The Use of Stable Isotope Ratio Analysis to Trace European Sea Bass (D. labrax) Originating from Different Farming Systems
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Experimental Design, Fish Sampling and Farms
2.2. Composition Analysis
2.3. Isotopic Measurements, Standards and Equations
2.4. Statistics
3. Results and Discussion
3.1. Biometric Measurements and Proximate Composition
3.2. Isotope Ratio Values in Feed and Animal Tissues
3.2.1. Influence of the Defatted Process on Isotopic Data of Fish Muscle Tissues
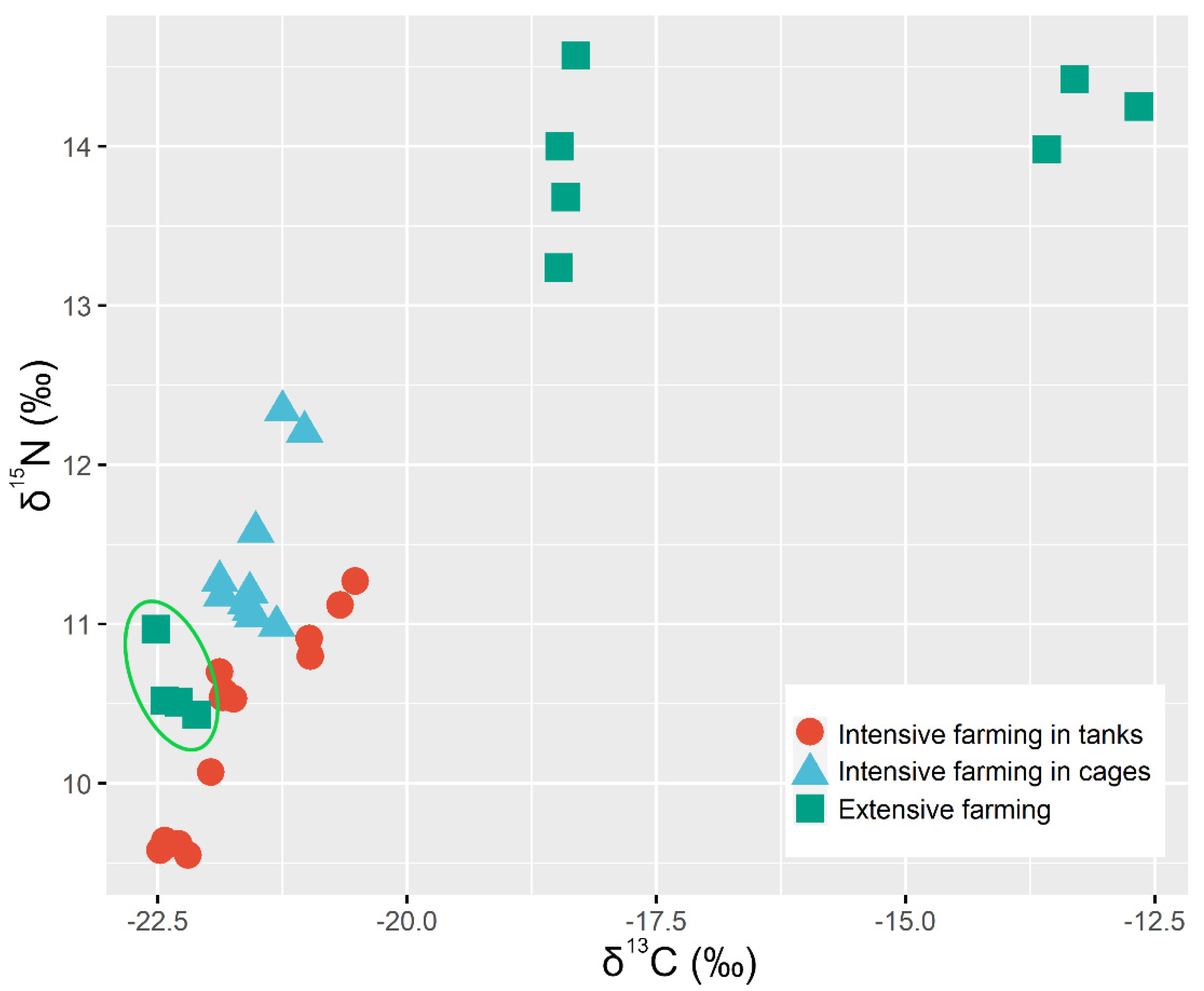
3.2.2. Discrimination of Farming System
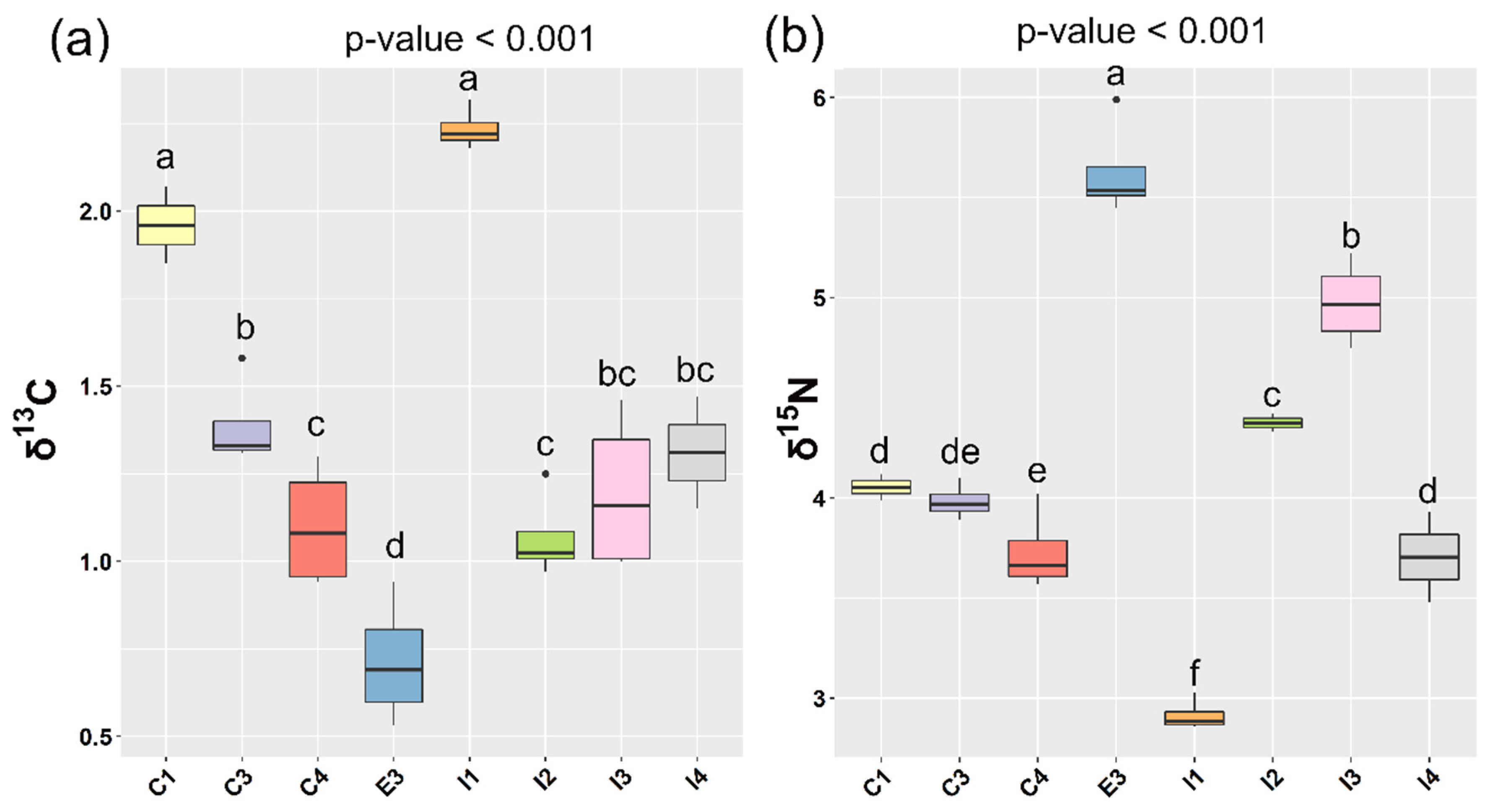
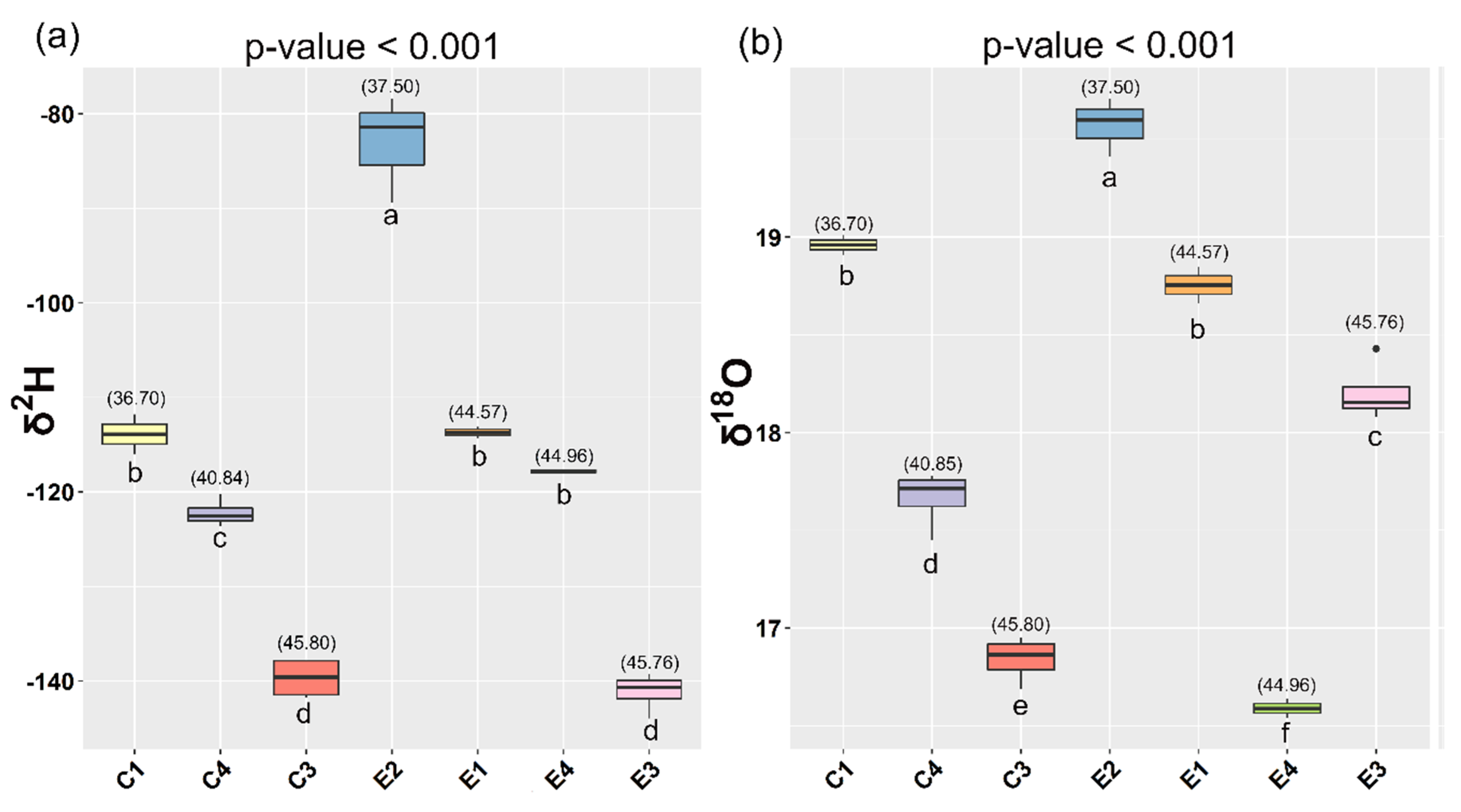
3.2.3. Geographical Origin Discrimination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; 200p. [Google Scholar]
- Fuentes, A.; Fernández-Segovia, I.; Serra, J.A.; Barat, J.M. Comparison of wild and cultured sea bass (Dicentrarchus labrax) quality. Food Chem. 2010, 119, 1514–1518. [Google Scholar] [CrossRef]
- Steffens, W. Aquaculture produces wholesome food: Cultured fish as a valuable source of n-3 fatty acids. Aquac. Int. 2015, 24, 787–802. [Google Scholar] [CrossRef]
- Van Rijswijk, W.; Frewer, L.J. Consumer perceptions of food quality and safety and their relation to traceability. Br. Food J. 2008, 110, 1034–1046. [Google Scholar] [CrossRef]
- Chiesa, L.; Panseri, S.; Bonacci, S.; Procopio, A.; Zecconi, A.; Arioli, F.; Cuevas, F.; Moreno-Rojas, J.M. Authentication of Italian PDO lard using NIR spectroscopy, volatile profile and fatty acid composition combined with chemometrics. Food Chem. 2016, 212, 296–304. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Segebarth, N.; Garmón-Lobato, S.; Holland, M.V.; Moreno-Rojas, J.M.; Fernández-Pierna, J.A.; Baeten, V.; Fuselli, S.R.; Gallo, B.; Berrueta, L.A.; et al. 1H-NMR and isotopic fingerprinting of olive oil and its unsaponifiable fraction: Geographical origin of virgin olive oils by pattern recognition. Eur. J. Lipid Sci. Technol. 2015, 117, 1991–2006. [Google Scholar] [CrossRef]
- Araghipour, N.; Colineau, J.; Koot, A.; Akkermans, W.; Rojas, J.M.M.; Beauchamp, J.; Wisthaler, A.; Maärk, T.D.; Downey, G.; Guillou, C.; et al. Geographical origin classification of olive oils by PTR-MS. Food Chem. 2008, 108, 374–383. [Google Scholar] [CrossRef]
- Mattarucchi, E.; Stocchero, M.; Moreno-Rojas, J.M.; Giordano, G.; Reniero, F.; Guillou, C. Authentication of Trappist Beers by LC-MS Fingerprints and Multivariate Data Analysis. J. Agric. Food Chem. 2010, 58, 12089–12095. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, F.; Pereira-Caro, G.; Pereira-Caro, G.; Ruiz-Moreno, M.J.; Montenegro, J.C.; Moreno-Rojas, J.M. A holistic approach to authenticate organic sweet oranges (Citrus Sinensis L. cv Osbeck) using different techniques and data fusion. Food Control. 2019, 104, 63–73. [Google Scholar] [CrossRef]
- Camin, F.; Bontempo, L.; Perini, M.; Tonon, A.; Breas, O.; Guillou, C.; Moreno-Rojas, J.; Gagliano, G. Control of wine vinegar authenticity through δ18O analysis. Food Control. 2013, 29, 107–111. [Google Scholar] [CrossRef]
- Ghidini, S.; Varrà, M.O.; Dall’Asta, C.; Badiani, A.; Ianieri, A.; Zanardi, E. Rapid authentication of European sea bass (Dicentrarchus labrax L.) according to production method, farming system, and geographical origin by near infrared spectroscopy coupled with chemometrics. Food Chem. 2019, 280, 321–327. [Google Scholar] [CrossRef]
- European Commission. Report from the Commission to the European Parliament and the Council Regarding Trans Fats in Foods and in the Overall Diet of the Union Population; COM: Brussels, Belgium, 2015. [Google Scholar]
- EUMOFA. Highlights the EU in the World EU Market Supply Consumption Trade EU Landings Aquaculture Production (2019 ed.). The EU Fish Market “Sea Bass” Intensive. Available online: https://www.eumofa.eu/documents/20178/314856/EN_The+EU+fish+market_2019.pdf (accessed on 29 May 2020).
- Bell, J.G.; Preston, T.; Henderson, R.J.; Strachan, F.; Bron, J.E.; Cooper, K.; Morrison, D.J. Discrimination of Wild and Cultured European Sea Bass (Dicentrarchus labrax) Using Chemical and Isotopic Analyses. J. Agric. Food Chem. 2007, 55, 5934–5941. [Google Scholar] [CrossRef] [PubMed]
- Grigorakis, K. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: A review. Aquaculture 2007, 272, 55–75. [Google Scholar] [CrossRef]
- Roncarati, A.; Sirri, F.; Di Domenico, A.; Brambilla, G.; Iamiceli, A.L.; Melotti, P.; Meluzzi, A. Survey of qualitative traits of European sea bass cultivated in different rearing systems. Eur. J. Lipid Sci. Technol. 2010, 112, 770–779. [Google Scholar] [CrossRef]
- Vidal, N.P.; Guillén, M.D.; Goicoechea, E.; Guillén, M.D. Farmed and wild sea bass (Dicentrarchus labrax) volatile metabolites: A comparative study by SPME-GC/MS. J. Sci. Food Agric. 2015, 96, 1181–1193. [Google Scholar] [CrossRef]
- Di Marco, P.; Petochi, T.; Marino, G.; Priori, A.; Finoia, M.; Tomassetti, P.; Porrello, S.; Giorgi, G.; Lupi, P.; Bonelli, A.; et al. Insights into organic farming of European sea bass Dicentrarchus labrax and gilthead sea bream Sparus aurata through the assessment of environmental impact, growth performance, fish welfare and product quality. Aquaculture 2017, 471, 92–105. [Google Scholar] [CrossRef]
- Mokrani, D.; Oumouna, M.; Cuesta, A.; Djamal, M.; Mustapha, O. Fish farming conditions affect to European sea bass (Dicentrarchus labrax L.) quality and shelf life during storage in ice. Aquaculture 2018, 490, 120–124. [Google Scholar] [CrossRef]
- Petrovic, M.; Kreisc, G.; Zrncic, S.; Oraic, D.; Dzafic, N.; Pleadin, J. Influence of season and farming location on the quality parameters of sea bass (Dicentrarchus labrax) and sea bream (Sparus aurata). Ital. J. Food Sci. 2015, 27, 151. [Google Scholar]
- Smichi, N.; Abdelmalek, B.E.; Kharrat, N.; Sila, A.; Bougatef, A.; Gargouri, Y.-T.; Fendri, A. The effects of storage on quality and nutritional aspects of farmed and wild sea bass (Dicentrachus labrax) muscle: In vitro oils digestibility evaluation. Fish. Res. 2017, 188, 74–83. [Google Scholar] [CrossRef]
- Vidal, N.P.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. Quality of farmed and wild sea bass lipids studied by 1H NMR: Usefulness of this technique for differentiation on a qualitative and a quantitative basis. Food Chem. 2012, 135, 1583–1591. [Google Scholar] [CrossRef]
- Xiccato, G.; Trocino, A.; Tulli, F.; Tibaldi, E. Prediction of chemical composition and origin identification of european sea bass (Dicentrarchus labrax L.) by near infrared reflectance spectroscopy (NIRS). Food Chem. 2004, 86, 275–281. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Tsitsika, E.V.; Panagiotaki, P. Implementation of quality control methods (physico-chemical, microbiological and sensory) in conjunction with multivariate analysis towards fish authenticity. Int. J. Food Sci. Technol. 2005, 40, 237–263. [Google Scholar] [CrossRef]
- Reilly, A. Overview of Food Fraud in the Fisheries Sector; FAO Fisheries and Aquaculture Circular No. 1165; FAO: Rome, Italy, 2018; 32p. [Google Scholar]
- El Sheikha, A.F.; Montet, D. How to Determine the Geographical Origin of Seafood? Crit. Rev. Food Sci. Nutr. 2014, 56, 306–317. [Google Scholar] [CrossRef] [Green Version]
- Deniro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Deniro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Moreno-Rojas, J.M.; Vasta, V.; Lanza, A.; Luciano, G.; Ladroue, V.; Guillou, C.; Priolo, A. Stable isotopes to discriminate lambs fed herbage or concentrate both obtained from C3plants. Rapid Commun. Mass Spectrom. 2008, 22, 3701–3705. [Google Scholar] [CrossRef] [PubMed]
- Hobson, K.A.; Clark, R.G. Assessing Avian Diets Using Stable Isotopes I: Turnover of13C in Tissues. Condor 1992, 94, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Michener, R.H.; Kaufman, L. Stable Isotope Ratios as Tracers in Marine Food Webs: An Update. Stable Isot. Ecol. Environ. Sci. 2008, 2, 238–282. [Google Scholar] [CrossRef]
- Kim, S.L.; Casper, D.R.; Galván-Magaña, F.; Ochoa-Díaz, R.; Hernández-Aguilar, S.B.; Koch, P.L. Carbon and nitrogen discrimination factors for elasmobranch soft tissues based on a long-term controlled feeding study. Environ. Boil. Fishes 2011, 95, 37–52. [Google Scholar] [CrossRef]
- Domi, N.; Bouquegneau, J.; Das, K. Feeding ecology of five commercial shark species of the Celtic Sea through stable isotope and trace metal analysis. Mar. Environ. Res. 2005, 60, 551–569. [Google Scholar] [CrossRef] [Green Version]
- Shiffman, D.S.; Frazier, B.S.; Kucklick, J.R.; Ábel, D.; Brandes, J.; Sancho, G. Feeding Ecology of the Sandbar Shark in South Carolina Estuaries Revealed through δ13C and δ15N Stable Isotope Analysis. Mar. Coast. Fish. 2014, 6, 156–169. [Google Scholar] [CrossRef] [Green Version]
- Dubois, S.F.; Orvain, F.; Marin-Léal, J.; Ropert, M.; Lefebvre, S. Small-scale spatial variability of food partitioning between cultivated oysters and associated suspension-feeding species, as revealed by stable isotopes. Mar. Ecol. Prog. Ser. 2007, 336, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Rojas, J.M.; Tulli, F.; Messina, M.; Tibaldi, E.; Guillou, C. Stable isotope ratio analysis as a tool to discriminate between rainbow trout (O. mykiss) fed diets based on plant or fish-meal proteins. Rapid Commun. Mass Spectrom. 2008, 22, 3706–3710. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the geographical origin of food: The application of multi-element and multi-isotope analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Camin, F.; Perini, M.; Bontempo, L.; Galeotti, M.; Tibaldi, E.; Piasentier, E. Stable isotope ratios of H, C, O, N and S for the geographical traceability of Italian rainbow trout (Oncorhynchus mykiss). Food Chem. 2018, 267, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Cossignani, L.; Cossignani, L.; Blasi, F.; Perini, M.; Barbero, A.; Pianezze, S.; Montesano, D. Characterisation and geographical traceability of Italian goji berries. Food Chem. 2019, 275, 585–593. [Google Scholar] [CrossRef]
- Wang, J.; Chen, T.; Zhang, W.; Zhao, Y.; Yang, S.; Chen, A. Tracing the geographical origin of rice by stable isotopic analyses combined with chemometrics. Food Chem. 2020, 313, 126093. [Google Scholar] [CrossRef]
- Benson, S.; Lennard, C.; Maynard, P.; Roux, C. Forensic applications of isotope ratio mass spectrometry—A review. Forensic Sci. Int. 2006, 157, 1–22. [Google Scholar] [CrossRef]
- Hong, E.; Lee, S.Y.; Jeong, J.Y.; Park, J.M.; Kim, B.H.; Kwon, K.; Chun, H.S. Modern analytical methods for the detection of food fraud and adulteration by food category. J. Sci. Food Agric. 2017, 97, 3877–3896. [Google Scholar] [CrossRef]
- Dempson, J.B.; Power, M. Use of stable isotopes to distinguish farmed from wild Atlantic salmon, Salmo salar. Ecol. Freshw. Fish 2004, 13, 176–184. [Google Scholar] [CrossRef]
- Ottavian, M.; Facco, P.; Fasolato, L.; Novelli, E.; Mirisola, M.; Perini, M.; Barolo, M. Use of Near-Infrared Spectroscopy for Fast Fraud Detection in Seafood: Application to the Authentication of Wild European Sea Bass (Dicentrarchus labrax). J. Agric. Food Chem. 2012, 60, 639–648. [Google Scholar] [CrossRef]
- Rojas, J.M.M.; Serra, F.; Giani, I.; Moretti, V.M.; Reniero, F.; Guillou, C. The use of stable isotope ratio analyses to discriminate wild and farmed gilthead sea bream (Sparus aurata). Rapid Commun. Mass Spectrom. 2006, 21, 207–211. [Google Scholar] [CrossRef]
- Serrano, R.; Blanes, M.; Orero, L. Stable isotope determination in wild and farmed gilthead sea bream (Sparus aurata) tissues from the western Mediterranean. Chemosphere 2007, 69, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Delgado, J.; Molina-Poveda, C.; Godínez-Siordia, D.E.; Villarreal, D.; Ricque-Marie, D.; Cruz-Suárez, L.E. Application of stable isotope analysis to differentiate shrimp extracted by industrial fishing or produced through aquaculture practices. Can. J. Fish. Aquat. Sci. 2014, 71, 1520–1528. [Google Scholar] [CrossRef] [Green Version]
- Thomas, F.; Jamin, E.; Wietzerbin, K.; Guérin, R.; Lees, M.; Morvan, E.; Billault, I.; Derrien, S.; Rojas, J.M.M.; Serra, F.; et al. Determination of Origin of Atlantic Salmon (Salmo salar): The Use of Multiprobe and Multielement Isotopic Analyses in Combination with Fatty Acid Composition to Assess Wild or Farmed Origin. J. Agric. Food Chem. 2008, 56, 989–997. [Google Scholar] [CrossRef]
- Sant’Ana, L.S.; Ducatti, C.; Ramires, D.G. Seasonal variations in chemical composition and stable isotopes of farmed and wild Brazilian freshwater fish. Food Chem. 2010, 122, 74–77. [Google Scholar] [CrossRef]
- Oliveira, E.J.V.M.; Sant’Ana, L.S.; Ducatti, C.; Denadai, J.C.; Kruliski, C.R.D.S. The use of stable isotopes for authentication of gadoid fish species. Eur. Food Res. Technol. 2010, 232, 97–101. [Google Scholar] [CrossRef]
- Lv, W.; Ju, T.; Dong, B.; Yu, B.; Yin, J. Effect of dietary stable isotopic ratios of carbon and nitrogen on the extent of their incorporation into tissues of rats. J. Anim. Sci. Biotechnol. 2012, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Kambikambi, M.J.; Chakona, A.; Kadye, W.T. The influence of diet composition and tissue type on the stable isotope incorporation patterns of a small-bodied southern African minnowEnteromius anoplus (Cypriniformes, Cyprinidae). Rapid Commun. Mass Spectrom. 2019, 33, 613–623. [Google Scholar] [CrossRef]
- Turchini, G.M.; Quinn, G.P.; Jones, P.L.; Palmeri, G.; Gooley, G. Traceability and Discrimination among Differently Farmed Fish: A Case Study on Australian Murray Cod. J. Agric. Food Chem. 2009, 57, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kumar, K.S.; Shin, K.-H. Applicability of stable C and N isotope analysis in inferring the geographical origin and authentication of commercial fish (Mackerel, Yellow Croaker and Pollock). Food Chem. 2015, 172, 523–527. [Google Scholar] [CrossRef]
- Bontempo, L.; Camin, F.; Larcher, R.; Nicolini, G.; Perini, M.; Rossmann, A. Coast and year effect on H, O and C stable isotope ratios of Tyrrhenian and Adriatic italian olive oils. Rapid Commun. Mass Spectrom. 2009, 23, 1043–1048. [Google Scholar] [CrossRef]
- Camin, F.; Larcher, R.; Nicolini, G.; Bontempo, L.; Bertoldi, D.; Perini, M.; Schlicht, C.; Schellenberg, A.; Thomas, F.; Heinrich, K.; et al. Isotopic and Elemental Data for Tracing the Origin of European Olive Oils. J. Agric. Food Chem. 2010, 58, 570–577. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Georgiou, C.A. Multi-element and multi-isotope-ratio analysis to determine the geographical origin of foods in the European Union. TrAC Trends Anal. Chem. 2012, 40, 38–51. [Google Scholar] [CrossRef]
- Schwägele, F. Traceability from a European perspective. Meat Sci. 2005, 71, 164–173. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Barnes, C.; Sweeting, C.J.; Jennings, S.; Barry, J.T.; Polunin, N.V.C. Effect of temperature and ration size on carbon and nitrogen stable isotope trophic fractionation. Funct. Ecol. 2007, 21, 356–362. [Google Scholar] [CrossRef]
- Bodin, N.; Le Loc’H, F.; Hily, C. Effect of lipid removal on carbon and nitrogen stable isotope ratios in crustacean tissues. J. Exp. Mar. Biol. Ecol. 2007, 341, 168–175. [Google Scholar] [CrossRef]
- Sweeting, C.; Barry, J.; Barnes, C.J.; Polunin, N.V.C.; Jennings, S.G. Effects of body size and environment on diet-tissue δ15N fractionation in fishes. J. Exp. Mar. Biol. Ecol. 2007, 340, 1–10. [Google Scholar] [CrossRef]
- Hussey, N.E.; Olin, J.A.; Kinney, M.J.; McMeans, B.C.; Fisk, A.T. Lipid extraction effects on stable isotope values (δ13C and δ15N) of elasmobranch muscle tissue. J. Exp. Mar. Biol. Ecol. 2012, 434, 7–15. [Google Scholar] [CrossRef]
- Ingram, T.; Matthews, B.; Harrod, C.; Stephens, T.; Grey, J.; Markel, R.; Mazumder, A. Lipid extraction has little effect on the d15N of aquatic consumers. Limnol. Oceanogr. Methods 2007, 5, 338–342. [Google Scholar] [CrossRef]
- Vasconi, M.; Lopez, A.; Galimberti, C.; Rojas, J.M.M.; Redondo, J.M.M.; Bellagamba, F.; Moretti, V.M. Authentication of farmed and wild european eel (Anguilla anguilla) by fatty acid profile and carbon and nitrogen isotopic analyses. Food Control. 2019, 102, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Boyd, C.E.; Sun, Z. Authentication of fishery and aquaculture products by multi-element and stable isotope analysis. Food Chem. 2016, 194, 1238–1244. [Google Scholar] [CrossRef]
- Park, H.J.; Park, T.H.; Lee, C.-I.; Kang, C.-K. Ontogenetic shifts in diet and trophic position of walleye pollock, Theragra chalcogramma, in the western East Sea (Japan Sea) revealed by stable isotope and stomach content analyses. Fish. Res. 2018, 204, 297–304. [Google Scholar] [CrossRef]
- Kusche, H.; Hillgruber, N.; Rößner, Y.; Focken, U. The effect of different fish feed compositions on δ13C and δ15N signatures of sea bass and its potential value for tracking mariculture-derived nutrients. Isot. Environ. Health Stud. 2017, 54, 28–40. [Google Scholar] [CrossRef]
- Gaye-Siessegger, J.; Focken, U.; Abel, H.; Becker, K. Starvation and low feeding levels result in an enrichment of13C in lipids and15N in protein of Nile tilapia Oreochromis niloticus L. J. Fish Biol. 2007, 71, 90–100. [Google Scholar] [CrossRef]
- Busetto, M.L.; Moretti, V.M.; Moreno-Rojas, J.M.; Caprino, F.; Giani, I.; Malandra, R.; Bellagamba, F.; Guillou, C. Authentication of Farmed and Wild Turbot (Psetta maxima) by Fatty Acid and Isotopic Analyses Combined with Chemometrics. J. Agric. Food Chem. 2008, 56, 2742–2750. [Google Scholar] [CrossRef]
- Gannes, L.Z.; Del Rio, C.M.; Koch, P. Natural Abundance Variations in Stable Isotopes and their Potential Uses in Animal Physiological Ecology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 119, 725–737. [Google Scholar] [CrossRef]
- Minagawa, M.; Wada, E. Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 1984, 48, 1135–1140. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Trocino, A.; Xiccato, G.; Majolini, D.; Tazzoli, M.; Tulli, F.; Tibaldi, E.; Messina, C.M.; Santulli, A. Levels of dioxin-like polychlorinated biphenyls (DL-PCBs) and metals in European sea bass from fish farms in Italy. Food Chem. 2012, 134, 333–338. [Google Scholar] [CrossRef]
- Valladares, S.; Planas, M. Application of Effective Day Degrees in the Assessment of Stable Isotope Patterns in Developing Seahorses under Different Temperatures. Animals 2020, 10, 1571. [Google Scholar] [CrossRef]
- Horacek, M.; Min, J.-S. Discrimination of Korean beef from beef of other origin by stable isotope measurements. Food Chem. 2010, 121, 517–520. [Google Scholar] [CrossRef]
- De Rijke, E.; Schoorl, J.; Cerli, C.; Vonhof, H.; Verdegaal, S.; Vivó-Truyols, G.; Lopatka, M.; Dekter, R.; Bakker, D.; Sjerps, M.J.; et al. The use of δ2H and δ18O isotopic analyses combined with chemometrics as a traceability tool for the geographical origin of bell peppers. Food Chem. 2016, 204, 122–128. [Google Scholar] [CrossRef] [Green Version]
| Farm Code | Latitude | Longitude | Farming System | Water Source | Temperature (°C) | Salinity (‰) | Feed Producer | Sampled Fish |
|---|---|---|---|---|---|---|---|---|
| C1 | 36.70° N | 15.12° E | Sea cage | Sea | 18 | 37 | Biomar | 10 |
| C3 | 45.80° N | 13.55° E | Sea cage | Sea/river | 12 | 34 | Skretting | 20 |
| C4 | 40.84° N | 17.47° E | Sea cage | Sea | 9.8 | 37 | Aller | 20 |
| E1 | 44.57° N | 12.33° E | Extensive | Sea | 16 | 28 | --- | 10 |
| E2 | 37.50° N | 12.48° E | Extensive | Sea | 16 | 37 | --- | 15 |
| E3 | 45.76° N | 13.17° E | Semi-intensive | Lagoon | 8 | 20 | Skretting | 20 |
| E4 | 44.96° N | 12.32° E | Extensive | Sea | 16 | 25 | --- | 10 |
| I1 | 42.42° N | 11.28° E | Inland in pond | Well | 18 | 24 | Skretting | 20 |
| I2 | 40.93° N | 14.03° E | Inland outdoor | Well | 18 | 28 | Biomar | 20 |
| I3 | 44.95° N | 12.32° E | Inland outdoor | Sea | 17 | 28 | Skretting | 20 |
| I4 | 37.98° N | 12.51° E | Inland outdoor | Sea | 18 | 37 | Biomar | 10 |
| E | I | C | MSE | |
|---|---|---|---|---|
| Biometric traits | ||||
| n. of samples | 55 | 70 | 50 | |
| Whole body weight (g) | 633.2 ± 136.4 | 613.7 ± 175.4 | 552.5 ± 146.2 | 30,964.2 |
| Total length (cm) | 38.0 ± 4.22 | 36.5 ± 3.40 | 36.3 ± 2.21 | 10.776 |
| Chemical composition (g/100 g) | ||||
| n. of samples | 55 | 70 | 50 | |
| Moisture | 76.25 ± 1.79 a | 69.54 ± 2.57 c | 71.64 ± 3.17 b | 7.004 |
| Protein Content | 19.21 ± 0.41 b | 19.73 ± 1.13 a | 19.31 ± 0.73 b | 0.875 |
| Lipid | 2.67 ± 1.80 c | 8.91 ± 2.79 a | 7.13 ± 3.01 b | 7.361 |
| Stable isotope data (‰) | ||||
| n. of samples | 11 | 14 | 10 | |
| δ13C | −16.17 ± 2.81 a | −21.87 ± 0.64 b | −21.52 ± 0.26 b | 1.927 |
| δ15N | 14.02 ± 0.45 a | 10.38 ± 0.57 b | 11.40 ± 0.49 b | 0.312 |
| Measure | Whole Muscle | Lipid Fraction | Defatted Muscle |
|---|---|---|---|
| δ13C (‰) | −21.74 ± 0.19 | −26.05 ± 0.17 | −19.88 ± 0.11 |
| δ15N (‰) | 11.12 ± 0.24 | 11.01 ± 0.22 | |
| δ2H (‰) | −124.43 ± 5.52 | −197.78 ± 2.37 | −85.52 ± 1.05 |
| Farming System | Farm Code | δ13C (‰) Diet | δ13C (‰) Group (Mean Value) | Δδ13C (‰) | δ15N (‰) Diet | δ15N (‰) Group (Mean Value) | Δδ15N (‰) |
|---|---|---|---|---|---|---|---|
| I | I1 | −24.06 | −21.82 | 2.24 | 7.67 | 10.62 | 2.95 |
| I2 | −23.45 | −22.38 | 1.07 | 5.22 | 9.60 | 4.38 | |
| I3 | −21.98 | −20.79 | 1.19 | 6.05 | 11.03 | 4.98 | |
| I4 | −23.44 | −22.13 | 1.31 | 6.14 | 9.84 | 3.70 | |
| C | C1 | −23.10 | −21.14 | 1.96 | 8.22 | 12.27 | 4.05 |
| C3 | −22.89 | −21.50 | 1.39 | 7.10 | 11.08 | 3.98 | |
| C4 | −22.82 | −21.72 | 1.10 | 7.56 | 11.29 | 3.73 | |
| E | E3 | −23.05 | −22.34 | 0.72 | 4.98 | 10.61 | 5.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tulli, F.; Moreno-Rojas, J.M.; Messina, C.M.; Trocino, A.; Xiccato, G.; Muñoz-Redondo, J.M.; Santulli, A.; Tibaldi, E. The Use of Stable Isotope Ratio Analysis to Trace European Sea Bass (D. labrax) Originating from Different Farming Systems. Animals 2020, 10, 2042. https://doi.org/10.3390/ani10112042
Tulli F, Moreno-Rojas JM, Messina CM, Trocino A, Xiccato G, Muñoz-Redondo JM, Santulli A, Tibaldi E. The Use of Stable Isotope Ratio Analysis to Trace European Sea Bass (D. labrax) Originating from Different Farming Systems. Animals. 2020; 10(11):2042. https://doi.org/10.3390/ani10112042
Chicago/Turabian StyleTulli, Francesca, José M. Moreno-Rojas, Concetta Maria Messina, Angela Trocino, Gerolamo Xiccato, José M. Muñoz-Redondo, Andrea Santulli, and Emilio Tibaldi. 2020. "The Use of Stable Isotope Ratio Analysis to Trace European Sea Bass (D. labrax) Originating from Different Farming Systems" Animals 10, no. 11: 2042. https://doi.org/10.3390/ani10112042
APA StyleTulli, F., Moreno-Rojas, J. M., Messina, C. M., Trocino, A., Xiccato, G., Muñoz-Redondo, J. M., Santulli, A., & Tibaldi, E. (2020). The Use of Stable Isotope Ratio Analysis to Trace European Sea Bass (D. labrax) Originating from Different Farming Systems. Animals, 10(11), 2042. https://doi.org/10.3390/ani10112042






