] [CrossRef]
- Slot, K.A.; Kastelijn, J.; Bachelot, A.; Kelly, P.A.; Binart, N.; Teerds, K.J. Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction 2006, 131, 525–532. [Google Scholar] [CrossRef]
- Vanacker, J.; Luyckx, V.; Dolmans, M.-M.; Des Rieux, A.; Jaeger, J.; Van Langendonckt, A.; Donnez, J.; Amorim, C.A. Transplantation of an alginate–matrigel matrix containing isolated ovarian cells: First step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 2012, 33, 6079–6085. [Google Scholar] [CrossRef]
- Martinez-Madrid, B.; Dolmans, M.-M.; Van Langendonckt, A.; Defrère, S.; Donnez, J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil. Steril. 2004, 82, 1390–1394. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Martinez-Madrid, B.; Gadisseux, E.; Guiot, Y.; Yuan, W.Y.; Torre, A.; Camboni, A.; Van Langendonckt, A.; Donnez, J. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction 2007, 134, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirshfield, A.N. Compensatory ovarian hypertrophy in the long-term hemicastrate rat: Size distribution of growing and atretic follicles. Biol. Reprod. 1983, 28, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Jorio, A.; Mariana, J.C.; Lahlou-Kassi, A. Development of the population of ovarian follicles during the prepubertal period in D‘man and Timahdite sheep. Anim. Reprod. Sci. 1991, 26, 239–250. [Google Scholar] [CrossRef]
- Figueiredo, J.R.; Hulshof, S.C.J.; Van den Hurk, R.; Nusgens, B.; Bevers, M.M.; Ectors, F.J.; Beckers, J.F. Preservation of oocyte and granulosa cell morphology in bovine preantral follicles cultured in vitro. Theriogenology 1994, 41, 1333–1346. [Google Scholar] [CrossRef]
- Braw-Tal, R.; Yossefi, S. Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. J. Reprod. Fertil. 1997, 109, 165–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Songsasen, N.; Comizzoli, P.; Nagashima, J.; Fujihara, M.; Wildt, D.E. The domestic dog and cat as models for understanding the regulation of ovarian follicle development in vitro. Reprod. Domest. Anim. 2012, 47, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Krohn, P.L. Transplantation of the Ovary. In The Ovary; Elsevier Science: Amsterdam, The Netherlands, 2013; Volume II, pp. 101–128. [Google Scholar]
- Keros, V.; Xella, S.; Hultenby, K.; Pettersson, K.; Sheikhi, M.; Volpe, A.; Hreinsson, J.; Hovatta, O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum. Reprod. 2009, 24, 1670–1683. [Google Scholar] [CrossRef] [Green Version]
- Dath, C.; Van Eyck, A.S.; Dolmans, M.M.; Romeu, L.; Delle Vigne, L.; Donnez, J.; Van Langendonckt, A. Xenotransplantation of human ovarian tissue to nude mice: Comparison between four grafting sites. Hum. Reprod. 2010, 25, 1734–1743. [Google Scholar] [CrossRef] [Green Version]
- Falanga, V.; Su Wen Qian, V.; Danielpour, D.; Katz, M.H.; Roberts, A.B.; Sporn, M.B. Hypoxia Upregulates the Synthesis of TGF-β1 by Human Dermal Fibroblasts. J. Investig. Dermatol. 1991, 97, 634–637. [Google Scholar] [CrossRef] [Green Version]
- Norman, J.T.; Clark, I.M.; Garcia, P.L. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000, 58, 2351–2366. [Google Scholar] [CrossRef] [Green Version]
- Donfack, N.J.; Alves, K.A.; Araújo, V.R.; Cordova, A.; Figueiredo, J.R.; Smitz, J.; Rodrigues, A.P.R. Expectations and limitations of ovarian tissue transplantation. Zygote 2017, 25, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Dissen, G.A.; Lara, H.E.; Fahrenbach, W.H.; Costa, M.E.; Ojeda, S.R. Immature rat ovaries become revascularized rapidly after autotransplantation and show a gonadotropin-dependent increase in angiogenic factor gene expression. Endocrinology 1994, 134, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Torrents, E.; Boiso, I.; Barri, P.N.; Veiga, A. Applications of ovarian tissue transplantation in experimental biology and medicine. Hum. Reprod. Update 2003, 9, 471–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eyck, A.-S.; Bouzin, C.; Feron, O.; Romeu, L.; Van Langendonckt, A.; Donnez, J.; Dolmans, M.-M. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil. Steril. 2010, 93, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Gosden, R.G.; Baird, D.T.; Wade, J.C.; Webb, R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C. Hum. Reprod. 1994, 9, 597–603. [Google Scholar] [CrossRef]
- Chen, S.U.; Lien, Y.R.; Chao, K.; Lu, H.F.; Ho, H.N.; Yang, Y.S. Cryopreservation of mature human oocytes by vitrification with ethylene glycol in straws. Fertil. Steril. 2000, 74, 804–808. [Google Scholar] [CrossRef]
- Lee, D.M.; Yeoman, R.R.; Battaglia, D.E.; Stouffer, R.L.; Zelinski-Wooten, M.B.; Fanton, J.W.; Wolf, D.P. Live birth after ovarian tissue transplant. Nature 2004, 428, 137–138. [Google Scholar] [CrossRef]
- Pacheco, F.; Oktay, K. Current Success and Efficiency of Autologous Ovarian Transplantation: A Meta-Analysis. Reprod. Sci. 2017, 24, 1111–1120. [Google Scholar] [CrossRef]
- Jensen, A.K.; Macklon, K.T.; Fedder, J.; Ernst, E.; Humaidan, P.; Andersen, C.Y. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: Focus on birth and perinatal outcome in 40 of these children. J. Assist. Reprod. Genet. 2017, 34, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Gunasena, K.T.; Villines, P.M.; Critser, E.S.; Critser, J.K. Live births after autologous transplant of cryopreserved mouse ovaries. Hum. Reprod. 1997, 12, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Gosden, R.G.; Boulton, M.I.; Grant, K.; Webb, R. Follicular development from ovarian xenografts in SCID mice. J. Reprod. Fertil. 1994, 101, 619–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassbender, M.; Hildebrandt, T.B.; Paris, M.C.J.; Colenbrander, B.; Jewgenow, K. High-Resolution Ultrasonography of Xenografted Domestic Cat Ovarian Cortex. J. Reprod. Dev. 2007, 53, 1023–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedemann, C.; Hribal, R.; Ringleb, J.; Bertelsen, M.F.; Rasmusen, K.; Andersen, C.Y.; Kristensen, S.G.; Jewgenow, K. Preservation of Primordial Follicles from Lions by Slow Freezing and Xenotransplantation of Ovarian Cortex into an Immunodeficient Mouse. Reprod. Domest. Anim. 2012, 47, 300–304. [Google Scholar] [CrossRef] [PubMed]




| Type | Treatment | Normal | Degenerated | Total | ||
|---|---|---|---|---|---|---|
| Total | % | Total | % | Follicles | ||
| Primordial | Fresh controls | 491 a | 87.8 a | 68 a | 12.2 a | 559 a |
| Cryo D0 | 471 a | 73.7 a | 168 a | 26.3 a | 639 a | |
| 7 days | 31 b | 31.3 b | 68 a | 68.7 b | 99 b | |
| 14 days | 2 b | 3.3 b | 59 a | 96.7 b | 61 b | |
| 28 days | 11 b | 26.8 b | 30 a | 73.2 b | 41 b | |
| 49 days | 0 b | 0.0 b | 10 b | 100.0 b | 10 b | |
| 63 days | 0 b | 0.0 b | 9 b | 100.0 b | 9 b | |
| Growing | Fresh controls | 29 ab | 96.7 | 1 | 3.3 | 30 ab |
| Cryo D0 | 36 b | 100.0 | 0 | 0.0 | 36 b | |
| 7 days | 2 ac | 40.0 | 3 | 60.0 | 5 ac | |
| 14 days | 0 c | 0 | 0 c | |||
| 28 days | 2 ac | 100.0 | 0 | 0.0 | 2 ac | |
| 49 days | 0 c | 0 | 0 c | |||
| 63 days | 0 c | 0 | 0 c | |||
| Treatment | % FLS/DFs | % FLS/TFs |
|---|---|---|
| Fresh controls | 1.4 (1/69) | 0.2 (1/589) |
| Cryo D0 | 8.9 (15/168) | 2.2 (15/675) |
| 7 days | 63.4 (45/71) | 43.3 (45/104) |
| 14 days | 100.0 (59/59) | 96.7 (59/61) |
| 28 days | 100.0 (30/30) | 69.8 (30/43) |
| 49 days | 100.0 (10/10) | 100.0 (10/10) |
| 63 days | 100.0 (9/9) | 100.0 (9/9) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, J.M.V.; Leonel, E.C.R.; Gonçalves, L.P.; Paiva, R.E.G.; Amaral, R.S.; Amorim, C.A.; Lucci, C.M. Function of Cryopreserved Cat Ovarian Tissue after Autotransplantation. Animals 2019, 9, 1065. https://doi.org/10.3390/ani9121065
Vilela JMV, Leonel ECR, Gonçalves LP, Paiva REG, Amaral RS, Amorim CA, Lucci CM. Function of Cryopreserved Cat Ovarian Tissue after Autotransplantation. Animals. 2019; 9(12):1065. https://doi.org/10.3390/ani9121065
Chicago/Turabian StyleVilela, Janice M. V., Ellen C. R. Leonel, Liudimila P. Gonçalves, Raísa E. G. Paiva, Rodrigo S. Amaral, Christiani A. Amorim, and Carolina M. Lucci. 2019. "Function of Cryopreserved Cat Ovarian Tissue after Autotransplantation" Animals 9, no. 12: 1065. https://doi.org/10.3390/ani9121065
APA StyleVilela, J. M. V., Leonel, E. C. R., Gonçalves, L. P., Paiva, R. E. G., Amaral, R. S., Amorim, C. A., & Lucci, C. M. (2019). Function of Cryopreserved Cat Ovarian Tissue after Autotransplantation. Animals, 9(12), 1065. https://doi.org/10.3390/ani9121065