Exploring Anhedonia in Kennelled Dogs: Could Coping Styles Affect Hedonic Preferences for Sweet and Umami Flavours?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Procedures
2.2.1. Determination of Coping Style: Human-Approach Test with a Feeding Opportunity
2.2.2. Perception of Sucrose Solutions
2.2.3. Perception of Monosodium Glutamate Solutions
2.3. Statistical Analysis
3. Results
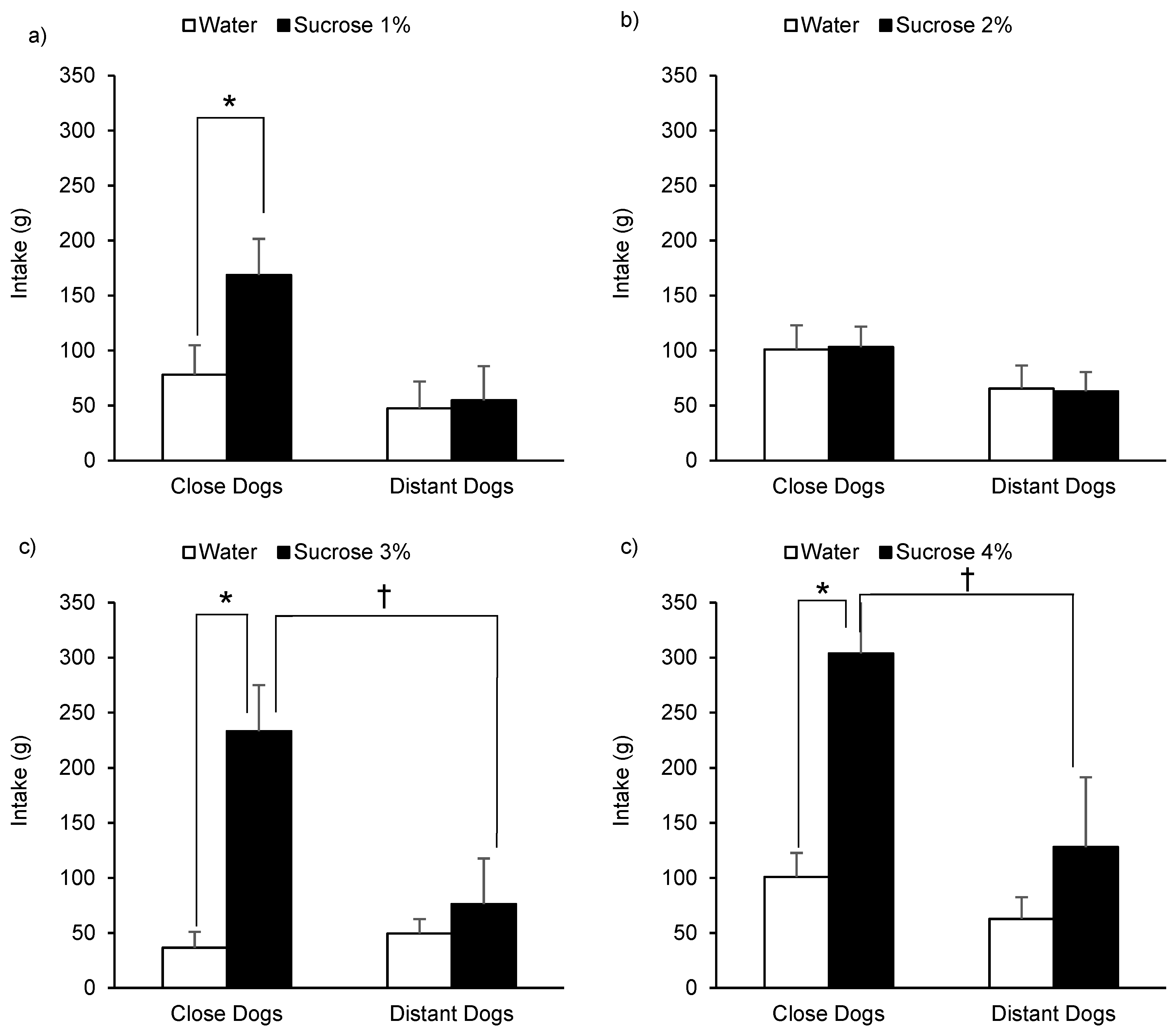
3.1. Sucrose Perception
3.2. Monosodium Glutamate Perception
4. Discussion
4.1. Coping Styles and Anhedonia
4.2. Sucrose Perception
4.3. MSG Perception
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balcombe, J. Animal Suffering and Welfare Animal pleasure and its moral significance. App. Anim. Behav. Sci. 2009, 118, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Huston, J.; Silva, M.; Komorowski, M.; Schulz, D.; Topic, B. Animal models of extinction-induced depression: Loss of reward and its consequences. Neurosci. Biobehav. Rev. 2013, 37, 2059–2070. [Google Scholar] [CrossRef]
- Cabanac, M. Physiological Role of Pleasure. Science 1971, 173, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Beaver, B. Canine Ingestive Behavior. In Canine Behavior Insights and Answers, 2nd ed.; Saunders: Philadelphia, PA, USA, 2009; pp. 223–243. [Google Scholar]
- Matthews, K.; Forbes, N.; Reid, I. Sucrose Consumption as an Hedonic Measure Following Chronic Unpredictable Mild Stress. Physiol. Behav. 1995, 57, 241–248. [Google Scholar] [CrossRef]
- Ho, N.; Sommers, M. Anhedonia: A Concept Analysis. Arch. Psychiat. Nurs. 2013, 27, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, A.; Miyoshi, M.; Imoto, T.; Ryoke, K.; Eatanabe, T. Chronic restraint stress in rats suppresses sweet and umami taste responses and lingual expression of T1R3 mRNA. Neurosci. Lett. 2010, 486, 211–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, J.; Solà-Oriol, D.; Manteca, X.; Pérez, J.F.; Dwyer, D.M. Anhedonia in pigs? Effects of social stress and restraint stress on sucrose preference. Physiol. Behav. 2015, 151, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Willner, P.; Richard, M.; Papp, M. Chronic mils stress-induced anhedonia: A realistic animal model of depression. Neurosci. Biobehav. Rev. 1992, 16, 525–534. [Google Scholar] [CrossRef]
- Koob, G.; Zimmer, A. Animal models of psychiatric disorders. In Handbook of Clinical Neurology, 3rd ed.; Aminoff, M., Boller, F., Swaab, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 106, pp. 137–166. [Google Scholar]
- Fureix, C.; Beaulieu, C.; Argaud, S.; Rochais, C.; Quinton, M.; Henry, S.; Hausberger, M.; Mason, G. Investigating anhedonia in a non-conventional species: Do some riding horses Equus caballus display symptoms of depression? Appl. Anim. Behav. Sci. 2015, 162, 26–36. [Google Scholar] [CrossRef]
- McCobb, E.C.; Patronek, G.J.; Marder, A.; Dinnage, J.D.; Stone, M.S. Assessment of stress levels among cats in four animal shelters. J. Am. Vet. Med. Assoc. 2005, 226, 548–555. [Google Scholar] [CrossRef]
- Harvey, N.D.; Moesta, A.; Kappel, S.; Wongsaengchan, C.; Harris, H.; Craigon, P.J.; Fureix, C. Could Greater Time Spent Displaying Waking Inactivity in the Home Environment Be a Marker for a Depression-Like State in the Domestic Dog? Animals 2019, 9, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luño, I.; Palacio, J.; García-Belenguer, S.; González-Martínez, Á.; Rosado, B. Perception of canine welfare concerns among veterinary students, practitioners, and behavior specialists in Spain. J. Vet. Med. Educ. 2017, 44, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.Y.; Paterson, M.; Phillips, C.J. Socioeconomic Influences on Reports of Canine Welfare Concerns to the Royal Society for the Prevention of Cruelty to Animals (RSPCA) in Queensland, Australia. Animals 2019, 9, 711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beerda, B.; Schilder, M.; van Hooff, J.; de Vries, H. Manifestations of chronic and acute stress in dogs. App. Anim. Behav. Sci. 1997, 52, 307–319. [Google Scholar] [CrossRef]
- Rooney, N.; Gaines, S.; Bradshaw, J. Behavioural and glucocorticoid responses of dogs (Canis familiaris) to kennelling: Investigating mitigation of stress by prior habituation. Physiol. Behav. 2007, 92, 847–854. [Google Scholar] [CrossRef]
- Rooney, N.; Gaines, S.; Hiby, E. A practitioner’s guide to working dog welfare. J. Vet. Behav. Clin. Appl. Res. 2009, 4, 127–134. [Google Scholar] [CrossRef]
- Pullen, A.; Ndende, R.; Stephen, J. Preferences for toy types and presentations in kennel housed dogs. App. Anim. Behav. Sci. 2010, 125, 151–156. [Google Scholar] [CrossRef]
- Hennessy, M. Using hypothalamic–pituitary–adrenal measures for assessing and reducing the stress of dogs in shelters. Appl. Anim. Behav. Sci. 2013, 149, 1–12. [Google Scholar] [CrossRef]
- Taylor, K.D.; Mills, D.S. The effect of the kennel environment on canine welfare: A critical review of experimental studies. Anim. Welf. 2007, 16, 435–447. Available online: https://www.ingentaconnect.com/contentone/ufaw/aw/2007/00000016/00000004/art00003 (accessed on 29 September 2020).
- Grigg, E.K.; Nibblett, B.M.; Robinson, J.Q.; Smits, J.E. Evaluating pair versus solitary housing in kennelled domestic dogs (Canis familiaris) using behaviour and hair cortisol: A pilot study. Vet. Rec. Open 2017, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Beerda, B.; Schilder, M.B.; van Hooff, J.A.; de Vries, H.W.; Mol, J.A. Behavioural and hormonal indicators of enduring environmental stress in dogs. Anim. Welf. 2000, 9, 49–62. Available online: https://www.ingentaconnect.com/contentone/ufaw/aw/2000/00000009/00000001/art00005 (accessed on 29 September 2020).
- Koolhaas, J.M.; van Reenen, C.G. Animal behavior and well-being symposium: Interaction between coping style/personality, stress and welfare: Relevance for domestic farm animals. J. Anim. Sci. 2016, 94, 2284–2296. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Korte, S.M.; de Boer, S.F.; van der Vegt, B.J.; van Reenen, C.G.; Hopster, H.; Blokhuis, H.J. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Coppens, C.M.; de Boer, S.F.; Koolhaas, J.M. Coping styles and behavioural flexibility: Towards underlying mechanisms. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010, 365, 4021–4028. [Google Scholar] [CrossRef]
- Carere, C.; Caramaschi, D.; Fawcett, T.W. Covariation between personalities and individual differences in copingwith stress: Converging evidence and hypotheses. Curr. Zool. 2010, 56, 728–740. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; de Boer, S.F.; Coppens, C.M.; Buwalda, B. Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Front. Neuroendocrinol. 2010, 31, 307–321. [Google Scholar] [CrossRef]
- Benus, R.F.; Koolhaas, J.M.; van Oortmerssen, G.A. Individual differences in behavioural reaction to a changing environment in mice and rats. Behaviour 1987, 100, 105–122. [Google Scholar] [CrossRef] [Green Version]
- Sluyter, F.; Korte, S.M.; Bohus, B.; van Oortmerssen, G.A. Behavioral stress response of genetically selected aggressive and nonaggressive wild house mice in the shock-probe/defensive burying test. Pharmacol. Biochem. Behav. 1996, 54, 113–116. [Google Scholar] [CrossRef]
- Bolhuis, J.E.; Schouten, W.G.; de Leeuw, J.A.; Schrama, J.W.; Wiegant, V.M. Individual coping characteristics, rearing conditions and behavioural flexibility in pigs. Behav. Brain Res. 2004, 152, 351–360. [Google Scholar] [CrossRef]
- Carere, C.; Groothuis, T.G.; Möstl, E.; Daan, S.; Koolhaas, J.M. Fecal corticosteroids in a territorial bird selected for different personalities: Daily rhythm and the response to social stress. Horm. Behav. 2003, 43, 540–548. [Google Scholar] [CrossRef]
- Øverli, Ø.; Korzan, W.J.; Höglund, E.; Winberg, S.; Bollig, H.; Watt, M.; Summers, C.H. Stress coping style predicts aggression and social dominance in rainbow trout. Horm. Behav. 2004, 45, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ruis, M.A.; te Brake, J.H.; Engel, B.; Buist, W.G.; Blokhuis, H.J.; Koolhaas, J.M. Adaptation to social isolation: Acute and long-term stress responses of growing gilts with different coping characteristics. Physiol. Behav. 2001, 73, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Veenema, A.H.; Cremers, T.I.; Jongsma, M.E.; Steenbergen, P.J.; de Boer, S.F.; Koolhaas, J.M. Differences in the effects of 5-HT 1A receptor agonists on forced swimming behavior and brain 5-HT metabolism between low and high aggressive mice. Psychopharmacology 2005, 178, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ijichi, C.L.; Collins, L.M.; Elwood, R.W. Evidence for the role of personality in stereotypy predisposition. Anim. Behav. 2013, 85, 1145–1151. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, X. Integration of animal behaviors under stresses with different time courses. Neural Regen. Res. 2014, 9, 1464–1473. [Google Scholar] [CrossRef]
- Axelsoon, E.; Ratnakumar, A.; Arendt, M.-L.; Maqbool, K.; Webster, M.; Perloski, M.; Liberg, O.; Arnemo, J.M.; Hedhammar, A.; Lindblad-Toh, K. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 2013, 495, 360–364. [Google Scholar] [CrossRef]
- Rolls, E. Functional neuroimaging of umami taste: What makes umami pleasant? Am. J. Clin. Nutr. 2009, 90, 804–813. [Google Scholar] [CrossRef]
- Alegría-Morán, R.A.; Guzmán-Pino, S.A.; Egaña, J.I.; Muñoz, C.; Figueroa, J. Food preferences in dogs: Effect of dietary composition and intrinsic variables on diet selection. Animals 2019, 9, 219. [Google Scholar] [CrossRef] [Green Version]
- Thodberg, K.; Jensen, K.H.; Herskin, M.S. A general reaction pattern across situations in prepubertal gilts. Appl. Anim. Behav. Sci. 1999, 63, 103–119. [Google Scholar] [CrossRef]
- Ruis, M.A.; te Brake, J.H.; van de Burgwal, J.A.; de Jong, I.C.; Blokhuis, H.J.; Koolhaas, J.M. Personalities in female domesticated pigs: Behavioural and physiological indications. Appl. Anim. Behav. Sci. 2000, 66, 31–47. [Google Scholar] [CrossRef]
- Kooij, E.E.V.; Kuijpers, A.H.; Schrama, J.W.; van Eerdenburg, F.J.C.M.; Schouten, W.G.P.; Tielen, M.J.M. Can we predict behaviour in pigs? Searching for consistency in behaviour over time and across situations. Appl. Anim. Behav. Sci. 2002, 75, 293–305. [Google Scholar] [CrossRef]
- Brown, J.A.; Dewey, C.; Delange, C.F.; Mandell, I.B.; Purslow, P.P.; Robinson, J.A.; Widowski, T.M. Reliability of temperament tests on finishing pigs in group-housing and comparison to social tests. Appl. Anim. Behav. Sci. 2009, 118, 28–35. [Google Scholar] [CrossRef]
- Svartberg, K.; Forkman, B. Personality traits in the domestic dog (Canis familiaris). Appl. Anim. Behav. Sci. 2002, 79, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Horváth, Z.; Igyártó, B.; Magyar, A.; Miloski, Á. Three different coping styles in police dogs exposed to a short- term challenge. Horm. Behav. 2007, 52, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Janczak, A.M.; Pedersen, L.J.; Rydhmer, L.; Bakken, M. Relation between early fear-and anxiety-related behaviour and maternal ability in sows. Appl. Anim. Behav. Sci. 2003, 82, 121–135. [Google Scholar] [CrossRef]
- Pecoraro, N.; Reyes, F.; Gomez, F.; Bhargava, A.; Dallman, M. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology 2004, 145, 3754–3762. [Google Scholar] [CrossRef]
- Maniam, J.; Morris, M. The link between stress and feeding behaviour. Neuropharmacology 2012, 63, 97–110. [Google Scholar] [CrossRef]
- Bowman, A.; Scottish, S.P.C.A.; Dowell, F.J.; Evans, N.P. The effect of different genres of music on the stress levels of kennelled dogs. Physiol. Behav. 2017, 171, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Beerda, B.; Schilder, M.B.; van Hooff, J.A.; de Vries, H.W.; Mol, J.A. Chronic stress in dogs subjected to social and spatial restriction. I. Behavioral responses. Physiol. Behav. 1999, 66, 233–242. [Google Scholar] [CrossRef]
- Hiby, E.F. The Welfare of Kennelled Domestic Dogs. Ph.D. Thesis, University of Bristol, Bristol, UK, 2005. [Google Scholar]
- Kovács, L.; Kézér, F.L.; Tőzsér, J.; Szenci, O.; Póti, P.; Pajor, F. Heart rate and heart rate variability in dairy cows with different temperament and behavioural reactivity to humans. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Silva, P.; Martins, C.; Engrola, S.; Marino, G. Individual differences in cortisol levels and behaviour of Senegalese sole (Solea senegalensis) juveniles: Evidence for coping styles. Appl. Anim. Behav. Sci. 2010, 124, 75–81. [Google Scholar] [CrossRef]
- Barnard, S.; Wells, D.L.; Hepper, P.G. Laterality as a Predictor of Coping Strategies in Dogs Entering a Rescue Shelter. Symmetry 2018, 10, 538. [Google Scholar] [CrossRef] [Green Version]
- Wechsler, B. Coping and coping strategies: A behavioural view. Appl. Anim. Behav. Sci. 1995, 43, 123–134. [Google Scholar] [CrossRef]
- Benus, R.F.; Koolhaas, J.M.; van Oortmerssen, G.A. Aggression and adaptation to the light-dark cycle: Role of intrinsic and extrinsic control. Physiol. Behav. 1988, 43, 131–137. [Google Scholar] [CrossRef]
- Groothuis, T.G.; Carere, C. Avian personalities: Characterization and epigenesis. Neurosci. Biobehav. Rev. 2005, 29, 137–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Palma, C.; Dufour, A.B.; Viggiano, E.; Palme, R.; Natoli, E.; Fantini, C.; Barillari, E. Evaluating the temperament in shelter dogs. Behaviour 2005, 142, 1307–1328. [Google Scholar] [CrossRef] [Green Version]
- Zidar, J.; Balogh, A.; Favati, A.; Jensen, P.; Leimar, O.; Løvlie, H. A comparison of animal personality and coping styles in the red junglefowl. Anim. Behav. 2017, 130, 209–220. [Google Scholar] [CrossRef]
- Sclafani, A. Oral and postoral determinants of food reward. Physiol. Behav. 2004, 81, 773–779. [Google Scholar] [CrossRef]
- Mccaughey, S. The taste of sugars. Neurosci. Biobehav. Rev. 2008, 32, 1024–1043. [Google Scholar] [CrossRef]
- Ventura, A.K.; Mennella, J.A. Innate and learned preferences for sweet taste during childhood. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, J.W.S. The Evolutionary Basis for the Feeding Behavior of Domestic Dogs (Canis familiaris) and Cats (Felis catus). J. Nutr. 2006, 136, 1927S–1931S. [Google Scholar] [CrossRef] [PubMed]
- Hewson-Hughes, A.K.; Hewson-Hughes, V.L.; Colyer, A.; Miller, A.T.; McGrane, S.J.; Hall, S.R.; Butterwick, R.F.; Simpson, S.J.; Raubenheimer, D. Geometric analysis of macronutrient selection in breeds of the domestic dog, Canis lupus familiaris. Behav. Ecol. 2013, 24, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Toyomasu, Y.; Mochiki, E.; Yanai, M.; Ogata, K.; Tabe, Y.; Ando, H.; Ohno, T.; Aihara, R.; Zai, H.; Kuwano, H. Intragastric monosodium L-glutamate stimulates motility of upper gut via vagus nerve in conscious dogs. Am. J. Physiol. Reg. Intergr. Comp. Physiol. 2010, 298, 1125–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumazawa, T.; Nakamura, M.; Kurihara, K. Canine taste nerve responses to umami substances. Physiol. Behav. 1991, 49, 875–881. [Google Scholar] [CrossRef]
- Figueroa, J.; Frías, D.; Solà-Oriol, D.; Tadich, T.; Franco-Rosselló, R.; Nuñez, V.; Dwyer, D.M. Palatability in pigs, the pleasure of consumption. J. Anim. Sci. 2019, 97, 2165–2174. [Google Scholar] [CrossRef]
- Uematsu, A.; Tsurugizawa, T.; Kitamura, A.; Ichikawa, R.; Iwatsuki, K.; Uneyama, H.; Torii, K. Evaluation of the ‘liking’ and ‘wanting’ properties of umami compound in rats. Physiol. Behav. 2011, 102, 553–558. [Google Scholar] [CrossRef]
- Davis, J.; Smith, G. Analysis of microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav. Neurosci. 1992, 106, 217–228. [Google Scholar] [CrossRef]
- Torres, S.; Nowson, C. Relationship between stress, eating behavior and obesity. Nutrition 2007, 23, 887–894. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna, D.; Carrasco, C.; Álvarez, D.; González, C.; Egaña, J.I.; Figueroa, J. Exploring Anhedonia in Kennelled Dogs: Could Coping Styles Affect Hedonic Preferences for Sweet and Umami Flavours? Animals 2020, 10, 2087. https://doi.org/10.3390/ani10112087
Luna D, Carrasco C, Álvarez D, González C, Egaña JI, Figueroa J. Exploring Anhedonia in Kennelled Dogs: Could Coping Styles Affect Hedonic Preferences for Sweet and Umami Flavours? Animals. 2020; 10(11):2087. https://doi.org/10.3390/ani10112087
Chicago/Turabian StyleLuna, Daniela, Carolina Carrasco, Daniela Álvarez, Catalina González, Juan Ignacio Egaña, and Jaime Figueroa. 2020. "Exploring Anhedonia in Kennelled Dogs: Could Coping Styles Affect Hedonic Preferences for Sweet and Umami Flavours?" Animals 10, no. 11: 2087. https://doi.org/10.3390/ani10112087




