The Current Trends in Using Nanoparticles, Liposomes, and Exosomes for Semen Cryopreservation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Seminal Plasma, Antioxidants, and Their Effect on Sperm Function
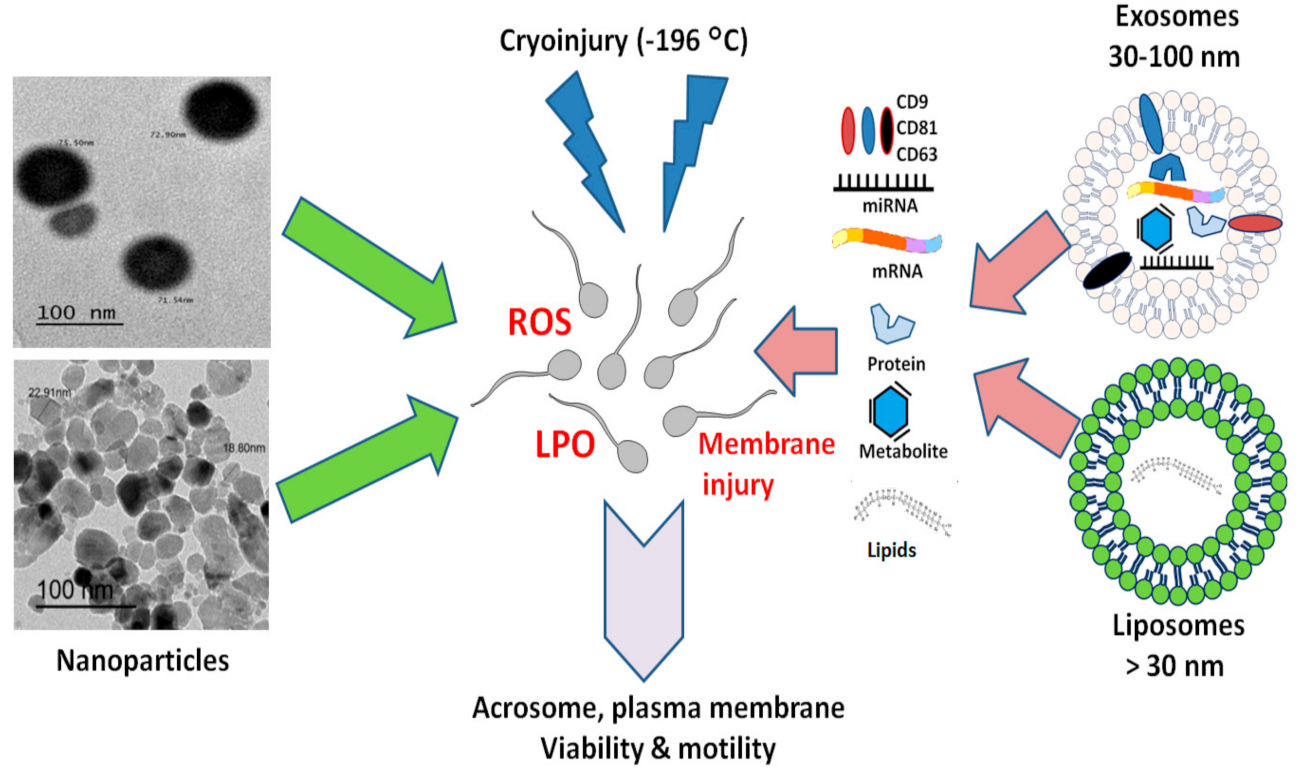
3. Nanoparticles (NPs)
3.1. Definition and Characterization of NPs
3.2. Metal Nanoparticles and Sperm Cryopreservation
3.3. Herbal Extract Nanoparticles and Sperm Cryopreservation
3.4. Vitamins Nanoparticles and Sperm Cryopreservation
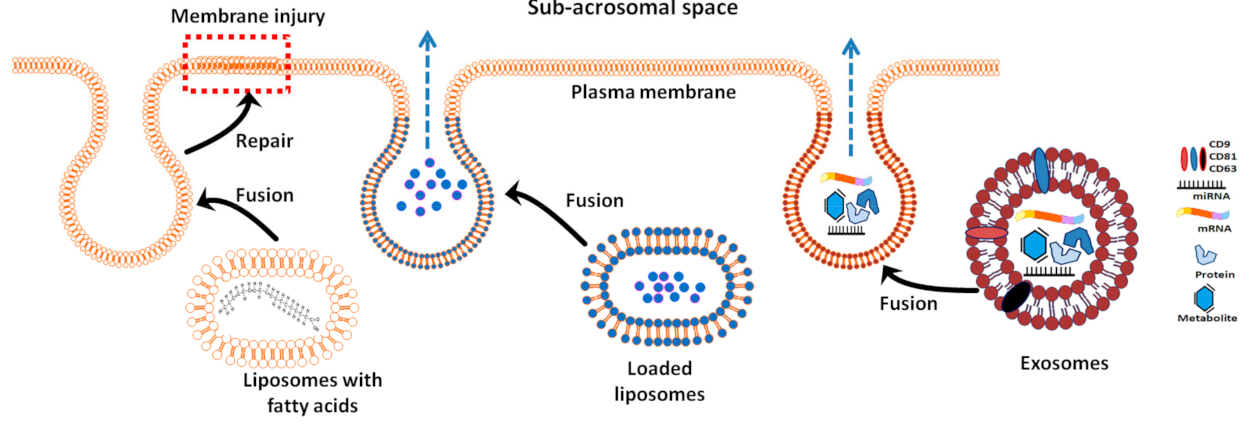
4. Artificial Exosome-Like Vesicles (Liposomes) for Semen Cryopreservation
5. Potential Uses of Exosomes in Semen Cryopreservation
5.1. Effect of Exosomes on Sperm Motility and Viability
5.2. Effect of Exosomes on Sperm Capacitation and Structural Integrity
5.3. Effect of Exosomes on Antioxidant Capacity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ntemka, A.; Tsakmakidis, I.A.; Kiossis, E.; Milovanović, A.; Boscos, C.M. Current status and advances in ram semen cryopreservation. J. Hell. Vet. Med. Soc. 2018, 69, 911. [Google Scholar] [CrossRef] [Green Version]
- Ferdinand, N.; Ngwa, T.D.; Augustave, K.; Dieudonné, B.P.H.; Willington, B.O.; D’Alex, T.C.; Pierre, K.; Joseph, T. Effect of egg yolk concentration in semen extender, pH adjustment of extender and semen cooling methods on bovine semen characteristics. Glob. Vet. 2014, 12, 292–298. [Google Scholar]
- Clulow, J.; Mansfield, L.; Morris, L.; Evans, G.; Maxwell, W. A comparison between freezing methods for the cryopreservation of stallion spermatozoa. Anim. Reprod. Sci. 2008, 108, 298–308. [Google Scholar] [CrossRef]
- Yousif, A.I.A. Studying the Negative Effects of Traditional Sperm Cryopreservation Methods in Ossimi and Finnish Rams Using Advanced Techniques. Ph.D. Thesis, Animal Production Department, Faculty of Agriculture, Masoura University, Mansoura, Egypt, 2018. [Google Scholar]
- Len, J.S.; Koh, W.S.D.; Tan, S.-X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Tatone, C.; Di Emidio, G.; Vento, M.; Ciriminna, R.; Artini, P.G. Cryopreservation and oxidative stress in reproductive cells. Gynecol. Endocrinol. 2010, 26, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Patist, A.; Zoerb, H. Preservation mechanisms of trehalose in food and biosystems. Colloids Surf. B Biointerfaces 2005, 40, 107–113. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, J.; Li, Q.; Jiang, Z.; Zhang, X. The cryoprotective effects of soybean lecithin on boar spermatozoa quality. Afr. J. Biotechnol. 2009, 8, 6476–6480. [Google Scholar]
- Akiyama, M. In vivo scavenging effect of ethylcysteine on reactive oxygen species in human semen. Jpn. J. Urol. 1999, 90, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villegas, J.; Kehr, K.; Soto, L.; Henkel, R.; Miska, W.; Sanchez, R. Reactive oxygen species induce reversible capacitation in human spermatozoa. Andrologia 2003, 35, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Hollan, S. Membrane fluidity of blood cells. Haematologia 1996, 27, 109–127. [Google Scholar]
- Huang, Y.-L.; Tseng, W.-C.; Lin, T.-H. In vitro effects of metal ions (Fe2+, Mn2+, Pb2+) on sperm motility and lipid peroxidation in human semen. J. Toxicol. Environ. Health A 2001, 62, 259–267. [Google Scholar] [CrossRef]
- Davydov, D.R. Microsomal monooxygenase in apoptosis: Another target for cytochrome c signaling? Trends Biochem. Sci. 2001, 26, 155–160. [Google Scholar] [CrossRef]
- Aitken, R.J.; Sawyer, D. The human spermatozoon—Not waving but drowning. In Advances in Male Mediated Developmental Toxicity; Springer: Boston, MA, USA; Berlin/Heidelberg, Germany, 2003; pp. 85–98. [Google Scholar]
- Khalifa, T.; El-Saidy, B. Pellet-freezing of Damascus goat semen in a chemically defined extender. Anim. Reprod. Sci. 2006, 93, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. The Amoroso Lecture The human spermatozoon–a cell in crisis? Reprod. Fertil. Dev. 1999, 115, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Nallella, K.P.; Allamaneni, S.S.; Said, T.M. Role of antioxidants in treatment of male infertility: An overview of the literature. Reprod. Biomed. Online 2004, 8, 616–627. [Google Scholar] [CrossRef]
- Arav, A.; Yavin, S.; Zeron, Y.; Natan, D.; Dekel, I.; Gacitua, H. New trends in gamete’s cryopreservation. Mol. Cell. Endocrinol. 2002, 187, 77–81. [Google Scholar] [CrossRef]
- Medeiros, C.; Forell, F.; Oliveira, A.; Rodrigues, J. Current status of sperm cryopreservation: Why isn’t it better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef]
- Petruska, P.; Capcarova, M.; Sutovsky, P. Antioxidant supplementation and purification of semen for improved artificial insemination in livestock species. Turk. J. Vet. Anim. Sci. 2014, 38, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Sikka, S.C. Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front. Biosci. 1996, 1, e78–e86. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.G.; Storey, B.T. Assessment of cell damage caused by spontaneous lipid peroxidation in rabbit spermatozoa. Biol. Reprod. 1984, 30, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Gil-Guzman, E.; Ollero, M.; Lopez, M.; Sharma, R.; Alvarez, J.; Thomas, A., Jr.; Agarwal, A. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum. Reprod. 2001, 16, 1922–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilodeau, J.F.; Chatterjee, S.; Sirard, M.A.; Gagnon, C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol. Reprod. Dev. 2000, 55, 282–288. [Google Scholar] [CrossRef]
- Ball, B.; Medina, V.; Gravance, C.; Baumber, J. Effect of antioxidants on preservation of motility, viability and acrosomal integrity of equine spermatozoa during storage at 5 C. Theriogenology 2001, 56, 577–589. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Ziaullah, M.; Ijaz, A.; Aleem, M.; Mahmood, A.; Rehman, H.; Bhatti, S.; Farooq, U.; Sohail, M. Optimal inclusion level of butylated hydroxytoluene in semen extender improves the quality of post-thawed canine sperm. Czech J. Anim. Sci. 2012, 57, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.J.; Brar, A.; Ahuja, C.; Sangha, S.; Chaudhary, K. A comparative study on lipid peroxidation, activities of antioxidant enzymes and viability of cattle and buffalo bull spermatozoa during storage at refrigeration temperature. Anim. Reprod. Sci. 2006, 96, 21–29. [Google Scholar] [CrossRef]
- Pagl, R.; Aurich, J.E.; Müller-Schlösser, F.; Kankofer, M.; Aurich, C. Comparison of an extender containing defined milk protein fractions with a skim milk-based extender for storage of equine semen at 5 °C. Theriogenology 2006, 66, 1115–1122. [Google Scholar] [CrossRef]
- Ortega, A.M.; Izquierdo, A.C.; Gómez, J.J.H.; Corichi, I.M.O.; Torres, V.M.M.; Méndez, J.d.J.V. Peroxidación lipídica y antioxidantes en la preservación de semen. Una revisión. Interciencia 2003, 28, 699–704. [Google Scholar]
- Bucak, M.N.; Tuncer, P.B.; Sarıözkan, S.; Başpınar, N.; Taşpınar, M.; Çoyan, K.; Bilgili, A.; Akalın, P.P.; Büyükleblebici, S.; Aydos, S. Effects of antioxidants on post-thawed bovine sperm and oxidative stress parameters: Antioxidants protect DNA integrity against cryodamage. Cryobiology 2010, 61, 248–253. [Google Scholar] [CrossRef]
- Gadea, J.; Sellés, E.; Marco, M.A.; Coy, P.; Matás, C.; Romar, R.; Ruiz, S. Decrease in glutathione content in boar sperm after cryopreservation: Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 2004, 62, 690–701. [Google Scholar] [CrossRef]
- Maxwell, W.; Stojanov, T. Liquid storage of ram semen in the absence or presence of some antioxidants. Reprod. Fertil. Dev. 1996, 8, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Baghshahi, H.; Riasi, A.; Mahdavi, A.; Shirazi, A. Antioxidant effects of clove bud (Syzygium aromaticum) extract used with different extenders on ram spermatozoa during cryopreservation. Cryobiology 2014, 69, 482–487. [Google Scholar] [CrossRef]
- Stradaioli, G.; Noro, T.; Sylla, L.; Monaci, M. Decrease in glutathione (GSH) content in bovine sperm after cryopreservation: Comparison between two extenders. Theriogenology 2007, 67, 1249–1255. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A. State-of-the-Art and Prospective of Nanotechnologies for Smart Reproductive Management of Farm Animals. Animals 2020, 10, 840. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.G.; Storey, B.T. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J. Androl. 1992, 13, 232–241. [Google Scholar] [PubMed]
- Partyka, J.; Sitarz, M.; Leśniak, M.; Gasek, K.; Jeleń, P. The effect of SiO2/Al2O3 ratio on the structure and microstructure of the glazes from SiO2–Al2O3–CaO–MgO–Na2O–K2O system. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 621–630. [Google Scholar] [CrossRef]
- Holland, M.K.; Alvarez, J.G.; Storey, B.T. Production of superoxide and activity of superoxide dismutase in rabbit epididymal spermatozoa. Biol. Reprod. 1982, 27, 1109–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perumal, P.; Vupru, K.; Khate, K. Effect of addition of melatonin on the liquid storage (5 °C) of Mithun (Bos frontalis) Semen. Int. J. Zool. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- El-Sisy, G.; El-Nattat, W.; El-Sheshtawy, R.J. Effect of superoxide dismutase and catalase on viability of cryopreserved buffalo spermatozoa. Glob. Vet. 2008, 2, 61–65. [Google Scholar]
- Roca, J.; Rodríguez, M.J.; Gil, M.A.; Carvajal, G.; Garcia, E.M.; Cuello, C.; Vazquez, J.M.; Martinez, E.A. Survival and in vitro fertility of boar spermatozoa frozen in the presence of superoxide dismutase and/or catalase. J. Androl. 2005, 26, 15–24. [Google Scholar] [PubMed]
- Abdulkareem, T.A.; Alzaidi, O.H. Effect of adding aqueous extract of Melissa officinalis leaves and some other antioxidants to milk–based extender on post-cooling and post-cryopreservative sperm’s individual motility and live sperm percentage of Holstein bulls. Al-Anbar J. Vet. Sci. 2018, 11, 37–53. [Google Scholar] [CrossRef]
- Abdulkareem, T.A.; Khalil, R.I.; Salman, A.H. Effect of adding Ferula hermonis Boiss roots and some antioxidants to Tris extender on post-cryopreserved sperm abnormalities percentage of Holstein bulls. Al-Anbar J. Vet. Sci. 2018, 11, 70–81. [Google Scholar]
- Eidan, S.M.; Abdulkareem, T.A.; Sultan, O.A.A. Influence of adding manganese to Tris extender on some post-cryopreservation semen attributes of Holstein bulls. Int. J. Appl. Agric. Sci. 2015, 1, 26–30. [Google Scholar]
- Ball, B.A.; Vo, A.T.; Baumber, J. Generation of reactive oxygen species by equine spermatozoa. Am. J. Vet. Res. 2001, 62, 508–515. [Google Scholar] [CrossRef]
- Ball, B.A.; Gravance, C.G.; Medina, V.; Baumber, J.; Liu, I.K. Catalase activity in equine semen. Am. J. Vet. Res. 2000, 61, 1026–1030. [Google Scholar] [CrossRef]
- Irvine, D.S. Glutathione as a treatment for male infertility. Rev. Reprod. 1996, 1, 6–12. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef]
- Bilodeau, J.-F.; Blanchette, S.; Gagnon, C.; Sirard, M.-A. Thiols prevent H2O2-mediated loss of sperm motility in cryopreserved bull semen. Theriogenology 2001, 56, 275–286. [Google Scholar] [CrossRef]
- Storey, B.T. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol. Hum. Reprod. 1997, 3, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Foote, R.; Brockett, C. Effect of sucrose, trehalose, hypotaurine, taurine, and blood serum on survival of frozen bull sperm. Cryobiology 1993, 30, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Aurich, J.; Schönherr, U.; Hoppe, H.; Aurich, C. Effects of antioxidants on motility and membrane integrity of chilled-stored stallion semen. Theriogenology 1997, 48, 185–192. [Google Scholar] [CrossRef]
- Ashrafi, I.; Kohram, H.; Ardabili, F.F. Antioxidative effects of melatonin on kinetics, microscopic and oxidative parameters of cryopreserved bull spermatozoa. Anim. Reprod. Sci. 2013, 139, 25–30. [Google Scholar] [CrossRef]
- Watson, P. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60, 481–492. [Google Scholar] [CrossRef]
- Varnet, P.; Aitken, R.; Drevet, J. Antioxidant strategies in the epidydimis. Mol. Cell. Endocrinol. 2004, 216, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bungum, M.; Humaidan, P.; Axmon, A.; Spano, M.; Bungum, L.; Erenpreiss, J.; Giwercman, A. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum. Reprod. 2007, 22, 174–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahin, M.A.; Khalil, W.A.; Saadeldin, I.M.; Swelum, A.A.-A.; El-Harairy, M.A. Comparison between the Effects of Adding Vitamins, Trace Elements, and Nanoparticles to SHOTOR Extender on the Cryopreservation of Dromedary Camel Epididymal Spermatozoa. Animals 2020, 10, 78. [Google Scholar] [CrossRef] [Green Version]
- Broekhuijse, M.; Gaustad, A.; Bolarin Guillén, A.; Knol, E. Efficient Boar Semen Production and Genetic Contribution: The Impact of Low Dose Artificial Insemination on Fertility. Reprod. Domest. Anim. 2015, 50, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.K.; Agarwal, A. Role of reactive oxygen species in male infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef]
- Feugang, J.M. Novel agents for sperm purification, sorting, and imaging. Mol. Reprod. Dev. 2017, 84, 832–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, T.M.; Land, J.A. Effects of advanced selection methods on sperm quality and ART outcome: A systematic review. Hum. Reprod. Update 2011, 17, 719–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozaien, M.; Elqusi, K.; Hassanen, E.; Hussin, A.; Alkhader, H.; El Tanbouly, S.; El-qassaby, S.; Zaki, H. A comparison of reproductive outcome using different sperm selection techniques; density gradient, testicular sperm, PICSI, and MACS for ICSI patients with abnormal DNA fragmentation index. Fertil. Steril. 2018, 110, 19–20. [Google Scholar] [CrossRef]
- Jain, S.; Park, S.B.; Pillai, S.R.; Ryan, P.L.; Willard, S.T.; Feugang, J.M. Applications of fluorescent quantum dots for reproductive medicine and disease detection. In Unraveling the Safety Profile of Nanoscale Particles and Materials—From Biomedical to Environmental Applications; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Huber, D.L.C. Synthesis, properties, and applications of iron nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef]
- Gessner, A.; Lieske, A.; Paulke, B.R.; Müller, R.H.J. Influence of surface charge density on protein adsorption on polymeric nanoparticles: Analysis by two-dimensional electrophoresis. Eur. J. Pharm. Biopharm. 2002, 54, 165–170. [Google Scholar] [CrossRef]
- Thorek, D.L.; Tsourkas, A. Size, charge and concentration dependent uptake of iron oxide particles by non-phagocytic cells. Biomaterials 2008, 29, 3583–3590. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Ping, Q.; Huang, G.; Xu, W.; Cheng, Y.; Han, X. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int. J. Pharm. 2006, 327, 153–159. [Google Scholar] [CrossRef]
- Chen, H.; Seiber, J.N.; Hotze, M. ACS Select on Nanotechnology in Food and Agriculture: A Perspective on Implications and Applications. J. Agric. Food Chem. 2014, 62, 1209–1212. [Google Scholar] [CrossRef]
- Albrecht, M.A.; Evans, C.W.; Raston, C.L. Green chemistry and the health implications of nanoparticles. Green Chem. 2006, 8, 417–432. [Google Scholar] [CrossRef]
- Davda, J.; Labhasetwar, V. Characterization of nanoparticle uptake by endothelial cells. Int. J. Pharm. 2002, 233, 51–59. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nano level. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkalina, N.; Charalambous, C.; Jones, C.; Coward, K. Nanotechnology in reproductive medicine: Emerging applications of nanomaterials. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 921–938. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based drug delivery systems. J. Occupat. Med. Toxicol. 2007, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Niżański, W.; Partyka, A.; Prochowska, S. Evaluation of spermatozoal function—Useful tools or just science. Reprod. Domest. Anim. 2016, 51, 37–45. [Google Scholar] [CrossRef]
- Durfey, C.L.; Burnett, D.D.; Liao, S.F.; Steadman, C.S.; Crenshaw, M.A.; Clemente, H.J.; Willard, S.T.; Ryan, P.L.; Feugang, J.M. Nanotechnology-based selection of boar spermatozoa: Growth development and health assessments of produced offspring. Livest. Sci. 2017, 205, 137–142. [Google Scholar] [CrossRef]
- Feugang, J.M.; Youngblood, R.C.; Greene, J.M.; Willard, S.T.; Ryan, P.L. Self-illuminating quantum dots for non-invasive bioluminescence imaging of mammalian gametes. J. Nanobiotechnol. 2015, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Odhiambo, J.F.; DeJarnette, J.; Geary, T.W.; Kennedy, C.E.; Suarez, S.S.; Sutovsky, M.; Sutovsky, P. Increased conception rates in beef cattle inseminated with nanopurified bull semen. Biol. Reprod. 2014, 97, 1–10. [Google Scholar]
- Ozsoy, N.; Can, A.; Mutlu, O.; Akev, N.; Yanardag, R. Oral zinc supplementation protects rat kidney tissue from oxidative stress in diabetic rats. Kafkas Univ. Vet. Fak. Derg. 2012, 18, 545–550. [Google Scholar]
- Heidari, J.; Seifdavati, J.; Mohebodini, H.; Sharifi, R.S.; Benemar, H.A. Effect of nano zinc oxide on post-thaw variables and oxidative status of Moghani ram semen. Kafkas Univ. Vet. Fak. Derg. 2018, 25, 71–76. [Google Scholar]
- Khalil, W.A.; El-Harairy, M.A.; Zeidan, A.E.; Hassan, M.A. Impact of selenium nano-particles in semen extender on bull sperm quality after cryopreservation. Theriogenology 2019, 126, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hozyen, H.; El-Shamy, A.; Farghali, A. In vitro supplementation of nano selenium minimizes freeze-thaw induced damage to ram spermatozoa. Int. J. Vet. Sci. 2019, 8, 249–254. [Google Scholar]
- Nateq, S.; Moghaddam, G.; Alijani, S.; Behnam, M. The effects of different levels of Nano selenium on the quality of frozen-thawed sperm in ram. J. Appl. Anim. Res. 2020, 48, 434–439. [Google Scholar] [CrossRef]
- Ismail, A.A.; Abdel-Khalek, A.; Khalil, W.; El-Harairy, M. Influence of adding green synthesized gold nanoparticles to tris-extender on sperm characteristics of cryopreserved goat semen. J. Anim. Poult. Prod. Mansoura Univ. 2020, 11, 39–45. [Google Scholar] [CrossRef]
- Tripathi, R.; Shrivastav, A.; Shrivastav, B. Biogenic gold nanoparticles: As a potential candidate for brain tumor directed drug delivery. Artif. Cells Nanomed. Biotechnol. 2015, 43, 311–317. [Google Scholar] [CrossRef]
- Moretti, E.; Terzuoli, G.; Renieri, T.; Iacoponi, F.; Castellini, C.; Giordano, C.; Collodel, G. In vitro effect of gold and silver nanoparticles on human spermatozoa. Andrologia 2013, 45, 392–396. [Google Scholar] [CrossRef]
- Nadri, T.; Towhidi, A.; Zeinoaldini, S.; Martínez-Pastor, F.; Mousavi, M.; Noei, R.; Tar, M.; Mohammadi Sangcheshmeh, A. Lecithin nanoparticles enhance the cryosurvival of caprine sperm. Theriogenology 2019, 133, 38–44. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Towhidi, A.; Zhandi, M.; Amoabediny, G.; Mohammadi-Sangcheshmeh, A.; Sharafi, M.; Hussaini, S.M.H. Comparison of two different antioxidants in a nano lecithin-based extender for bull sperm cryopreservation. Anim. Reprod. Sci. 2019, 209, 106171. [Google Scholar] [CrossRef]
- Jahanbin, R.; Yazdanshenas, P.; Amin, A.M.; Mohammadi, S.A.; Varnaseri, H.; Chamani, M.; Nazaran, M.H.; Bakhtiyarizadeh, M.R. Effect of zinc nano-complex on bull semen quality after freeze-thawing process. J. Anim. Prod. 2016, 17, 371–380. [Google Scholar]
- Ismail, A.A.; Abdel-Khalek, A.-K.E.; Khalil, W.A.; Yousif, A.I.; Saadeldin, I.M.; Abomughaid, M.M.; El-Harairy, M.A. Effects of mint, thyme, and curcumin extract nanoformulations on the sperm quality, apoptosis, chromatin decondensation, enzyme activity, and oxidative status of cryopreserved goat semen. Cryobiology 2020. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Hassan, M.A.; Mohammed, A.K.; Alhimaidi, A.R.; Al-Gabri, N.; Al-Khaldi, K.O.; Swelum, A.A. The Effect of Adding Different Levels of Curcumin and Its Nanoparticles to Extender on Post-Thaw Quality of Cryopreserved Rabbit Sperm. Animals 2020, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Shokry, D.M.; Badr, M.R.; Orabi, S.H.; Khalifa, H.K.; El-Seedi, H.R.; Abd Eldaim, M.A. Moringa oleifera leaves extract enhances fresh and cryopreserved semen characters of Barki rams. Theriogenology 2020, 153, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Elsheshtawy, R.; Elnattat, W.S. Assessment of buffalo semen preservability using tris extender enriched with Moringa oleifera extract. Egypt. J. Vet. Sci. 2020, 51, 235–239. [Google Scholar] [CrossRef]
- El-Harairy, M.; Abdel-Khalek, A.; Khalil, W.; Khalifa, E.; El-Khateeb, A.; Abdulrhmn, A. Effect of aqueous extracts of Moringa oleifera leaves or Arctium lappa roots on lipid peroxidation and membrane integrity of ram sperm preserved at cool temperature. J. Anim. Poult. Prod. Mansoura Univ. 2016, 7, 467–473. [Google Scholar] [CrossRef]
- Tvrdá, E.; Greifová, H.; Mackovich, A.; Hashim, F.; Lukáč, N. Curcumin offers antioxidant protection to cryopreserved bovine semen. Czech J. Anim. Sci. 2018, 63, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Tvrdá, E.; Halenár, M.; Greifová, H.; Mackovich, A.; Hashim, F.; Lukáč, N. The effect of curcumin on cryopreserved bovine semen. Int. J. Anim. Vet. Sci. 2016, 10, 707–711. [Google Scholar]
- Abadjieva, D.; Yotov, S.; Mladenova, V.; Lauberte, L.; Kalvanov, I.; Krasilnikova, J.; Telesheva, G.; Kistanova, E. Positive effect of natural antioxidant oregonin from Alnus incana bark on ram semen quality stored at 5 °C for 48 h. Res. Vet. Sci. 2020, 131, 153–158. [Google Scholar] [CrossRef]
- Sobeh, M.; Hassan, S.; El Raey, M.; Khalil, W.; Hassan, M.; Wink, M. Polyphenolics from Albizia harveyi exhibit antioxidant activities and counteract oxidative damage and ultra-structural changes of cryopreserved bull semen. Molecules 2017, 22, 1993. [Google Scholar] [CrossRef] [Green Version]
- Merati, Z.; Farshad, A. Ginger and echinacea extracts improve the quality and fertility potential of frozen-thawed ram epididymal spermatozoa. Cryobiology 2020, 92, 138–145. [Google Scholar] [CrossRef]
- Sanchez-Rubio, F.; Soria-Meneses, P.J.; Jurado-Campos, A.; Bartolome-Garcia, J.; Gomez-Rubio, V.; Soler, A.J.; Arroyo-Jimenez, M.M.; Santander-Ortega, M.J.; Plaza-Oliver, M.; Lozano, M.V.; et al. Nanotechnology in reproduction: Vitamin E nanoemulsions for reducing oxidative stress in sperm cells. Free Radic. Biol. Med. 2020, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Stremersch, S.; Vandenbroucke, R.E.; Van Wonterghem, E.; Hendrix, A.; De Smedt, S.C.; Raemdonck, K. Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J. Control. Release 2016, 232, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortazavi, S.-H.; Eslami, M.; Farrokhi-Ardabili, F. Comparison of different carrier-compounds and varying concentrations of oleic acid on freezing tolerance of ram spermatozoa in tris-citric acid-egg yolk plasma semen diluent. Anim. Reprod. Sci. 2020, 219, 106533. [Google Scholar] [CrossRef]
- Najafi, A.; Taheri, R.A.; Mehdipour, M.; Farnoosh, G.; Martínez-Pastor, F. Lycopene-loaded nanoliposomes improve the performance of a modified Beltsville extender broiler breeder roosters. Anim. Reprod. Sci. 2018, 195, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Kia, H.D.; Mehdipour, M.; Hamishehkar, H.; Alvarez-Rodriguez, M. Effect of quercetin loaded liposomes or nanostructured lipid carrier (NLC) on post-thawed sperm quality and fertility of rooster sperm. Theriogenology 2020, 152, 122–128. [Google Scholar] [CrossRef]
- Mehdipour, M.; Daghigh Kia, H.; Nazari, M.; Najafi, A. Effect of lecithin nanoliposome or soybean lecithin supplemented by pomegranate extract on post-thaw flow cytometric, microscopic and oxidative parameters in ram semen. Cryobiology 2017, 78, 34–40. [Google Scholar] [CrossRef]
- Medina-León, A.Z.; Domínguez-Mancera, B.; Cazalez-Penino, N.; Cervantes-Acosta, P.; Jácome-Sosa, E.; Romero-Salas, D.; Barrientos-Morales, M. Cryopreservation of horse semen with a liposome and trehalose added extender. Aust. J. Vet. Sci. 2019, 51, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Pillet, E.; Labbe, C.; Batellier, F.; Duchamp, G.; Beaumal, V.; Anton, M.; Desherces, S.; Schmitt, E.; Magistrini, M. Liposomes as an alternative to egg yolk in stallion freezing extender. Theriogenology 2012, 77, 268–279. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, M.; Kumar, D.; Balhara, A.K.; Yadav, S.P.; Singh, P.; Yadav, P.S. Liposome-based semen extender is suitable alternative to egg yolk-based extender for cryopreservation of buffalo (Bubalus bubalis) semen. Anim. Reprod. Sci. 2015, 159, 38–45. [Google Scholar] [CrossRef]
- Mafolo, K.S.; Pilane, C.M.; Chitura, T.; Nedambale, T.L. Use of phosphatidylcholine in Tris-based extender with or without egg yolk to freeze Bapedi ram semen. S. Afr. J. Anim. Sci. 2020, 50, 389–396. [Google Scholar] [CrossRef]
- Luna-Orozco, J.R.; Gonzalez-Ramos, M.A.; Calderon-Leyva, G.; Gaytan-Aleman, L.R.; Arellano-Rodriguez, F.; Angel-Garcia, O.; Veliz-Deras, F.G. Comparison of different diluents based on liposomes and egg yolk for ram semen cooling and cryopreservation. Iran. J. Vet. Res. 2019, 20, 126–130. [Google Scholar] [PubMed]
- He, L.; Bailey, J.L.; Buhr, M.M. Incorporating Lipids into Boar Sperm Decreases Chilling Sensitivity but Not Capacitation Potential1. Biol. Reprod. 2001, 64, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röpke, T.; Oldenhof, H.; Leiding, C.; Sieme, H.; Bollwein, H.; Wolkers, W.F. Liposomes for cryopreservation of bovine sperm. Theriogenology 2011, 76, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Saez, F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction 2013, 146, R21–R35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köse, G.T.; Arica, M.Y.; Hasirci, V. Low-Molecular-Weight Heparin-Conjugated Liposomes with Improved Stability and Hemocompatibility. Drug Deliv. 1998, 5, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Purdy, P.H.; Graham, J.K. Membrane Modification Strategies for Cryopreservation. Cryopreserv. Freeze. Dry. Protoc. 2015, 1257, 337–342. [Google Scholar] [CrossRef]
- Quinn, P.J.; Chow, P.Y.W.; White, I.G. Evidence that phospholipid protects ram spermatozoa from cold shock at a plasma membrane site. Reproduction 1980, 60, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.A.; Rakha, B.A.; Akhter, S. Cryopreservation of bull semen in OptiXcell® and conventional extenders: Comparison of semen quality and fertility. Anim. Sci. Pap. Rep. 2017, 35, 317–328. [Google Scholar]
- Ansari, M.S.; Rakha, B.A.; Akhter, S.; Ashiq, M. OPTIXcell improves the postthaw quality and fertility of buffalo bull sperm. Theriogenology 2016, 85, 528–532. [Google Scholar] [CrossRef]
- Abdel-Aziz Swelum, A.; Saadeldin, I.M.; Ba-Awadh, H.; Al-Mutary, G.M.; Moumen, A.F.; Alowaimer, A.N.; Abdalla, H. Efficiency of Commercial Egg Yolk-Free and Egg Yolk-Supplemented Tris-Based Extenders for Dromedary Camel Semen Cryopreservation. Animals 2019, 9, 999. [Google Scholar] [CrossRef] [Green Version]
- Al-Bulushi, S.; Manjunatha, B.M.; Bathgate, R.; Rickard, J.P.; de Graaf, S.P. Liquid storage of dromedary camel semen in different extenders. Anim. Reprod. Sci. 2019, 207, 95–106. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- D’Souza-Schorey, C.; Clancy, J.W. Tumor-derived microvesicles: Shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012, 26, 1287–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machtinger, R.; Laurent, L.C.; Baccarelli, A.A. Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 2015, 22, 182–193. [Google Scholar] [CrossRef]
- Gould, S.J.; Raposo, G. As we wait: Coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20389. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.M.; El-Kares, R.; Taranta, A.; Bellomo, F.; Emma, F.; Besouw, M.; Levtchenko, E.; Toelen, J.; van den Heuvel, L.; Chu, L.; et al. Stem Cell Microvesicles Transfer Cystinosin to Human Cystinotic Cells and Reduce Cystine Accumulation In Vitro. PLoS ONE 2012, 7, e42840. [Google Scholar] [CrossRef]
- Charrier, A.; Chen, R.; Chen, L.; Kemper, S.; Hattori, T.; Takigawa, M.; Brigstock, D.R. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery 2014, 156, 548–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shtam, T.A.; Kovalev, R.A.; Varfolomeeva, E.; Makarov, E.M.; Kil, Y.V.; Filatov, M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013, 11, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, S.G.; Lee, W.H.; Huang, M.; Dey, D.; Kodo, K.; Sanchez-Freire, V.; Gold, J.D.; Wu, J.C. Cross Talk of Combined Gene and Cell Therapy in Ischemic Heart Disease: Role of Exosomal MicroRNA Transfer. Circulation 2014, 130, S60–S69. [Google Scholar] [CrossRef] [Green Version]
- Tomasoni, S.; Longaretti, L.; Rota, C.; Morigi, M.; Conti, S.; Gotti, E.; Capelli, C.; Introna, M.; Remuzzi, G.; Benigni, A. Transfer of Growth Factor Receptor mRNA Via Exosomes Unravels the Regenerative Effect of Mesenchymal Stem Cells. Stem Cell Dev. 2013, 22, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saadeldin, I.M.; Oh, H.J.; Lee, B.C. Embryonic-maternal cross-talk via exosomes: Potential implications. Stem Cell Cloning Adv. Appl. 2015, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Saadeldin, I.M.; Kim, S.J.; Choi, Y.B.; Lee, B.C. Improvement of Cloned Embryos Development by Co-Culturing with Parthenotes: A Possible Role of Exosomes/Microvesicles for Embryos Paracrine Communication. Cell. Reprogram. 2014, 16, 223–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qamar, A.Y.; Fang, X.; Kim, M.J.; Cho, J. Improved post-thaw quality of canine semen after treatment with exosomes from conditioned medium of adipose-derived mesenchymal stem cells. Animals 2019, 9, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Shen, J.; Wang, Y.; Pan, C.; Pang, W.; Diao, H.; Dong, W. Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane. Oncotarget 2016, 7, 58832–58847. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H.; Yang, J.-M.; Park, S.-B.; Kwak, S.-J.; Park, J.-S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, R.; Murtaza, G. Comparative bioavailability and pharmacokinetics of flurbiprofen 200 mg SR pellets from India and France. Adv. Clin. Exp. Med. 2011, 20, 599–604. [Google Scholar]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.-P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of the first phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Shedden, K.; Xie, X.T.; Chandaroy, P.; Chang, Y.T.; Rosania, G.R. Expulsion of small molecules in vesicles shed by cancer cells: Association with gene expression and chemosensitivity profiles. Cancer Res. 2003, 63, 4331–4337. [Google Scholar]
- Mokarizadeh, A.; Rezvanfar, M.-A.; Dorostkar, K.; Abdollahi, M. Mesenchymal stem cell derived microvesicles: Trophic shuttles for enhancement of sperm quality parameters. Reprod. Toxicol. 2013, 42, 78–84. [Google Scholar] [CrossRef]
- Mahiddine, F.Y.; Kim, J.W.; Qamar, A.Y.; Ra, J.C.; Kim, S.H.; Jung, E.J.; Kim, M.J. Conditioned Medium from Canine Amniotic Membrane-Derived Mesenchymal Stem Cells Improved Dog Sperm Post-Thaw Quality-Related Parameters. Animals 2020, 10, 1899. [Google Scholar] [CrossRef]
- Mahiddine, F.Y.; Qamar, A.Y.; Kim, M.J. Canine amniotic membrane derived mesenchymal stem cells exosomes addition in canine sperm freezing medium. J. Anim. Reprod. Biotechnol. 2020, 35, 268–272. [Google Scholar] [CrossRef]
- Simon, L.; Murphy, K.; Shamsi, M.; Liu, L.; Emery, B.; Aston, K.; Hotaling, J.; Carrell, D. Paternal influence of sperm DNA integrity on early embryonic development. Hum. Reprod. 2014, 29, 2402–2412. [Google Scholar] [CrossRef] [PubMed]
- Franchi, A.; Moreno-Irusta, A.; Domínguez, E.M.; Adre, A.J.; Giojalas, L.C. Extracellular vesicles from oviductal isthmus and ampulla stimulate the induced acrosome reaction and signaling events associated with capacitation in bovine spermatozoa. J. Cell. Biochem. 2019, 121, 2877–2888. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Monteiro Melo Ferraz, M.; Nagashima, J.B.; Noonan, M.J.; Crosier, A.E.; Songsasen, N. Oviductal Extracellular Vesicles Improve Post-Thaw Sperm Function in Red Wolves and Cheetahs. Int. J. Mol. Sci. 2020, 21, 3733. [Google Scholar] [CrossRef] [PubMed]
- Naresh, S.; Atreja, S.K. The protein tyrosine phosphorylation during in vitro capacitation and cryopreservation of mammalian spermatozoa. Cryobiology 2015, 70, 211–216. [Google Scholar] [CrossRef]
- Alcântara-Neto, A.S.; Schmaltz, L.; Caldas, E.; Blache, M.-C.; Mermillod, P.; Almiñana, C. Porcine oviductal extracellular vesicles interact with gametes and regulate sperm motility and survival. Theriogenology 2020, 155, 240–255. [Google Scholar] [CrossRef]
| Animal Species | Nanoparticle (NPs) | The Effects | Reference |
|---|---|---|---|
| Goat bucks | Nano-lecithin | Improved motility, viability, and hypo-osmotic swelling test and lower apoptosis. | [91] |
| Bulls | Nano-lecithin-based extender with glutathione peroxidase | Enhanced plasma membrane integrity and reduced malondialdehyde (MDA) concentration. | [92] |
| Bulls | Selenium NPs | Improved kinematic sperm quality, antioxidative defense, and decreased apoptotic and necrotic cells. | [85] |
| Zinc NPs | Improved plasma membrane integrity and mitochondrial functions. | [93] | |
| Camel | Selenium NPs Zinc NPs | Improved sperm functions (progressive motility, vitality, sperm membrane integrity). Maintained ultrastructural morphology and decreased apoptosis. Increased antioxidative defense. | [60] |
| Goat | Mint, thyme, and curcumin nanoformulations (NFs) | Improved progressive motility, vitality, and plasma membrane integrity; antioxidative defense; chromatin decondensation. Decreased apoptosis. | [94] |
| Goat | Green synthesized gold nanoparticles (GSGNPs) | Improved motility, survivability, membrane integrity, acrosome integrity, and antioxidative defense. | [88] |
| Rabbit | Curcumin NPs | Enhanced sperm motility and antioxidative defense. Reduced apoptotic and necrotic spermatozoa. | [95] |
| Species | Sources of EVs/Exosomes | Condition of Storage | The Improved Parameters | References |
|---|---|---|---|---|
| Pig | Seminal plasma | 17 °C for 10 days | Viability, motility, plasma membrane integrity, antioxidant capacity, and MDA reduction | [138] |
| Pig | Oviduct-derived | Freezing | Survival and motility | [150] |
| Rat | Bone marrow-derived mesenchymal stem cells | Freezing | Viability, motility, total antioxidant capacity, and increased surface adhesion molecules | [143] |
| Dog | Amniotic-derived mesenchymal stem cells and conditioned medium | Cooling and freezing | Viability, motility | [144,145] |
| Dog | Adipose-derived mesenchymal stem cells | Freezing | Viability, motility | [137] |
| Red wolves and cheetahs | Oviduct-derived | Freezing | Motility and acrosome integrity | [148] |
| Bovine | Oviduct-derived | Freezing | Viability | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadeldin, I.M.; Khalil, W.A.; Alharbi, M.G.; Lee, S.H. The Current Trends in Using Nanoparticles, Liposomes, and Exosomes for Semen Cryopreservation. Animals 2020, 10, 2281. https://doi.org/10.3390/ani10122281
Saadeldin IM, Khalil WA, Alharbi MG, Lee SH. The Current Trends in Using Nanoparticles, Liposomes, and Exosomes for Semen Cryopreservation. Animals. 2020; 10(12):2281. https://doi.org/10.3390/ani10122281
Chicago/Turabian StyleSaadeldin, Islam M., Wael A. Khalil, Mona G. Alharbi, and Seok Hee Lee. 2020. "The Current Trends in Using Nanoparticles, Liposomes, and Exosomes for Semen Cryopreservation" Animals 10, no. 12: 2281. https://doi.org/10.3390/ani10122281






