Cellular and Molecular Events that Occur in the Oocyte during Prolonged Ovarian Storage in Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Oocyte Collection and In Vitro Maturation
2.2. In Vitro Fertilization (IVF)
2.3. In Vitro Culture (IVC)
2.4. Early Apoptosis Assay
2.5. Measurement of Glutathione (GSH) and Reactive Oxygen Species (ROS)
2.6. DNA Fragmentation Assay
2.7. Measurement of Caspase-3 Activity
2.8. Mitochondrial Membrane Potential Analysis
2.9. Assessment of Mitochondrial Distribution
2.10. Quantification of Transcript Abundance
2.11. In Vitro Maturation and Fertilization Assessment
2.12. Flow Cytometry Analysis of Cumulus Cells
2.13. Statistical Analysis
3. Results
3.1. Effect of Ovarian Transport Time on Oocyte Viability and Quality
3.2. Effect of Ovarian Transport Time on the In Vitro Maturation and Fertilization Potential of Oocytes
3.3. Effect of Ovarian Transport Time on Cumulus Cells from Cumulus–Oocyte Complexes (COCs)
3.4. Effect of Ovarian Transport Time on In Vitro Embryo Development and Blastocyst Quality
3.5. Effect of Medium Type During Ovary Transport on Oocyte Developmental Competence and Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Amiridis, G.S.; Cseh, S. Assisted reproductive technologies in the reproductive management of small ruminants. Anim. Reprod. Sci. 2012, 130, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Garde, J.J.; Martínez-Pastor, F.; Gomendio, M.; Malo, A.F.; Soler, A.J.; Fernández-Santos, M.R.; Esteso, M.C.; García, A.J.; Anel, L.; Roldán, E.R.S. The application of reproductive technologies to natural populations of red deer. Reprod. Domest. Anim. 2006, 41, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Paramio, M.-T.; Izquierdo, D. Recent advances in in vitro embryo production in small ruminants. Theriogenology 2016, 86, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Sirard, M.A.; Desrosier, S.; Assidi, M. In vivo and in vitro effects of FSH on oocyte maturation and developmental competence. Theriogenology 2007, 68, S71–S76. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.Y. Evaluation of oocyte quality: Morphological, cellular and molecular predictors. Reprod. Fertil. Dev. 2007, 19, 1–12. [Google Scholar] [CrossRef]
- Wongsrikeao, P.; Otoi, T.; Karja, N.W.K.; Agung, B.; Nii, M.; Nagai, T. Effects of ovary storage time and temperature on DNA fragmentation and development of porcine oocytes. J. Reprod. Dev. 2005, 51, 87–97. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Grazul-Bilska, A.T.; Redmer, D.A. Angiogenesis in the female reproductive organs: Pathological implications. Int. J. Exp. Pathol. 2002, 83, 151–164. [Google Scholar] [CrossRef]
- King, T.C. Cell Injury, Cellular Responses to Injury, and Cell Death. In Elsevier’s Integrated Pathology; Mosby Elsevier: Maryland Heights, MO, USA, 2007; pp. 1–20. ISBN 978-0-323-04328-1. [Google Scholar]
- Tellado, M.N.; Alvarez, G.M.; Dalvit, G.C.; Cetica, P.D. The Conditions of Ovary Storage Affect the Quality of Porcine Oocytes. Adv. Reprod. Sci. 2014, 2, 56–67. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Ajofrín, I.; Iniesta-Cuerda, M.; Peris-Frau, P.; Martín-Maestro, A.; Medina-Chávez, D.; Maside, C.; Fernández-Santos, M.; Ortiz, J.; Montoro, V.; Garde, J.; et al. Beneficial Effects of Melatonin in the Ovarian Transport Medium on In Vitro Embryo Production of Iberian Red Deer (Cervus Elaphus hispanicus). Animals 2020, 10, 763. [Google Scholar] [CrossRef]
- García-Álvarez, O.; Maroto-Morales, A.; Berlinguer, F.; Fernández-Santos, M.R.; Esteso, M.C.; Mermillod, P.; Ortiz, J.A.; Ramon, M.; Pérez-Guzmán, M.D.; Garde, J.J.; et al. Effect of storage temperature during transport of ovaries on in vitro embryo production in Iberian red deer (Cervus elaphus hispanicus). Theriogenology 2011, 75, 65–72. [Google Scholar] [CrossRef]
- Takahashi, Y.; First, N.L. In vitro development of bovine ane-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogelology 1992, 37, 963–978. [Google Scholar] [CrossRef]
- Sánchez-Ajofrín, I.; Iniesta-Cuerda, M.; Sánchez-Calabuig, M.J.; Peris-Frau, P.; Martín-Maestro, A.; Ortiz, J.A.; del Rocío Fernández-Santos, M.; Garde, J.J.; Gutiérrez-Adán, A.; Soler, A.J. Oxygen tension during in vitro oocyte maturation and fertilization affects embryo quality in sheep and deer. Anim. Reprod. Sci. 2020, 213, 106279. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Álvarez, P.; Rizos, D.; Rath, D.; Lonergan, P.; Gutiérrez-Adán, A. Can Bovine In Vitro-Matured Oocytes Selectively Process X- or Y-Sorted Sperm Differentially? Biol. Reprod. 2008, 79, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Halici, Z.; Karaca, M.; Keles, O.N.; Borekci, B.; Odabasoglu, F.; Suleyman, H.; Cadirci, E.; Bayir, Y.; Unal, B. Protective effects of amlodipine on ischemia-reperfusion injury of rat ovary: Biochemical and histopathologic evaluation. Fertil. Steril. 2008, 90, 2408–2415. [Google Scholar] [CrossRef]
- Amorim, C.A. Artificial ovary. In Principles and Practice of Fertility Preservation; Cambridge University Press: Cambridge, UK, 2011; pp. 448–458. ISBN 9784431559634. [Google Scholar]
- Taylor, M.J. Biology of Cell Survival in the Cold: The Basis for Biopreservation of Tissues and Organs. In Advances in Biopreservation; CRC Press: New York, NY, USA, 2006; pp. 15–63. ISBN 1420004220. [Google Scholar]
- Pedersen, H.G.; Watson, E.D.; Telfer, E.E. Effect of ovary holding temperature and time on equine granulosa cell apoptosis, oocyte chromatin configuration and cumulus morphology. Theriogenology 2004, 62, 468–480. [Google Scholar] [CrossRef]
- Wolfe, B.A.; Wildt, D.E. Development to blastocysts of domestic cat oocytes matured and fertilized in vitro after prolonged cold storage. J. Reprod. Fertil. 1996, 106, 135–141. [Google Scholar] [CrossRef]
- Otera, H.; Mihara, K. Mitochondrial dynamics: Functional link with apoptosis. Int. J. Cell Biol. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Gottlieb, E.; Armour, S.M.; Harris, M.H.; Thompson, C.B. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003, 10, 709–717. [Google Scholar] [CrossRef]
- Susin, S.A.; Zamzami, N.; Kroemer, G. Mitochondria as regulators of apoptosis: Doubt no more. Biochim. Biophys. Acta Bioenerg. 1998, 1366, 151–165. [Google Scholar] [CrossRef]
- Yang, M.; Antoine, D.; Weemhoff, J.; Jenkins, R.; Farhood, A.; Park, B.; Jaeschke, H. Biomarkers Distinguish Apoptotic and Necrotic Cell Death During Hepatic ischemia/reperfusion Injury in Mice. Liver Transpl. 2014, 20, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Garrity, M.M.; Burgart, L.J.; Riehle, D.L.; Hill, E.M.; Sebo, T.J.; Witzig, T. Identifying and quantifying apoptosis: Navigating technical pitfalls. Mod. Pathol. 2003, 16, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Lemasters, J.J. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 2003, 125, 1246–1257. [Google Scholar] [CrossRef]
- Zhou, T.; Chuang, C.C.; Zuo, L. Molecular Characterization of Reactive Oxygen Species in Myocardial Ischemia-Reperfusion Injury. Biomed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Clanton, T.L. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J. Appl. Physiol. 2007, 102, 2379–2388. [Google Scholar] [CrossRef]
- Paradis, S.; Charles, A.L.; Meyer, A.; Lejay, A.; Scholey, J.W.; Chakfé, N.; Zoll, J.; Geny, B. Chronology of mitochondrial and cellular events during skeletal muscle ischemia-reperfusion. Am. J. Physiol. Cell Physiol. 2016, 310, C968–C982. [Google Scholar] [CrossRef]
- Zhou, T.; Prather, E.R.; Garrison, D.E.; Zuo, L. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef]
- Reader, K.L.; Stanton, J.A.L.; Juengel, J.L. The role of oocyte organelles in determining developmental competence. Biology 2017, 6, 35. [Google Scholar] [CrossRef]
- Castaneda, C.A.; Kaye, P.; Pantaleon, M.; Phillips, N.; Norman, S.; Fry, R.; D’Occhio, M.J. Lipid content, active mitochondria and brilliant cresyl blue staining in bovine oocytes. Theriogenology 2013, 79, 417–422. [Google Scholar] [CrossRef]
- Torner, H.; Ghanem, N.; Ambros, C.; Hölker, M.; Tomek, W.; Phatsara, C.; Alm, H.; Sirard, M.A.; Kanitz, W.; Schellander, K.; et al. Molecular and subcellular characterisation of oocytes screened for their developmental competence based on glucose-6-phosphate dehydrogenase activity. Reproduction 2008, 135, 197–212. [Google Scholar] [CrossRef]
- Gaulden, M.E. Maternal age effect: The enigma of Down syndrome and other trisomic conditions. Mutat. Res. Genet. Toxicol. 1992, 296, 69–88. [Google Scholar] [CrossRef]
- Van Blerkom, J.; Antczak, M.; Schrader, R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: Association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum. Reprod. 1997, 12, 1047–1055. [Google Scholar] [CrossRef]
- Khosravi-Farsani, S.; Sobhani, A.; Amidi, F.; Mahmoudi, R. Mouse oocyte vitrification: The effects of two methods on maturing germinal vesicle breakdown oocytes. J. Assist. Reprod. Genet. 2010, 27, 233–238. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Ritter, L.J.; Armstrong, D.T. Oocyte-somatic cell interactions during follicle development in mammals. Anim. Reprod. Sci. 2004, 82–83, 431–446. [Google Scholar] [CrossRef]
- Febretrisiana, A.; Setiadi, M.A.; Karja, N.W.K. Nuclear maturation rate of sheep oocytes in vitro: Effect of storage duration and ovary temperature. J. Indones. Trop. Anim. Agric. 2015, 40, 93–99. [Google Scholar] [CrossRef]
- Hinrichs, K.; Choi, Y.H.; Love, L.B.; Varner, D.D.; Love, C.C.; Walckenaer, B.E. Chromatin Configuration Within the Germinal Vesicle of Horse Oocytes: Changes Post Mortem and Relationship to Meiotic and Developmental Competence1. Biol. Reprod. 2005, 72, 1142–1150. [Google Scholar] [CrossRef]
- Blondin, P.; Coenen, K.; Guilbault, L.; Sirard, M.-A. In vitro production of bovine embryos: Developmental competence is acquired before maturation. Theriogenology 1997, 47, 1061–1075. [Google Scholar] [CrossRef]
- Ribeiro, B.I.; Love, L.B.; Choi, Y.H.; Hinrichs, K. Transport of equine ovaries for assisted reproduction. Anim. Reprod. Sci. 2008, 108, 171–179. [Google Scholar] [CrossRef]
- Luu, V.V.; Hanatate, K.; Tanihara, F.; Sato, Y.; Do, L.T.K.; Taniguchi, M.; Otoi, T. The effect of relaxin supplementation of in vitro maturation medium on the development of cat oocytes obtained from ovaries stored at 4 °C. Reprod. Biol. 2013, 13, 122–126. [Google Scholar] [CrossRef]
- Goodarzi, A.; Zare Shahneh, A.; Kohram, H.; Sadeghi, M.; Moazenizadeh, M.H.; Fouladi-Nashta, A.; Dadashpour Davachi, N. Effect of melatonin supplementation in the long-term preservation of the sheep ovaries at different temperatures and subsequent in vitro embryo production. Theriogenology 2018, 106, 265–270. [Google Scholar] [CrossRef]
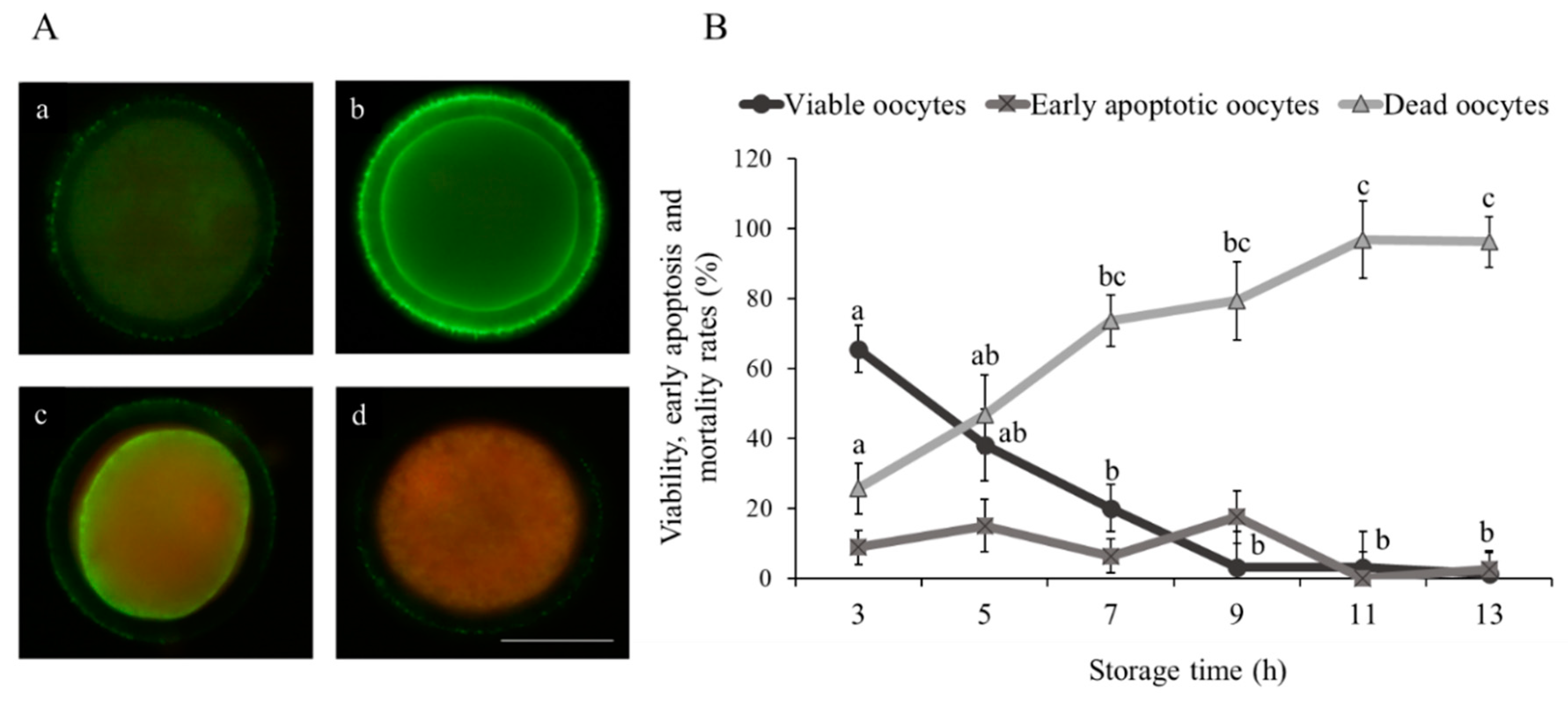
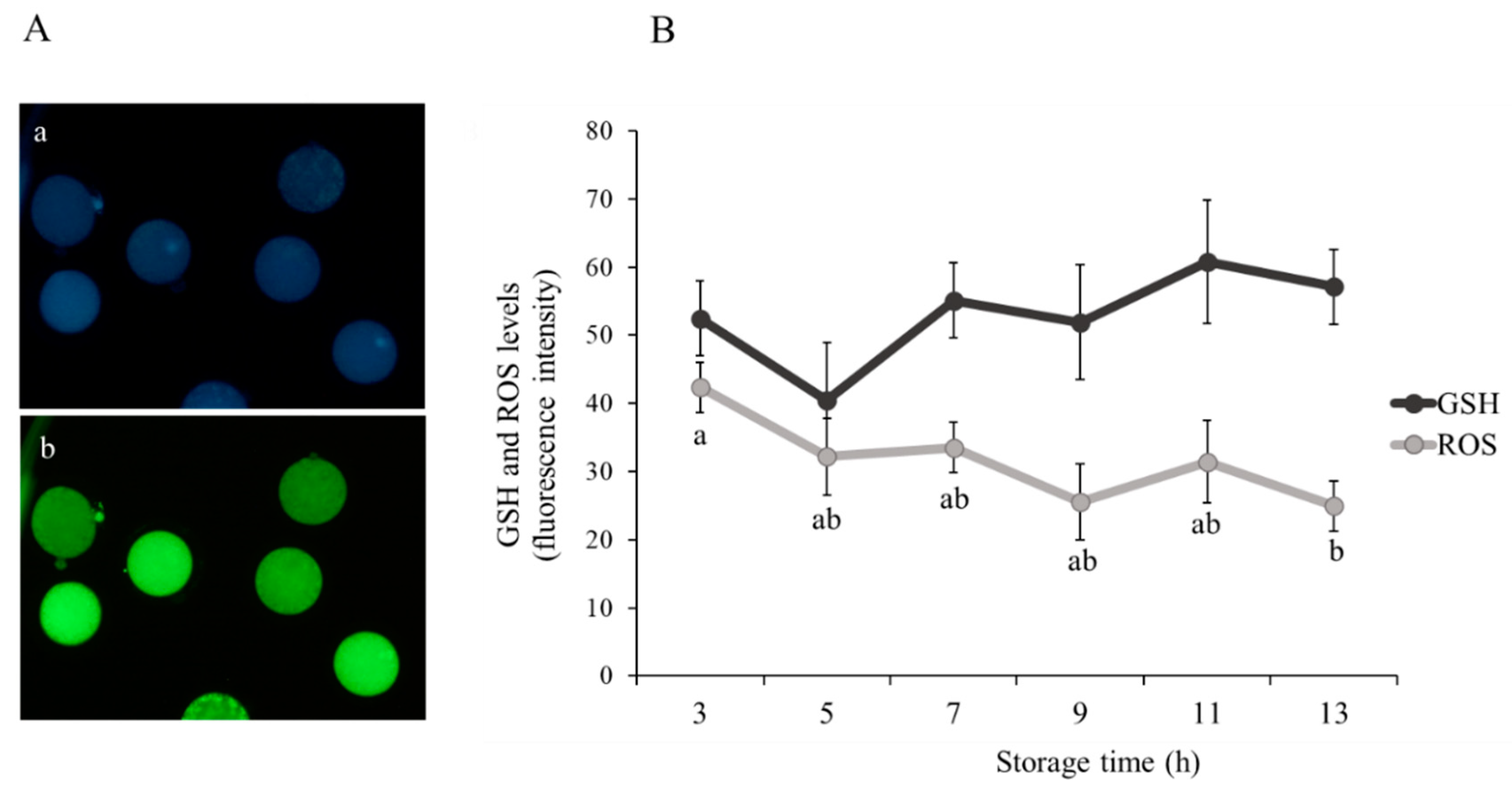
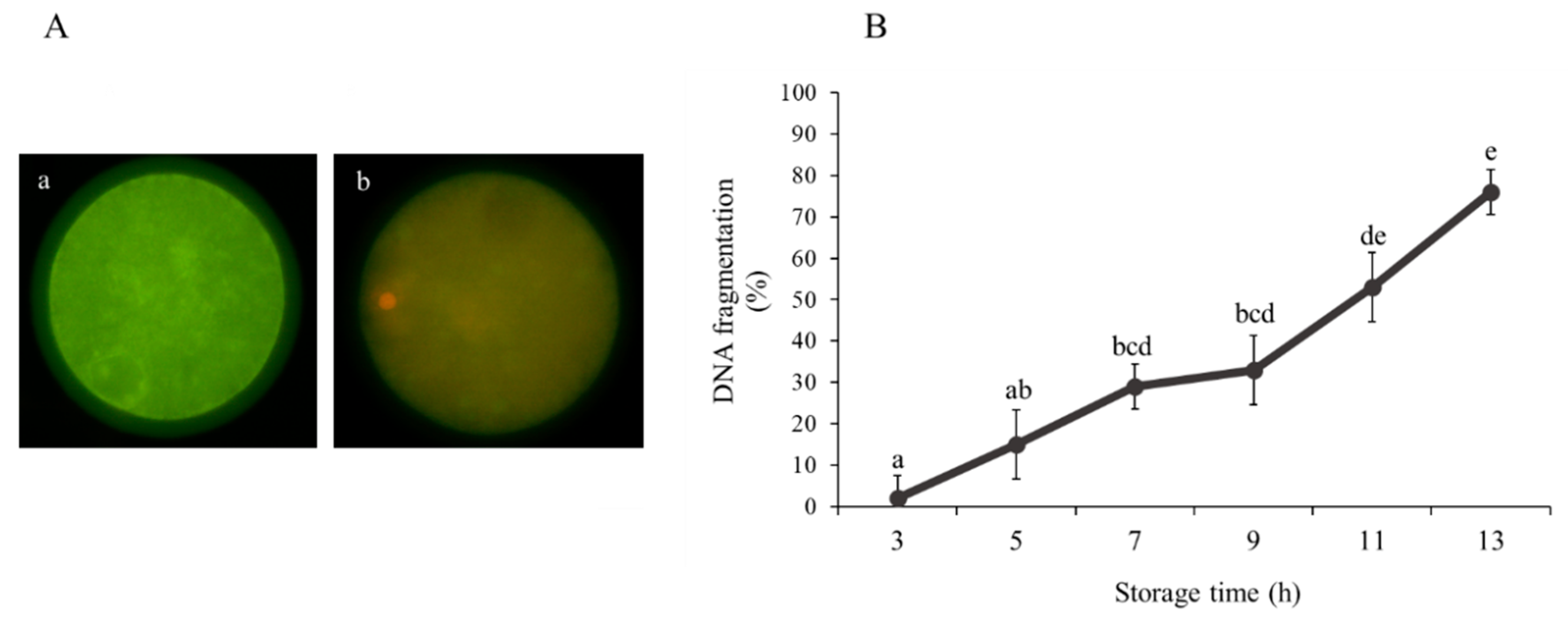
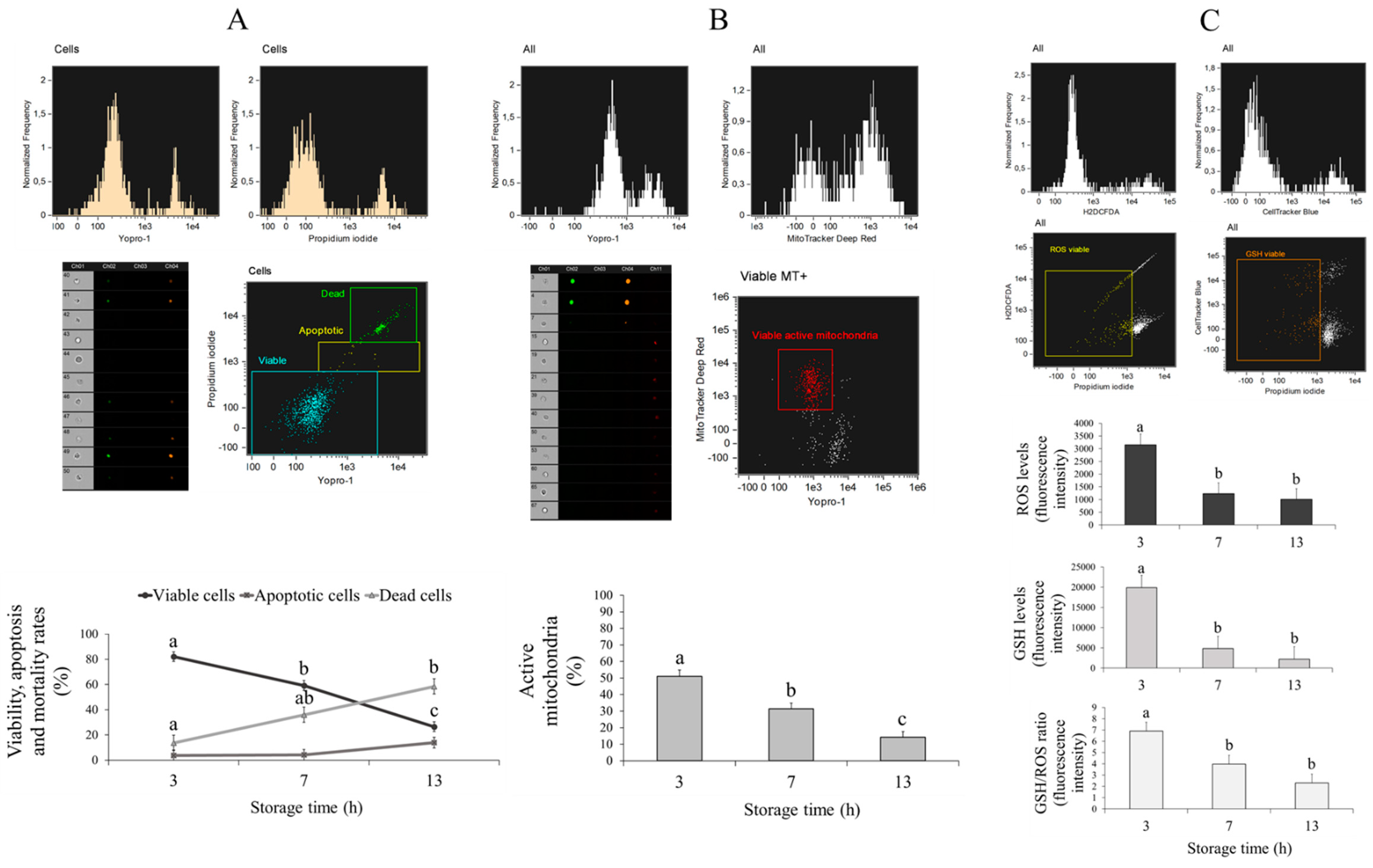
| Storage Time (h) | Total Oocyte (n) | Maturation MII (%) | Fertilization 2PN (%) |
|---|---|---|---|
| 3 | 187 | 68.33 ± 6.20 a | 44.49 ± 4.47 a |
| 7 | 144 | 30.07 ± 6.53 b | 15.31 ± 4.70 b |
| 13 | 140 | 4.69 ± 6.53 c | 1.56 ± 4.70 b |
| Storage Time (h) | Total Oocyte (n) | Cleaved Embryo at 48 hpi (%) | Expanded Blastocyst (%) | |
|---|---|---|---|---|
| Total | Cleaved | |||
| 3 | 555 | 65.96 ± 5.23 a | 26.68 ± 2.19 a | 40.82 ± 4.58 a |
| 7 | 519 | 18.16 ± 5.23 b | 4.66 ± 2.19 b | 13.23 ± 4.58 b |
| 13 | 386 | 2.59 ± 5.23 b | 0.25 ± 2.19 b | 6.25± 4.58 b |
| Treatment | Total Oocyte (n) | Maturation MII (%) | Fertilization 2PN (%) | Cleaved Embryo at 48 hpi (%) | Total Expanded Blastocyst (%) | Expanded Blastocyst/ Cleaved (%) | TUNEL-Positive Blastomeres (%) |
|---|---|---|---|---|---|---|---|
| TCM199 | 934 | 28.27 ± 5.30 b | 13.64 ± 3.83 b | 23.81 ± 4.27 b | 9.14 ± 1.78 | 13.15 ± 3.74 b | 13.94 ± 1.22 |
| Saline solution | 997 | 43.46 ± 5.15 a | 27.27 ± 3.71 a | 34.00 ± 4.27 a | 11.93 ± 1.78 | 27.05 ± 3.74 a | 11.42 ± 0.76 |
| Treatment | Viable Cells (%) | Apoptotic Cells (%) | Dead Cells (%) | Active Mitochondria (%) | GSH Levels (Fluorescence Intensity) | ROS Levels (Fluorescence Intensity) |
|---|---|---|---|---|---|---|
| TCM199 | 44.97 ± 3.87 b | 9.59 ± 3.29 | 45.07 ± 5.31 b | 26.42 ± 3.46 b | 8347.83 ± 2338.17 | 1591.14 ± 324.92 |
| Saline solution | 66.87 ± 3.87 a | 5.07 ± 3.29 | 27.08 ± 5.31 a | 38.97 ± 3.61 a | 9597.23 ± 2338.17 | 2007.19 ± 324.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Maestro, A.; Sánchez-Ajofrín, I.; Maside, C.; Peris-Frau, P.; Medina-Chávez, D.-A.; Cardoso, B.; Navarro, J.C.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Cellular and Molecular Events that Occur in the Oocyte during Prolonged Ovarian Storage in Sheep. Animals 2020, 10, 2414. https://doi.org/10.3390/ani10122414
Martín-Maestro A, Sánchez-Ajofrín I, Maside C, Peris-Frau P, Medina-Chávez D-A, Cardoso B, Navarro JC, Fernández-Santos MR, Garde JJ, Soler AJ. Cellular and Molecular Events that Occur in the Oocyte during Prolonged Ovarian Storage in Sheep. Animals. 2020; 10(12):2414. https://doi.org/10.3390/ani10122414
Chicago/Turabian StyleMartín-Maestro, Alicia, Irene Sánchez-Ajofrín, Carolina Maside, Patricia Peris-Frau, Daniela-Alejandra Medina-Chávez, Beatriz Cardoso, José Carlos Navarro, María Rocío Fernández-Santos, José Julián Garde, and Ana Josefa Soler. 2020. "Cellular and Molecular Events that Occur in the Oocyte during Prolonged Ovarian Storage in Sheep" Animals 10, no. 12: 2414. https://doi.org/10.3390/ani10122414
APA StyleMartín-Maestro, A., Sánchez-Ajofrín, I., Maside, C., Peris-Frau, P., Medina-Chávez, D.-A., Cardoso, B., Navarro, J. C., Fernández-Santos, M. R., Garde, J. J., & Soler, A. J. (2020). Cellular and Molecular Events that Occur in the Oocyte during Prolonged Ovarian Storage in Sheep. Animals, 10(12), 2414. https://doi.org/10.3390/ani10122414





