Analysis of Animal Well-Being When Supplementing Drinking Water with Tramadol or Metamizole during Chronic Pancreatitis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Analysis of Animal Distress and Water Consumption
2.3. Graphs and Statistical Analysis
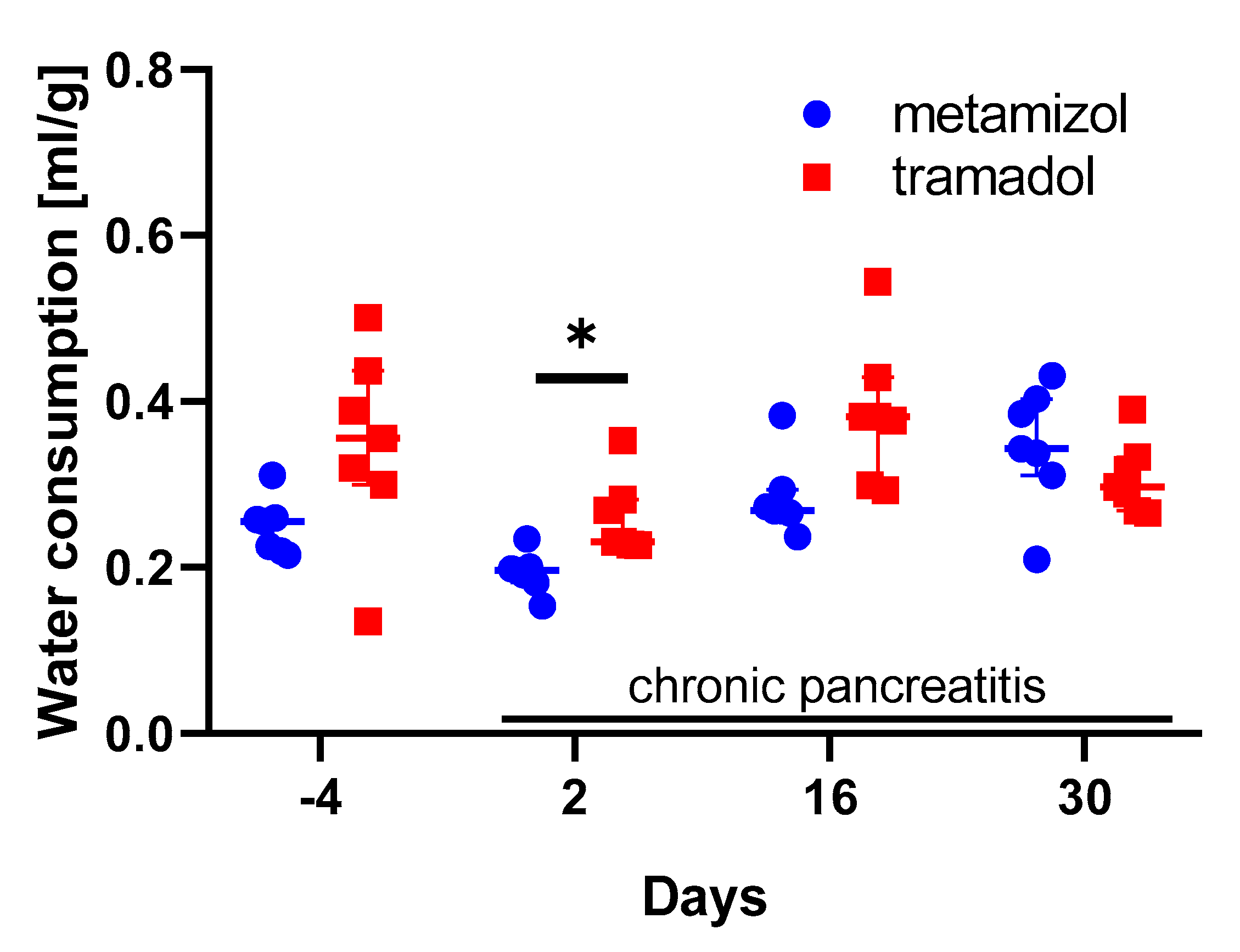
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prescott, M.J.; Lidster, K. Improving quality of science through better animal welfare: The NC3Rs strategy. Lab. Anim. 2017, 46, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L. Ethical and IACUC Considerations Regarding Analgesia and Pain Management in Laboratory Rodents. Comp. Med. 2019, 69, 443–450. [Google Scholar] [CrossRef] [PubMed]
- European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32010L0063&from=DE (accessed on 25 September 2020).
- Bekker, A.; Kloepping, C.; Collingwood, S. Meloxicam in the management of post-operative pain: Narrative review. J. Anaesthesiol. Clin. Pharmacol. 2018, 34, 450–457. [Google Scholar] [CrossRef]
- Stumpf, F.; Algül, H.; Thoeringer, C.K.; Schmid, R.M.; Wolf, E.; Schneider, M.R.; Dahlhoff, M. Metamizol Relieves Pain Without Interfering With Cerulein-Induced Acute Pancreatitis in Mice. Pancreas 2016, 45, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, A.; Kumstel, S.; Zhang, X.; Liebig, M.; Wendt, E.H.U.; Eichberg, J.; Palme, R.; Thum, T.; Vollmar, B.; Zechner, D. A novel multi-parametric analysis of non-invasive methods to assess animal distress during chronic pancreatitis. Sci. Rep. 2019, 9, 14084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, W.; Yuan, J.; Zhang, C.; Bai, Z.; Zhou, W.; Yan, J.; Li, X. Parenteral analgesics for pain relief in acute pancreatitis: A systematic review. Pancreatology 2013, 13, 201–206. [Google Scholar] [CrossRef]
- Peiró, A.M.; Martínez, J.; Martínez, E.; de Madaria, E.; Llorens, P.; Horga, J.F.; Pérez-Mateo, M. Efficacy and tolerance of metamizole versus morphine for acute pancreatitis pain. Pancreatology 2008, 8, 25–29. [Google Scholar] [CrossRef]
- Singh, V.K.; Yadav, D.; Garg, P.K. Diagnosis and Management of Chronic Pancreatitis: A Review. JAMA 2019, 322, 2422–2434. [Google Scholar] [CrossRef]
- Kumar, N.S.; Muktesh, G.; Samra, T.; Sarma, P.; Samanta, J.; Sinha, S.K.; Dhaka, N.; Yadav, T.D.; Gupta, V.; Kochhar, R. Comparison of efficacy of diclofenac and tramadol in relieving pain in patients of acute pancreatitis: A randomized parallel group double blind active controlled pilot study. Eur. J. Pain 2020, 24, 639–648. [Google Scholar] [CrossRef]
- Evangelista, V.R.; Draganov, D.I.; Rapp, C.; Avenel, F.; Steiner, G.; Arras, M.; Bergadano, A. Preliminary pharmacokinetics of tramadol hydrochloride after administration via different routes in male and female B6 mice. Vet. Anaesth. Analg. 2018, 45. [Google Scholar] [CrossRef]
- Jirkof, P.; Leucht, K.; Cesarovic, N.; Caj, M.; Nicholls, F.; Rogler, G.; Arras, M.; Hausmann, M. Burrowing is a sensitive behavioural assay for monitoring general wellbeing during dextran sulfate sodium colitis in laboratory mice. Lab. Anim. 2013, 47, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Oliver, V.L.; Thurston, S.E.; Lofgren, J.L. Using Cageside Measures to Evaluate Analgesic Efficacy in Mice (Mus musculus) after Surgery. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 186–201. [Google Scholar] [PubMed]
- Jirkof, P. Burrowing and nest building behavior as indicators of well-being in mice. J. Neurosci. Methods 2014, 234, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lofgren, J.; Miller, A.L.; Lee, C.C.S.; Bradshaw, C.; Flecknell, P.; Roughan, J. Analgesics promote welfare and sustain tumour growth in orthotopic 4T1 and B16 mouse cancer models. Lab. Anim. 2018, 52, 351–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matson, D.J.; Broom, D.C.; Carson, S.R.; Baldassari, J.; Kehne, J.; Cortright, D.N. Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J. Pharmacol. Exp. Ther. 2007, 320, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.A.; Doods, H.; Lamla, T.; Pekcec, A. Pharmacological characterization of intraplantar Complete Freund’s Adjuvant-induced burrowing deficits. Behav. Brain Res. 2016, 301, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Brase, D.A.; Loh, H.H.; Way, E.L. Comparison of the effects of morphine on locomotor activity, analgesia and primary and protracted physical dependence in six mouse strains. J. Pharmacol. Exp. Ther. 1977, 201, 368–374. [Google Scholar] [PubMed]
- Flecknell, P. Analgesics in Small Mammals. Vet. Clin. N. Am. Exot. Anim. Pract. 2018, 21, 83–103. [Google Scholar] [CrossRef]
- Foley, P.L.; Kendall, L.V.; Turner, P.V. Clinical Management of Pain in Rodents. Comp. Med. 2019, 69, 468–489. [Google Scholar] [CrossRef]
- Sadler, A.M.; Bailey, S.J. Repeated daily restraint stress induces adaptive behavioural changes in both adult and juvenile mice. Physiol. Behav. 2016, 167, 313–323. [Google Scholar] [CrossRef]
- Balcombe, J.P.; Barnard, N.D.; Sandusky, C. Laboratory routines cause animal stress. Contemp. Top. Lab. Anim. Sci. 2004, 43, 42–51. [Google Scholar] [PubMed]
- Meijer, M.K.; Spruijt, B.M.; van Zutphen, L.F.M.; Baumans, V. Effect of restraint and injection methods on heart rate and body temperature in mice. Lab. Anim. 2006, 40, 382–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjendal, K.; Ottesen, J.L.; Olsson, I.A.S.; Sørensen, D.B. Burrowing and nest building activity in mice after exposure to grid floor, isoflurane or ip injections. Physiol. Behav. 2019, 206, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Kröger, M.; Thümmler, J.; Tietze, L.; Palme, R.; Touma, C. Impact of three commonly used blood sampling techniques on the welfare of laboratory mice: Taking the animal’s perspective. PLoS ONE 2020, 15, e0238895. [Google Scholar] [CrossRef] [PubMed]
- Goldkuhl, R.; Hau, J.; Abelson, K.S.P. Effects of voluntarily-ingested buprenorphine on plasma corticosterone levels, body weight, water intake, and behaviour in permanently catheterised rats. In Vivo 2010, 24, 131–135. [Google Scholar]
- Coppola, D.M.; Slotnick, B. Odor-Cued Bitter Taste Avoidance. Chem. Senses 2018, 43, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Lemon, C.H.; Norris, J.E.; Heldmann, B.A. The TRPA1 Ion Channel Contributes to Sensory-Guided Avoidance of Menthol in Mice. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Hovard, A.; Teilmann, A.; Hau, J.; Abelson, K. The applicability of a gel delivery system for self-administration of buprenorphine to laboratory mice. Lab. Anim. 2015, 49, 40–45. [Google Scholar] [CrossRef]
- Molina-Cimadevila, M.J.; Segura, S.; Merino, C.; Ruiz-Reig, N.; Andrés, B.; de Madaria, E. Oral self-administration of buprenorphine in the diet for analgesia in mice. Lab. Anim. 2014, 48, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Taylor, B.F.; Ramirez, H.E.; Battles, A.H.; Andrutis, K.A.; Neubert, J.K. Analgesic Activity of Tramadol and Buprenorphine after Voluntary Ingestion by Rats (Rattus norvegicus). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 74–82. [Google Scholar]
- Hestehave, S.; Munro, G.; Pedersen, T.B.; Abelson, K.S.P. Antinociceptive effects of voluntarily ingested buprenorphine in the hot-plate test in laboratory rats. Lab. Anim. 2017, 51, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Jasiecka, A.; Maślanka, T.; Jaroszewski, J.J. Pharmacological characteristics of metamizole. Pol. J. Vet. Sci. 2014, 17, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Baskın, V.; Bilge, S.S.; Bozkurt, A.; Akyüz, B.; Ağrı, A.E.; Güzel, H.; İlkaya, F. Effect of nonsteroidal anti-inflammatory drugs on colorectal distension-induced visceral pain. Indian J. Pharmacol. 2016, 48, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Zajaczkowska, R.; Popiolek-Barczyk, K.; Pilat, D.; Rojewska, E.; Makuch, W.; Wordliczek, J.; Mika, J. Involvement of microglial cells in the antinociceptive effects of metamizol in a mouse model of neuropathic pain. Pharmacol. Biochem. Behav. 2018, 175, 77–88. [Google Scholar] [CrossRef]
- Guadarrama-Enríquez, O.; González-Trujano, M.E.; Ventura-Martínez, R.; Rodríguez, R.; Ángeles-López, G.E.; Reyes-Chilpa, R.; Baenas, N.; Moreno, D.A. Broccoli sprouts produce abdominal antinociception but not spasmolytic effects like its bioactive metabolite sulforaphane. Biomed. Pharmacother. 2018, 107, 1770–1778. [Google Scholar] [CrossRef]
- Dhesi, M.; Maldonado, K.A.; Maani, C.V. Tramadol; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Bassiony, M.M.; Salah El-Deen, G.M.; Yousef, U.; Raya, Y.; Abdel-Ghani, M.M.; El-Gohari, H.; Atwa, S.A. Adolescent tramadol use and abuse in Egypt. Am. J. Drug Alcohol Abus. 2015, 41, 206–211. [Google Scholar] [CrossRef]
- Abdel-Hamid, I.A.; Andersson, K.-E.; Waldinger, M.D.; Anis, T.H. Tramadol Abuse and Sexual Function. Sex. Med. Rev. 2016, 4, 235–246. [Google Scholar] [CrossRef]
- Lopopolo, M.; Affaitati, G.; Fabrizio, A.; Massimini, F.; Lapenna, D.; Giamberardino, M.A.; Costantini, R. Effects of tramadol on viscero-visceral hyperalgesia in a rat model of endometriosis plus ureteral calculosis. Fundam. Clin. Pharmacol. 2014, 28, 331–341. [Google Scholar] [CrossRef]
- Whittaker, A.L.; Lymn, K.A.; Wallace, G.L.; Howarth, G.S. Differential Effectiveness of Clinically-Relevant Analgesics in a Rat Model of Chemotherapy-Induced Mucositis. PLoS ONE 2016, 11, e0158851. [Google Scholar] [CrossRef] [Green Version]
- Drewes, A.M.; Bouwense, S.A.W.; Campbell, C.M.; Ceyhan, G.O.; Delhaye, M.; Demir, I.E.; Garg, P.K.; van Goor, H.; Halloran, C.; Isaji, S.; et al. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology 2017, 17, 720–731. [Google Scholar] [CrossRef]
- Majumder, S.; Chari, S.T. Chronic pancreatitis. Lancet 2016, 387, 1957–1966. [Google Scholar] [CrossRef]
- Kleeff, J.; Whitcomb, D.C.; Shimosegawa, T.; Esposito, I.; Lerch, M.M.; Gress, T.; Mayerle, J.; Drewes, A.M.; Rebours, V.; Akisik, F.; et al. Chronic pancreatitis. Nat. Rev. Dis. Primers 2017, 3, 17060. [Google Scholar] [CrossRef] [PubMed]
- Kumstel, S.; Tang, G.; Zhang, X.; Kerndl, H.; Vollmar, B.; Zechner, D. Grading Distress of Different Animal Models for Gastrointestinal Diseases Based on Plasma Corticosterone Kinetics. Animals 2019, 9, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tordoff, M.G. Taste solution preferences of C57BL/6J and 129X1/SvJ mice: Influence of age, sex, and diet. Chem. Senses 2007, 32, 655–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tordoff, M.G.; Bachmanov, A.A. Mouse taste preference tests: Why only two bottles? Chem. Senses 2003, 28, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Sclafani, A. Enhanced sucrose and Polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiol. Behav. 2006, 87, 745–756. [Google Scholar] [CrossRef]
- Kumstel, S.; Wendt, E.H.U.; Eichberg, J.; Talbot, S.R.; Häger, C.; Zhang, X.; Abdelrahman, A.; Schönrogge, M.; Palme, R.; Bleich, A.; et al. Grading animal distress and side effects of therapies. Ann. N. Y. Acad. Sci. 2020, 20–34. [Google Scholar] [CrossRef] [Green Version]
- Touma, C.; Sachser, N.; Möstl, E.; Palme, R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 2003, 130, 267–278. [Google Scholar] [CrossRef]
- Touma, C.; Palme, R.; Sachser, N. Analyzing corticosterone metabolites in fecal samples of mice: A noninvasive technique to monitor stress hormones. Horm. Behav. 2004, 45, 10–22. [Google Scholar] [CrossRef]
- Schneider, H.R.; Stadler, P.A.; Stütz, P.; Troxler, F.; Seres, J. Synthesis and properties of bromocriptine (author’s transl). Experientia 1977, 33. [Google Scholar] [CrossRef]
- Paster, E.V.; Villines, K.A.; Hickman, D.L. Endpoints for mouse abdominal tumor models: Refinement of current criteria. Comp. Med. 2009, 59, 234–241. [Google Scholar] [PubMed]
- Kumstel, S.; Vasudevan, P.; Palme, R.; Zhang, X.; Wendt, E.H.U.; David, R.; Vollmar, B.; Zechner, D. Benefits of non-invasive methods compared to telemetry for distress analysis in a murine model of pancreatic cancer. J. Adv. Res. 2020, 21, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kumstel, S.; Tang, G.; Talbot, S.R.; Seume, N.; Abshagen, K.; Vollmar, B.; Zechner, D. A rational approach of early humane endpoint determination in a murine model for cholestasis. ALTEX 2020, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deacon, R. Assessing burrowing, nest construction, and hoarding in mice. J. Vis. Exp. 2012, e2607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küster, T.; Zumkehr, B.; Hermann, C.; Theurillat, R.; Thormann, W.; Gottstein, B.; Hemphill, A. Voluntary ingestion of antiparasitic drugs emulsified in honey represents an alternative to gavage in mice. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 219–223. [Google Scholar] [PubMed]
- Abelson, K.S.P.; Jacobsen, K.R.; Sundbom, R.; Kalliokoski, O.; Hau, J. Voluntary ingestion of nut paste for administration of buprenorphine in rats and mice. Lab. Anim. 2012, 46, 349–351. [Google Scholar] [CrossRef] [Green Version]
- Hojman, P.; Eriksen, J.; Gehl, J. Tet-On induction with doxycycline after gene transfer in mice: Sweetening of drinking water is not a good idea. Anim. Biotechnol. 2007, 18, 183–188. [Google Scholar] [CrossRef]
- Jirkof, P.; Durst, M.; Klopfleisch, R.; Palme, R.; Thöne-Reineke, C.; Buttgereit, F.; Schmidt-Bleek, K.; Lang, A. Administration of Tramadol or Buprenorphine via the drinking water for post-operative analgesia in a mouse-osteotomy model. Sci. Rep. 2019, 9, 10749. [Google Scholar] [CrossRef] [Green Version]
- Coudereau, J.P.; Stain, F.; Drion, N.; Sandouk, P.; Monier, C.; Debray, M.; Scherrmann, J.M.; Bourre, J.M.; Francès, H. Effect of social isolation on the metabolism of morphine and its passage through the blood-brain barrier and on consumption of sucrose solutions. Psychopharmacology 1999, 144, 198–204. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Tordoff, M.G.; Beauchamp, G.K. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem. Senses 2001, 26, 905–913. [Google Scholar] [CrossRef]
- Jirkof, P.; Tourvieille, A.; Cinelli, P.; Arras, M. Buprenorphine for pain relief in mice: Repeated injections vs sustained-release depot formulation. Lab. Anim. 2015, 49, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.M.; Holtzman, M.; Kim, T.; Kharasch, E.D. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology 2011, 115, 1251–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Muñoz, F.J.; Moreno-Rocha, L.A.; Bravo, G.; Guevara-López, U.; Domínguez-Ramírez, A.M.; Déciga-Campos, M. Enhancement of antinociception but not constipation by combinations containing tramadol and metamizole in arthritic rats. Arch. Med. Res. 2013, 44. [Google Scholar] [CrossRef]
- Rawal, N.; Allvin, R.; Amilon, A.; Ohlsson, T.; Hallén, J. Postoperative analgesia at home after ambulatory hand surgery: A controlled comparison of tramadol, metamizol, and paracetamol. Anesth. Analg. 2001, 92, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, G.; Stankov, G.; Zerle, G.; Schinzel, S.; Brune, K. Observer-blind study with metamizole versus tramadol and butylscopolamine in acute biliary colic pain. Arzneimittelforschung 1993, 43, 1216–1221. [Google Scholar] [PubMed]
- Stankov, G.; Schmieder, G.; Zerle, G.; Schinzel, S.; Brune, K. Double-blind study with dipyrone versus tramadol and butylscopolamine in acute renal colic pain. World J. Urol. 1994, 12. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rocha, L.A.; López-Muñoz, F.J.; Medina-López, J.R.; Domínguez-Ramírez, A.M. Effect of tramadol on metamizol pharmacokinetics and pharmacodynamics after single and repeated administrations in arthritic rats. Saudi Pharm. J. 2016, 24, 674–684. [Google Scholar] [CrossRef] [Green Version]
- Suljević, I.; Hadžiavdić, M.; Šurković, I.; Suljević, O.; Turan, M.; Mušija, E. The preemptive effect of tramadol and metamizole on the intensity of postoperative pain. Med. Glas. 2020, 17, 285–289. [Google Scholar] [CrossRef]
- Abdel-Zaher, A.O.; Abdel-Rahman, M.S.; Elwasei, F.M. Protective effect of Nigella sativa oil against tramadol-induced tolerance and dependence in mice: Role of nitric oxide and oxidative stress. Neurotoxicology 2011, 32. [Google Scholar] [CrossRef]
- Kayser, V.; Besson, J.M.; Guilbaud, G. Effects of the analgesic agent tramadol in normal and arthritic rats: Comparison with the effects of different opioids, including tolerance and cross-tolerance to morphine. Eur. J. Pharmacol. 1991, 195, 37–45. [Google Scholar] [CrossRef]
- Miranda, H.F.; Pinardi, G. Antinociception, tolerance, and physical dependence comparison between morphine and tramadol. Pharmacol. Biochem. Behav. 1998, 61. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, G.; Nierath, W.-F.; Palme, R.; Vollmar, B.; Zechner, D. Analysis of Animal Well-Being When Supplementing Drinking Water with Tramadol or Metamizole during Chronic Pancreatitis. Animals 2020, 10, 2306. https://doi.org/10.3390/ani10122306
Tang G, Nierath W-F, Palme R, Vollmar B, Zechner D. Analysis of Animal Well-Being When Supplementing Drinking Water with Tramadol or Metamizole during Chronic Pancreatitis. Animals. 2020; 10(12):2306. https://doi.org/10.3390/ani10122306
Chicago/Turabian StyleTang, Guanglin, Wiebke-Felicitas Nierath, Rupert Palme, Brigitte Vollmar, and Dietmar Zechner. 2020. "Analysis of Animal Well-Being When Supplementing Drinking Water with Tramadol or Metamizole during Chronic Pancreatitis" Animals 10, no. 12: 2306. https://doi.org/10.3390/ani10122306
APA StyleTang, G., Nierath, W.-F., Palme, R., Vollmar, B., & Zechner, D. (2020). Analysis of Animal Well-Being When Supplementing Drinking Water with Tramadol or Metamizole during Chronic Pancreatitis. Animals, 10(12), 2306. https://doi.org/10.3390/ani10122306




