Effects of Oxytetracycline and Gentamicin Therapeutic Doses on Hematological, Biochemical and Hematopoietic Parameters in Cyprinus carpio Juveniles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
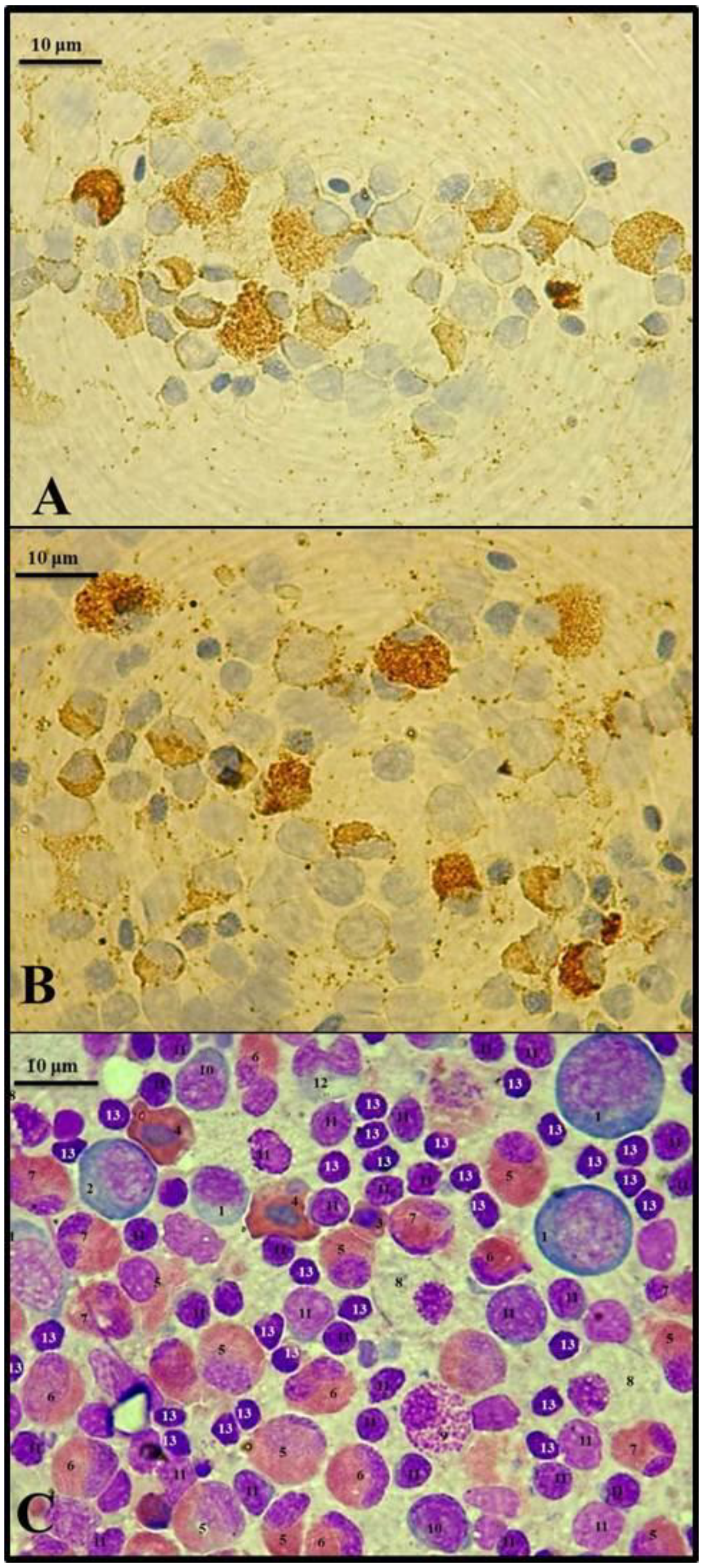
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Elia, A.C.; Ciccotelli, V.; Pacini, N.; Dörr, A.J.M.; Gili, M.; Natali, M.; Gasco, L.; Prearo, M.; Abete, M.C. Transferability of oxytetracycline (OTC) from feed to carp muscle and evaluation of the antibiotic effects on antioxidant systems in liver and kidney. Fish Physiol. Biochem. 2014, 40, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.F.; Santos, E.B.; Esteves, V.I. Oxytetracycline in intensive aquaculture: Water quality during and after its administration, environmental fate, toxicity and bacterial resistance. Rev. Aquac. 2019, 11, 1176–1194. [Google Scholar] [CrossRef]
- Bojarski, B.; Batoryna, M.; Bień, M.; Drąg-Kozak, E.; Formicki, G.; Jakubiak, M.; Socha, M.; Tombarkiewicz, B. Assessment of gentamicin effect on oxidoreductive balance and microstructure of trunk kidney in Prussian carp (Carassius gibelio). Ann. Wars. Univ. Life Sci. Sggw Anim. Sci. 2019, 58, 115–123. [Google Scholar] [CrossRef]
- Hari, R.; Taherunnisa, S.; Raut, S.Y.; Mutalik, S.; Koteshwara, K.B. Challenges in the development of analytical test procedure for aminoglycosides: A critical review. J. Appl. Pharm. Sci. 2019, 9, 145–152. [Google Scholar]
- Gbylik-Sikorska, M.; Posyniak, A.; Mitrowska, K.; Gajda, A.; Błądek, T.; Sniegocki, T.; Zmudzki, J. Occurrence of veterinary antibiotics and chemotherapeutics in fresh water, sediment, and fish of the rivers and lakes in Poland. Bull. Vet. Insting. Pulawy 2014, 58, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Bojarski, B.; Kot, B.; Witeska, M. Antibacterials in aquatic environment and their toxicity to fish. Pharmaceuticals 2020, 13, 189. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dolz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef]
- Linares, J.F.; Gustafsson, I.; Baquero, F.; Martinez, J.L. Antibiotics as intermicrobial signaling agents instead of weapons. Proc. Natl. Acad. Sci. USA 2006, 103, 19484–19489. [Google Scholar] [CrossRef] [Green Version]
- Manage, P.M. Heavy use of antibiotics in aquaculture: Emerging human and animal health problems—A review. Sri Lanka J. Aquat. Sci. 2018, 23, 13–27. [Google Scholar] [CrossRef]
- Seyfried, E.E.; Newton, R.J.; Rubert, K.F.; Pedersen, J.A.; McMahon, K.D. Occurrence of tetracycline resistance genes in aquaculture facilities with varying use of oxytetracycline. Microb. Ecol. 2010, 59, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Tamminen, M.; Karkman, A.; Lohmus, A.; Muziasari, W.I.; Takasu, H.; Wada, S.; Suzuki, S.; Virta, M. Tetracycline resistance genes persist at aquaculture farms in the absence of selection pressure. Environ. Sci. Technol. 2010, 45, 386–391. [Google Scholar] [CrossRef]
- Reda, R.M.; Ibrahim, R.E.; Ahmed, E.N.G.; El-Bouhy, Z.M. Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. Egypt. J. Aquat. Res. 2013, 39, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, S.; Antunes, S.C.; Nunes, B.; Correia, A.T. Histological alterations in gills and liver of rainbow trout (Oncorhynchus mykiss) after exposure to the antibiotic oxytetracycline. Environ. Toxicol. Pharmacol. 2017, 53, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Grondel, G.L.; Gloudemans, A.G.M.; van Muiswinkel, W.B. The influence of antibiotics on the immune system. I.I. Modulation of fish leukocyte responses in culture. Vet. Immunol. Immunopathol. 1985, 9, 251–260. [Google Scholar] [CrossRef]
- Botelho, R.G.; Christofolettib, C.A.; Correiac, J.E.; Ansoarc, Y.; Olindad, R.A.; Tornisielo, V.L. Genotoxic responses of juvenile tilapia (Oreochromis niloticus) exposed to florfenicol and oxytetracycline. Chemosphere 2015, 132, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Antunes, S.C.; Correia, A.T.; Nunes, B. Rainbow trout (Oncorhynchus mykiss) pro-oxidant and genotoxic responses following acute and chronic exposure to the antibiotic oxytetracycline. Ecotoxicology 2017, 26, 104–117. [Google Scholar] [CrossRef]
- Pes, T.S.; Saccol, E.M.H.; Londero, E.P.; Bressan, C.A.; Ourique, G.M.; Rizzetti, T.M.; Prestes, O.D.; Zanella, R.; Baldisserotto, B.; Pavanato, M.A. Protective effect of quercetin against oxidative stress induced byoxytetracycline in muscle of silver catfish. Aquaculture 2018, 484, 120–125. [Google Scholar] [CrossRef]
- Oliveira, R.; McDonough, S.; Ladewig, J.C.L.; Soares, A.M.V.M.; Nogueira, A.J.A.; Domingues, I. Effects of oxytetracycline and amoxicillin ondevelopment and biomarkers activities of zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2013, 36, 903–912. [Google Scholar] [CrossRef]
- Barros-Becker, F.; Romero, J.; Pulgar, A.; Feijoo, C.G. Persistent oxytetracycline exposure induces an inflammatory process that improves regenerative capacity in zebrafish larvae. PLoS ONE 2012, 7, e36827. [Google Scholar] [CrossRef] [Green Version]
- Omoregie, E.; Oyebanji, S.M. Oxytetracycline-induced blood disorder in juvenile Nile tilapia Oreochromis niloticus (Trewavas). J. World Aquac. Soc. 2002, 33, 377–382. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Yousefi, M. Beneficial effects of thyme (Thymus vulgaris) extract on oxytetracycline-induced stress response, immunosuppression, oxidative stress and enzymatic changes in rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2019, 25, 298–309. [Google Scholar] [CrossRef]
- Maklakova, M.E.; Kondratieva, I.A.; Mikhailova, E.S.; Stupin, R.V.; Khapchaev, S.H.; Kasumya, A.O. Effect of antibiotics on immunophysiological status and their taste attractiveness for rainbow trout Parasalmo (=Oncorhynchus) mykiss (Salmoniformes, Salmonidae). J. Ichthyol. 2011, 51, 1133–1142. [Google Scholar] [CrossRef]
- Ambili, T.R.; Saravanan, M.; Ramesh, M.; Abhijith, D.B.; Poopal, R.K. Toxicological effects of the antibiotic oxytetracycline to an Indian major carp Labeo rohita. Arch. Environ. Contam. Toxicol. 2013, 64, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Reda, R.M.; Mahmoud, R.; Selima, K.M.; El-Araby, I.E. Effects of dietary acidifiers on growth, hematology, immune responseand disease resistance of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2016, 50, 255–262. [Google Scholar] [CrossRef]
- El-Adawy, M.; Abd El-Aziz, M.; El-Shazly Ali, N.G.; Abu El-Magd, M. Dietary propionic acid enhances antibacterial and immunomodulatory effects of oxytetracycline on Nile tilapia, Oreochromis niloticus. Environ. Sci. Pollut. Res. 2018, 25, 34200–34211. [Google Scholar] [CrossRef] [PubMed]
- Serezli, R.; Cagirgan, H.; Okumus, I.; Akhan, S.; Balta, F. The effect of oxytetracycline on non-specific immune response in sea bream (Sparus aurata L. 1758). Turk. J. Vet. Anim. Sci. 2005, 29, 31–35. [Google Scholar]
- Nakano, T.; Hayashi, S.; Nagamine, N. Effect of excessive doses of oxytetracycline on stress-related biomarker expression in coho salmon. Environ. Sci. Pollut. Res. 2015, 1, 7121–7128. [Google Scholar] [CrossRef]
- Cernaro, V.; Sfacteria, A.; Rifici, C.; Marci, F.; Maricchiolo, G.; Lacquaniti, A. Renoprotective effect of erythropoietin in zebrafish after administration of gentamicin: An immunohistochemical study for β-catenin and c-kit expression. J. Nephrol. 2017, 30, 385–391. [Google Scholar] [CrossRef]
- Reimschuessel, R.; Chamie, S.J.; Kinnel, M. Evaluation of gentamicin induced nephrotoxicosis in toadfish. J. Am. Vet. Med. Assoc. 1996, 209, 137–139. [Google Scholar]
- Reimschuessel, R.; Williams, D. Development of new nephrons in adult kidneys following gentamicin-induced nephrotoxicity. Ren. Fail. 1995, 17, 101–106. [Google Scholar] [CrossRef]
- Augusto, J.; Smith, B.; Smith, S.; Robertson, J.; Reimschuessel, R. Gentamicin-induced nephrotoxicity and nephroneogenesis in Oreochromis nilotica, a tilapian fish. Dis. Aquat. Org. 1996, 26, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Wooster, A.; Bowser, P.R. Comparative blood chemistry and histopathology of tilapia infected with Vibrio vulnificus or Streptococcus iniae or exposed to carbon tetrachloride, gentamicin, or copper sulfate. Aquaculture 2004, 239, 421–443. [Google Scholar] [CrossRef]
- Van Trump, W.J.; Coombs, S.; Duncan, K.; McHenry, M.J. Gentamicin is ototoxic to all hair cells in the fish lateral line system. Hear. Res. 2010, 261, 42–50. [Google Scholar] [CrossRef]
- Trushenski, J.T.; Aardsma, M.P.; Barry, K.J.; Bowker, J.D.; Jackson, C.J.; Jakaitis, M.; McClure, L.R.; Rombenso, A.N. Oxytetracycline does not cause growth promotion in finfish. J. Anim. Sci. 2018, 96, 1667–1677. [Google Scholar] [CrossRef]
- Hassani, M.; Lazaro, R.; Perez, C.; Condon, S.; Pagan, R. Thermostability of oxytetracycline, tetracycline, and doxycycline at ultrahigh temperatures. J. Agric. Food Chem. 2008, 56, 2676–2680. [Google Scholar] [CrossRef]
- Jones, J.; Kinnel, M.; Christenson, R.; Reimschuessel, R. Gentamicin concentrations in toadfish and goldfish serum. J. Aquat. Anim. Health 1997, 9, 211–215. [Google Scholar] [CrossRef]
- Svobodova, Z.; Pravda, D.; Palackova, J. Unified Methods of Haematological Examination of Fish; Methods No. 20; Research Institute of Fish Culture and Hydrobiology: Vodnany, Czech Republic, 1991. [Google Scholar]
- Studnicka, B.; Siwicki, A.K.; Ryba, B. Phagocytic ability of neutrophils in carp (Cyprinus carpio L.). Isr. J. Aquac. Bamidgeh 1985, 37, 123–128. [Google Scholar]
- Fijan, N. Morphogenesis of blood cell lineages in channel catfish. J. Fish Biol. 2002, 60, 999–1014. [Google Scholar] [CrossRef]
- Fijan, N. Composition of main haematopoietic compartments in normal and bled channel catfish. J. Fish Biol. 2002, 60, 1142–1154. [Google Scholar] [CrossRef]
- Kondera, E. Haematopoiesis in the head kidney of common carp (Cyprinus carpio L.): A morphological study. Fish Physiol. Biochem. 2011, 37, 355–362. [Google Scholar] [CrossRef]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [Green Version]
- Awad, Y.M.; Kim, S.C.; El-Azeem, A.M.; Kim, K.H.; Kim, K.R.; Kim, K.; Jeon, C.; Lee, S.S.; Ok, Y.S. Veterinary antibiotics contamination in water, sediment, and soil near a swine manure composting facility. Environ. Earth Sci. 2014, 71, 1433–1440. [Google Scholar] [CrossRef]
- Kim, K.R.; Owens, G.; Kwon, S.I.; So, K.H.; Lee, D.B.; Ok, Y.S. Occurrence and environmental fate of veterinary antibiotics in the terrestrial environment. Water Air Soil Pollut. 2011, 214, 163–174. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Kondera, E.; Witeska, M. Cadmium and copper reduce hematopoietic potential in common carp (Cyprinus carpio L.) head kidney. Fish Physiol. Biochem. 2013, 39, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Kondera, E.; Teodorczuk, B.; Ługowska, K.; Witeska, M. Effect of glyphosate-based herbicide on hematological and hemopoietic parameters in common carp (Cyprinus carpio L.). Fish Physiol. Biochem. 2018, 44, 1011–1018. [Google Scholar] [CrossRef]
- Rodrigues, S.; Antunes, S.C.; Nunes, B.; Correia, A.T. Histopathological effects in gills and liver of Sparus aurata following acute and chronic exposures to erythromycin and oxytetracycline. Environ. Sci. Pollut. Res. 2019, 26, 15481–15495. [Google Scholar] [CrossRef]
- Marking, L.L.; Howe, G.E.; Crowther, J.R. Toxicity of erythromycin, oxytetracycline, and tetracycline administered to lake trout in water baths, by injection, or by feeding. Progress. Fish-Cult. 1988, 50, 197–201. [Google Scholar] [CrossRef]
- Hentschel, D.M.; Park, K.M.; Cilenti, L.; Zervos, A.S.; Drummond, I.; Bonventre, J.V. Acute renal failure in zebrafish: A novel system to study a complex disease. Am. J. Physiol. Ren. Physiol. 2005, 288, 923–929. [Google Scholar] [CrossRef] [Green Version]
| Parameter | Experimental Groups | |||
|---|---|---|---|---|
| Control 1 | Control 2 | OTC | G | |
| Ht [%] | 30.7 ± 3.4 a | 33.4 ± 3.2 a | 32.7 ± 3.8 a | 30.9 ± 4.0 a |
| Hb [g/L] | 84.8 ± 13.0 a | 98.1 ± 13.2 a | 89.4 ± 10.4 a | 93.5 ± 7.5 a |
| RBC [106/µL] | 1.60 ± 0.14 a | 1.71 ± 0.31 a | 1.74 ± 0.24 a | 1.65 ± 0.13 a |
| MCV [fL] | 193.0 ± 28.9 a | 199.0 ± 29.6 a | 190.6 ± 25.2 a | 188.4 ± 25.8 a |
| MCH [pg] | 53.4 ± 9.8 a | 58.5 ± 9.6 a | 52.2 ± 7.8 a | 57.1 ± 6.2 a |
| MCHC [g/L] | 276.0 ± 19.6 a | 293.7 ± 29.2 a | 274.5 ± 23.9 a | 306.6 ± 43.9 a |
| Erythroblasts [%] | 0.1 ± 0.2 a | 0.2 ± 0.2 a | 0.2 ± 0.2 a | 0.1 ± 0.2 a |
| Abnormal erythrocytes [%] | 3.2 ± 2.4 a | 2.9 ± 2.4 a | 2.4 ± 2.2 a | 2.7 ± 2.2 a |
| WBC [103/µL] | 77.0 ± 25.7 a | 57.9 ± 21.0 a | 69.9 ± 19.7 a | 62.9 ± 21.4 a |
| Lymphocytes [%] | 96.0 ± 5.8 a | 97.0 ± 3.6 a | 95.8 ± 3.9 a | 93.7 ± 5.0 a |
| Neutrophils [%] | 1.6 ± 2.1 a | 2.0 ± 3.3 a | 2.5 ± 2.6 a | 4.9 ± 6.0 a |
| Monocytes [%] | 1.8 ± 4.0 a | 0.7 ± 0.8 a | 0.9 ± 1.0 a | 1.1 ± 1.4 a |
| TC [103/µL] | 5.4 ± 7.7 a | 4.2 ± 5.6 a | 4.7 ± 6.0 a | 5.4 ± 6.3 a |
| Glucose [mg/dL] | 65.3 ± 7.2 a | 54.7 ± 8.0 b | 70.2 ± 8.5 a | 55.1 ± 12.4 b |
| NBT [g/L] | 0.69 ± 0.21 a | 0.77 ± 0.17 a | 1.49 ± 0.18 b | 1.03 ± 0.27 ab |
| ALT [U/L] | 74.0 ± 13.8 a | 57.5 ± 14.9 a | 81.1 ± 38.7 a | 70.0 ± 31.8 a |
| AST [U/L] | 67.0 ± 8.9 a | 65.4 ± 27.4 a | 86.0 ± 51.1 a | 91.1 ± 68.0 a |
| Cell Types | Experimental Groups | |||
|---|---|---|---|---|
| Control 1 | Control 2 | OTC | G | |
| PCNA-positive [%] | 7.6 ± 1.3 ab | 7.4 ± 1.2 a | 8.5 ± 1.7 ab | 9.5 ± 1.9 b |
| Cas-positive [%] | 6.9 ± 1.1 a | 6.5 ± 1.3 a | 8.1 ± 1.7 ab | 8.8 ± 1.5 b |
| PCNA/Cas | 1.2 ± 0.4 a | 1.2 ± 0.3 a | 1.1 ± 0.4 a | 1.1 ± 0.2 a |
| Early blast cells | 8.9 ± 2.8 a | 8.9 ± 2.6 a | 9.9 ± 2.8 a | 10.7 ± 3.2 a |
| Erythroid [%] | 6.2 ± 1.6 a | 6.4 ± 1.6 a | 7.5 ± 1.7 a | 6.8 ± 2.7 a |
| Erythroblasts | 2.6 ± 1.1 a | 3.0 ± 1.2 a | 3.8 ± 1.0 a | 3.4 ± 2.0 a |
| Erythrocytes | 3.5 ± 1.1 a | 3.5 ± 0.9 a | 3.8 ± 1.0 a | 3.4 ± 1.2 a |
| Lymphoid [%] | 51.0 ± 5.3 a | 48.7 ± 4.7 ab | 46.3 ± 8.1 ab | 41.0 ± 7.3 b |
| Prolymphocytes | 2.5 ± 1.8 a | 2.7 ± 1.2 a | 2.3 ± 1.0 a | 3.0 ± 2.4 a |
| Lymphocytes | 47.2 ± 5.8 a | 44.6 ± 4.7 ab | 42.3 ± 8.5 ab | 37.5 ± 7.1 b |
| Plasmocytes | 1.3 ± 0.6 a | 1.4 ± 0.6 a | 1.8 ± 0.6 a | 1.6 ± 0.8 a |
| Neutrophilic [%] | 20.1 ± 1.5 a | 20.6 ± 1.2 a | 23.7 ± 1.6 b | 26.1 ± 1.8 b |
| Myelocytes + metamyelocytes | 15.4 ± 1.8 a | 17.9 ± 1.5 ab | 18.7 ± 2.5 b | 20.5 ± 3.0 b |
| Band + segmented neutrophils | 4.5 ± 1.4 ab | 2.7 ± 1.4 a | 4.9 ± 1.9 ab | 5.6 ± 2.5 b |
| Monocytoid [%] | 2.3 ± 1.5 a | 1.8 ± 1.1 a | 1.4 ± 0.6 a | 1.2 ± 0.5 a |
| Promonocytes | 1.8 ± 1.1 a | 1.3 ± 0.8 a | 1.0 ± 0.4 a | 0.8 ± 0.4 a |
| Monocytes | 0.5 ± 0.5 a | 0.5 ± 0.5 a | 0.4 ± 0.4 a | 0.4 ± 0.2 a |
| Basophilic [%] | 4.5 ± 1.5 a | 5.3 ± 1.9 a | 4.4 ± 2.7 a | 5.1 ± 2.7 a |
| Proglanulocytes + metagranulocytes | 2.3 ± 1.6 a | 2.6 ± 1.1 a | 2.4 ± 1.7 a | 3.5 ± 2.5 a |
| Basophils | 1.9 ± 0.7 a | 2.7 ± 1.6 a | 2.0 ± 0.6 a | 1.6 ± 0.9 a |
| Eosinophilic [%] | 0.1 ± 0.3 a | 0.2 ± 0.3 a | 0.2 ± 0.2 a | 0.3 ± 0.3 a |
| Thrombocytoid [%] | 6.2 ± 1.7 a | 5.9 ± 2.0 a | 6.0 ± 2.0 a | 6.3 ± 1.9 a |
| Unclassified cells | 1.3 ± 0.6 a | 1.4 ± 0.4 a | 1.5 ± 0.6 a | 1.6 ± 0.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondera, E.; Bojarski, B.; Ługowska, K.; Kot, B.; Witeska, M. Effects of Oxytetracycline and Gentamicin Therapeutic Doses on Hematological, Biochemical and Hematopoietic Parameters in Cyprinus carpio Juveniles. Animals 2020, 10, 2278. https://doi.org/10.3390/ani10122278
Kondera E, Bojarski B, Ługowska K, Kot B, Witeska M. Effects of Oxytetracycline and Gentamicin Therapeutic Doses on Hematological, Biochemical and Hematopoietic Parameters in Cyprinus carpio Juveniles. Animals. 2020; 10(12):2278. https://doi.org/10.3390/ani10122278
Chicago/Turabian StyleKondera, Elżbieta, Bartosz Bojarski, Katarzyna Ługowska, Barbara Kot, and Małgorzata Witeska. 2020. "Effects of Oxytetracycline and Gentamicin Therapeutic Doses on Hematological, Biochemical and Hematopoietic Parameters in Cyprinus carpio Juveniles" Animals 10, no. 12: 2278. https://doi.org/10.3390/ani10122278





